Introduction
Pancreatic carcinoma causes more than 20,000 deaths
every year in Japan, with an overall 5-year survival rate of less
than 5% (1,2). For patients with localized disease,
radical surgery may afford long-term benefits. However, even in
patients who undergo resection, the reported 5-year survival rate
remains in the range of 7–24%, and in most series the median
survival is only approximately 1 year, indicating that surgery
alone is usually inadequate. Even after curative resection,
patients with pancreatic cancer face a 50–80% local recurrence rate
and a 25–50% chance of developing distant metastases (3).
Gemcitabine, a deoxycytidine analogue that competes
for incorporation into DNA, thereby inhibiting its synthesis, is
the key drug for the treatment of pancreatic cancer. Compared to
resection alone, adjuvant chemotherapy with gemcitabine improves,
although to a limited degree, survival in patients with resectable
pancreatic adenocarcinoma (4).
However, a major drawback of adjuvant therapy for pancreatic cancer
is the impossibility of administering the designated therapy to
20–30% of patients as a result of post-operative complications,
delayed surgical recovery or early disease recurrence (5,6).
Hepatic arterial infusion (HAI) of chemotherapeutic
agents is a treatment option for patients with primary or
metastatic hepatic malignancies confined to the liver. The use of
HAI chemotherapy is based on sound physiology and pharmacology.
First, liver metastases that grow beyond 2–3 mm depend on the
hepatic artery for vascularization, whereas normal liver tissues
are perfused by the portal vein (7,8).
Second, HAI therapy allows drug delivery to hepatic metastases not
achievable by systemic administration, especially drugs with a high
systemic clearance (9). Third,
first-pass hepatic extraction of certain drugs results in lower
systemic concentrations, and hence less systemic toxicities
(10). Phase I studies of HAI
chemotherapy with gemcitabine in patients with liver malignancies
were recently reported (11–13).
Herein, we report a case of a patient with
pancreatic head cancer and post-operative liver metastases,
determined to be an unsuitable candidate for systemic chemotherapy
due to leukocytopenia. The patient was treated safely by HAI
consisting of gemcitabine and 5-FU.
Case report
In May 2008, a 61-year-old woman underwent
pancreaticoduodenectomy for pancreatic head cancer. Pathological
examination revealed invasive ductal carcinoma of the pancreas head
with three metastatic regional lymph nodes. Tumor stage was found
to be T1,-N1b,-M0, stage III according to the International Union
Against Cancer (UICC) classification. The patient received
pre-operative chemotherapy with gemcitabine and oral S-1. Although
two cycles of chemotherapy were originally planned, the second
cycle was not administered because the patient developed
leukocytopenia. The leukocyte count returned to within the normal
range pre-operatively, and the post-operative course was
uneventful. However, adjuvant systemic chemotherapy could not be
administered as the patient again developed leukocytopenia
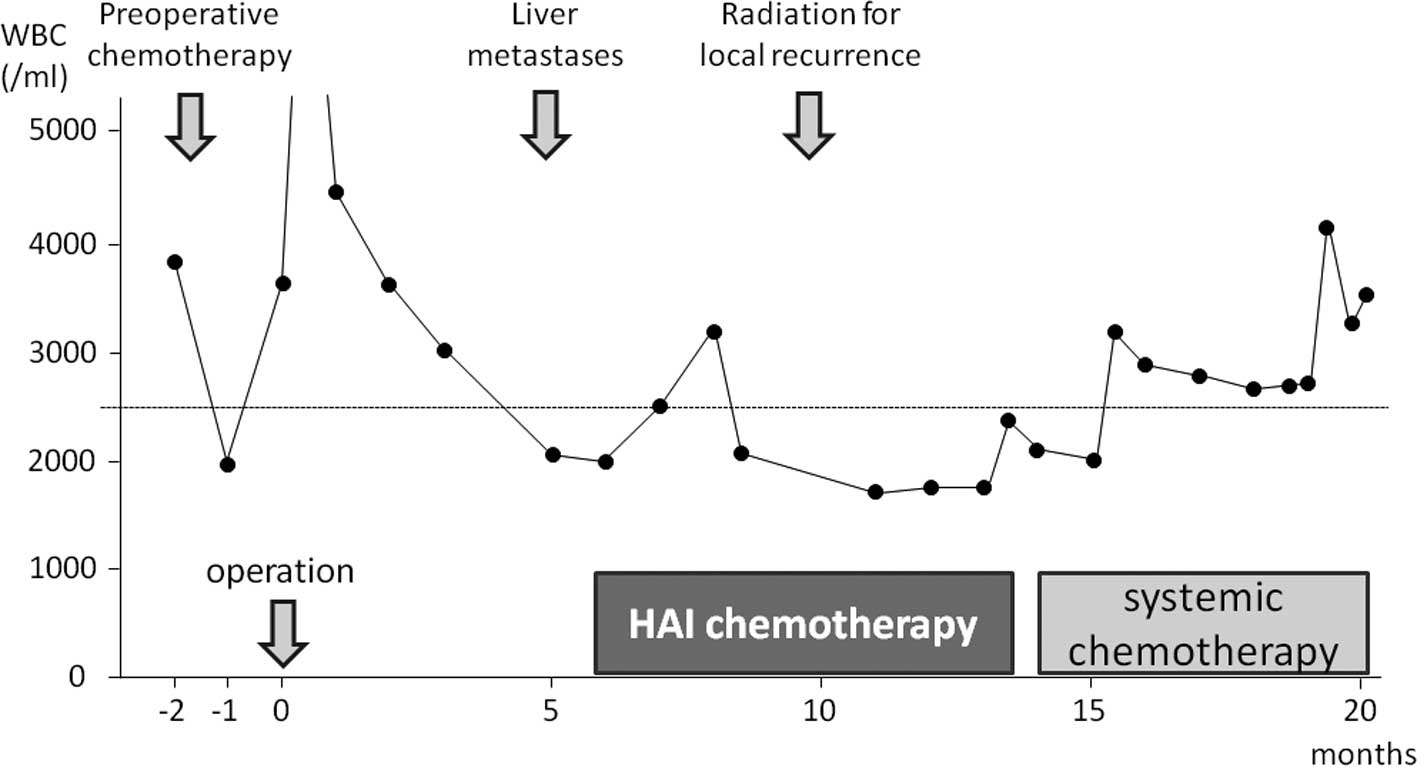
(Fig. 1). Five months after
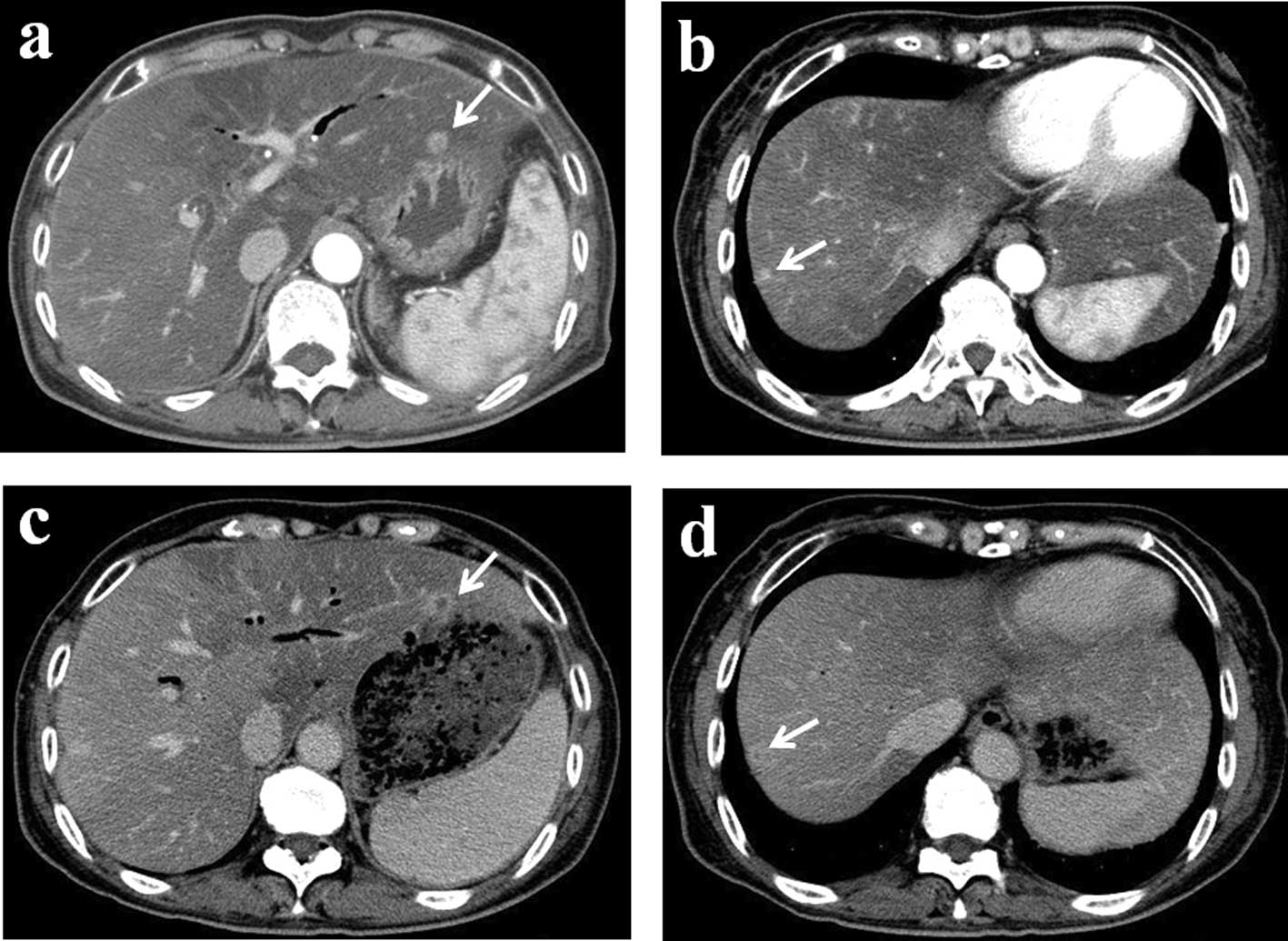
surgery, a follow-up computed tomography (CT) scan revealed two
liver metastases (Fig. 2a and b).
Due to the leukocytopenia, intravenous systemic chemotherapy was
contraindicated. In the apparent absence of recurrence, excepting
the liver metastases, we decided to administer HAI chemotherapy,
which had already been administered following the curative surgery
and has been shown to be associated with a lower incidence of
systemic side effects. Written informed consent was obtained from
the patient, and the treatment was undertaken with the approval of
the Medical Ethics Committee of Kanazawa University Hospital.
An intrahepatic arterial catheter was percutaneously
implanted after hepatic arteriography performed by right femoral
puncture. The catheter was then connected to a subcutaneous
implantable port system, located in the lower right abdominal area.
Gemcitabine (400 mg) was dissolved in 50 ml of saline for
administration by bedside pump over 30 min. After gemcitabine
infusion, 250 mg of 5-FU dissolved in 50 ml of saline was infused
continuously over 24 h from days 1 to 5. This comprised 1 cycle of
therapy. Each treatment cycle was continued biweekly from hospital
days 1 to 6.
After 10 cycles, a follow-up contrast-enhanced CT
revealed no decrease in the size of the metastatic tumors [stable
disease according to the Response Evaluation Criteria in Solid
Tumors (RECIST) guidelines]; however, tumor vascularity was reduced
(Fig. 2c and d). HAI treatment was
continued up to the 13th cycle without any severe side effects.
However, after the 10th treatment cycle (5 months from the start of
HAI chemotherapy), local recurrence was detected. Moreover, due to
partial thrombosis of the hepatic artery, it was necessary to
remove the HAI catheter and subcutaneous implantable port system
after the 13th cycle. At this time, the leukocyte count had
returned to within the normal range, and the patient was
administered systemic chemotherapy (Fig. 1). Systemic chemotherapy was
continued at an outpatient clinic for 22 months after the
institution of HAI treatment.
Discussion
Pancreatic cancer is a fatal disease, with a 5-year
survival rate of less than 5%. Surgery remains the only curative
option. Therefore, in order to improve the prognosis for patients
with carcinoma of the pancreatic head, we usually perform radical
pancreatic resection, including wide lymph node dissection and
complete removal of the extra pancreatic nerve plexus of the
superior mesenteric artery or celiac axis (14–16).
Compared to resection alone, adjuvant chemotherapy improves,
although to a limited degree, survival in patients with resectable
pancreatic adenocarcinoma (4).
However, a major drawback of adjuvant therapy for pancreatic cancer
is the impossibility of administering the designated therapy to
20–30% of patients as a result of post-operative complications,
delayed surgical recovery or early disease recurrence (5,6).
Theoretically, these issues may be addressed by the use of
neoadjuvant therapy, in order to ensure that more patients are able
to receive potentially beneficial adjuvant treatment. Recently,
several clinical studies of neoadjuvant chemo-(radio)-therapy in
pancreatic cancer have been reported (20–24).
In this case, we attempted two pre-operative cycles of chemotherapy
with gemcitabine and oral S-1. However, only 1 cycle of
chemotherapy was administered due to the development of
leukocytopenia.
HAI chemotherapy has been studied most extensively
in patients with liver metastases from colorectal cancer treated
using fluorodeoxyuridine or 5-FU. Despite the significantly higher
response rates to HAI than to intravenous infusion, most of these
studies did not report a significant prolongation of survival;
nonetheless, meta-analyses were performed (25,26).
Arterial infusion chemotherapy with gemcitabine and 5-FU has
previously been reported for locally advanced pancreatic cancer and
liver metastases from pancreatic cancer (10,27,28). Moreover, in certain Phase I
studies, HAI chemotherapy with gemcitabine was well tolerated when
administered at doses of up to 1,000 mg/m2 infused over
400 min (8,9). Super-selective HAI delivers high
doses of chemotherapeutic agents into the tumor vessels, producing
increased regional levels with greater effectiveness and a lower
incidence/severity of systemic side effects. Thus, as in the
presented case, HAI chemotherapy may be indicated for
liver-directed treatment.
According to the pharmacokinetics of gemcitabine,
when 1,000 mg/m2 of gemcitabine is injected by
intravenous infusion over 30 min, the average maximum plasma
concentrations is 21,865±4,165 ng/ml by 15 min. It is reported that
the flow volume of the proper hepatic artery is approximately 330
ml/ min (25). When 400 mg of
gemcitabine is infused into the proper hepatic artery over 30 min,
the local plasma concentration in the liver is approximately 40,000
ng/ml by 30 min. On the other hand, the plasma concentration of 250
mg of 5-FU infused into the proper hepatic artery over 24 h has
been shown to be 0.5 μg/ml. This concentration is equal to the
concentration obtained following administration of 30 mg/kg (1,350
mg for this patient) of 5-FU over 24 h (26). Vogl et al reported that the
maximum tolerated dose of hepatic intraarterial chemotherapy with
gemcitabine was 1,400 mg/m2 (8). Maruyama et al reported that
when 1,000–1,500 mg of 5-FU was infused into the hepatic artery
over 5 h, the maximum plasma concentration was 0.48 μg/ml, on
average, and no grade 3 adverse effects were noted. In this case,
gemcitabine was infused at 400 mg due to the presense of
leukocytopenia (27).
At the time of presentation of liver metastases,
systemic chemotherapy could not be administered to our patient due
to the presence of leukocytopenia. After the 13th treatment cycle,
the HAI catheter and subcutaneous implantable port system were
removed due totube trouble. However, the leukocyte count had been
restored to normal range by this time, and the patient was
administered systemic chemotherapy.
In conclusion, HAI chemotherapy is useful and safe
for the treatment of malignant tumors confined to the liver, even
in patients in a poor general condition. A Phase I study of HAI
chemotherapy with gemcitabine and 5-FU is underway in patients with
pancreatic cancer with post-operative metastases confined to the
liver.
References
|
1.
|
Ministry of Health, Labor and Welfare: The
Dynamic Statistics of the Population in 2005. http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei05/hyo7/htmluri.
|
|
2.
|
Ishii H, Furuse J, Boku N, et al: Phase II
study of gemcitabine chemotherapy alone for locally advanced
pancreatic carcinoma: JCOG0506. Jpn J Clin Oncol. 40:573–579. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Evans DB, Abbruzzese JL and Willett CG:
Cancer of the pancreas. Cancer: Priciples and Practice of Oncology.
De Vita VT Jr, Hellman S and Rosenberg SA: 6th edition. Lippincott,
Williams and Wilkins; Philadelphia: pp. 1126–1161. 2001
|
|
4.
|
Oettle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs. observation in patients
undergo curative-intent resection of pancreatic cancer: a
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar
|
|
5.
|
Aloia TE, Lee JE, Vauthey JN, et al:
Delayed recovery after pancreaticoduodenectomy: a major factor
impairing the delivery of adjuvant chemotherapy? J Am Coll Surg.
204:347–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sandy H, Bruckner H, Cooperman A, et al:
Survival advantage of combined chemoradiotherapy compared with
resection as the initial treatment of patients with regional
pancreatic carcinoma. An outcomes trial. Cancer. 89:314–327. 2000.
View Article : Google Scholar
|
|
7.
|
Ensminger WD, Rosowsky A and Raso V: A
clinical pharmacological evaluation of hepatic arterial infusions
of 5-fluoro-2-deoxyuridine and 5-fluoroufacil. Cancer Res.
38:3789–3792. 1978.PubMed/NCBI
|
|
8.
|
Vogl TJ, Schwarz W, Eichler K, et al:
Hepatic intraarterial chemotherapy with gemcitabine in patients
with unresectable cholangiocarcinoma and liver metastases of
pancreatic cancer: a clinical study on maximum tolerable dose and
treatment efficacy. J Cancer Res Clin Oncol. 132:745–755. 2006.
View Article : Google Scholar
|
|
9.
|
Tse AN, Wu N, Patel D, et al: A phase I
study of gemcitabine given via intrahepatic pump for primary or
metastatic hepatic malignancies. Cancer Chemother Pharmacol.
64:935–944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Van Riel JM, Peters GJ, Mammatas LH, et
al: A phase I and pharmacokinetic study of gemcitabine given by
24-h hepatic arterial infusion. Euro J Cancer. 45:2519–2527.
2009.PubMed/NCBI
|
|
11.
|
Nagakawa T, Nagamori M, Futakami F, et al:
Result of extensive surgery for pancreatic carcinoma. Cancer.
77:640–645. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Noto M, Miwa K, Kitagawa H, et al:
Pancreas head carcinoma. Frequency of invasion to soft tissue
adherent to the superior mesenteric artery. Am J Surg Pathol.
29:1056–1061. 2005.PubMed/NCBI
|
|
13.
|
Traverso LW: Pancreatic cancer: surgery
alone is not sufficient. Surg Endosc. 20(Suppl 2): 446–449. 2006.
View Article : Google Scholar
|
|
14.
|
Spitz FR, Abbruzzese JL, Lee JE, et al:
Preoperative and postoperative chemoradiation strategies in
patients treated with pancreaticoduodenectomy for adenocarcinoma of
the pancreas. J Clin Oncol. 15:928–937. 1997.PubMed/NCBI
|
|
15.
|
Hoffman JP, Lipsitz S, Pisansky T, et al:
Phase II trial of preoperative radiation therapy and chemotherapy
for patients with localized, resectable adenocarcinoma of the
pancreas: an Eastern Cooperative Oncology Group Study. J Clin
Oncol. 16:317–323. 1998.
|
|
16.
|
Palmer DH, Stocken DD, Hewitt H, et al: A
randomized phase 2 trial of neoadjuvant chemotherapy in resectable
pancreatic cancer: gemicitabine alone versus gemcitabine combined
with cisplatin. Ann Surg Oncol. 14:2088–2096. 2007. View Article : Google Scholar
|
|
17.
|
Golcher H, Brunner T, Grabenbauer G, et
al: Preoperative chemoradiation in adenocarcinoma of the pancreas.
A single centre experience advocating a new treatment strategy.
EJSO. 34:756–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Turrini O, Viret F, Zabotto LM, et al:
Neoadjuvant 5 fluorouracilcisplatin chemoradiation effect on
survival in patients with resectable pancreatic head
adenocarcinoma: a ten-year single institution experience. Oncology.
76:413–419. 2009.PubMed/NCBI
|
|
19.
|
Takai S, Satoi S, Yanagimoto H, et al:
Neoadjuvant chemoradiation in patients with potentially resectable
pancreatic cancer. Pancreas. 36:26–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Satoi S, Yanaagimoto H, Toyokawa H, et al:
Surgical results after prepoerative chemoradiation therapy for
patienes with pancreatic cancer. Pancreas. 38:282–288. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Cohen AD and Kemeny NE: An update on
hepatic arterial infusion chemotherapy for colorectal cancer.
Oncologist. 8:553–566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mocellin S, Pilati P, Lise M, et al:
Meta-analysis of hepatic arterial infusion for unresectable liver
metastases from colorectal cancer: the end of an era? J Clin Oncol.
25:5649–5654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Homma H, Akiyama T, Mezawa S, et al:
Advanced pancreatic carcinoma showing a complete response to
arterial infusion chemotherapy. Int J Clin Oncol. 9:197–201. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Miyanishi K, Ishiwatari H and Hayashi T: A
phase I trial lf arterial infusion chemotherapy with gemcitabine
and 5-fluorouracil for unresectable advanced pancreatic cancer
after vascular supply distribution via superselective embolization.
Jpn J Clin Oncol. 38:268–274. 2008. View Article : Google Scholar
|
|
25.
|
Mogami K, Ichihara T, Sato T, et al: A
case of the papilla lf vater accompanied witn a stricture of the
celiac artery by the median arcuate ligament. Jpn J Gastroenterol
Surg. 41:1588–1593. 2008. View Article : Google Scholar
|
|
26.
|
Kikuchi K and Kanno H: Comparison for
blood levels and clinical effects between tablet and other dosage
forms of 5-fluorouracil (5-FU). Gan To Kagaku Ryouho. 6:559–565.
1979.
|
|
27.
|
Maruyama S, Ando M and Watayo T:
Concentration of 5-FU after hepatic artery infusion chemotherapy
for liver metastases of colorectal cancer. Gan To Kagaku Ryouho.
30:1635–1638. 2003.PubMed/NCBI
|