Introduction
Of the three leading causes of death in Japan –
malignant neoplasms, cardiovascular diseases and cerebrovascular
diseases – malignant neoplasms have been the leading cause of death
in Japan since 1981. For the last 30 years, liver cancer has been
the third leading cause of death by malignant neoplasms in men and,
during the past decade, has ranked fifth in women (1–3).
Hepatocellular carcinoma (HCC) accounts for 85–90% of cases of
primary liver cancer, and chronic hepatitis B and C infections are
the main cause of HCC. However, the prevalence of HCC in Japan in
the liver of patients that are both hepatitis B surface antigen
(HBsAg)- and hepatitis C virus (HCV)-RNA-negative has been
increasing over the last 12 years (4).
Epidemiological findings have recently been reported
proposing a link between type 2 diabetes mellitus (DM) and cancer
in various organs (5,6). The possibility that DM may increase
the risk of liver cancer, as well as cancer at other sites, has
been raised in a number of cohorts and case-control studies
(7–10). We carried out this retrospective
study to determine the prevalence of type 2 DM in Japanese patients
with HCC.
Patients and methods
Patients
A total of 1,251 patients with HCC diagnosed between
January 1991 and December 2005 at the liver disease centers of the
National Nagasaki Medical Center and Nagasaki University Hospital
were consecutively recruited for this study. Informed consent was
obtained from all patients. The diagnosis of HCC was based on the
elevation of serum α-fetoprotein or des-γ-carboxy prothrombin
levels, characteristic image findings obtained using
ultrasonography, computerized tomography, magnetic resonance
imaging and hepatic angiography, and/or histological diagnosis
using tumor biopsy samples.
Etiology of HCC
The HCC cases were categorized according to etiology
into four groups: HCC-B, hepatitis B virus surface antigen
(HBsAg)-positive and hepatitis C virus (HCV)-RNA-negative; HCC-C,
HCV-RNA-positive and HBsAg-negative; HCC-BC, both HBsAg- and
HCV-RNA-positive; and HCC-nonBC, both HBsAg- and HCV-RNA-negative.
A diagnosis of chronic HCV infection was based on the presence of
both serum anti-HCV antibody and HCV-RNA detected by polymerase
chain reaction (PCR), while a diagnosis of chronic hepatitis B
virus (HBV) infection was based on the presence of HBsAg.
Diagnosis of type 2 DM
Type 2 DM was diagnosed on the basis of the presence
of hyperglycemia (≥200 mg/dl) in at least two postabsorptive
samples, overt glycosuria, or both; or active treatment with
insulin, oral hypoglycemic agents, or both. No consideration was
given to minor alterations in glucose metabolism, such as impaired
glucose tolerance based on an oral glucose tolerance test, in
accordanc with World Health Organization criteria.
Statistical analysis
Data were analyzed by the Mann-Whitney U test for
continuous ordinal data, and by the χ2 test with Yates’
correction and Fisher’s exact test for associations between two
qualitative variables. p<0.05 was considered statistically
significant. Data analysis was performed with SPSS version 16.0 for
Windows.
Results
Clinical features of the studied
patients
As shown in Table
I, of the 1,251 patients with HCC, 20% (248/1,251) were
diagnosed with HCC-B, whereas 65% (809/1,251) had HCC-C and an
additional 2% (29/1,251) had HCC associated with both viruses. In
the remaining 165 patients (13%), no association was found between
HCC and either of the viruses. Analyzing the patients with HCC by
category revealed the male/female ratio in HCC-B, HCC-C, HCC-BC and
HCC-nonBC to be 3.4, 2.3, 1.9 and 2.8, respectively. The
male/female ratio in HCC-C was less than that in HCC-B. In
addition, the median age of patients diagnosed with HCC-B, HCC-C,
HCC-BC and HCC-nonBC was 57, 67, 65 and 67 years, respectively. The
median age of patients diagnosed with HCC-B was significantly lower
than that of the patients with other types of HCC. Among the
patients with HCC, 25% (310/1,251) had type 2 DM, 3% (34/1,251)
HCC-B, 16% (209/1,251) HCC-C, 1% (6/1,251) HCC-BC and 5% (61/1,251)
HCC-nonBC.
 | Table I.Characteristics of the HCC
patients. |
Table I.
Characteristics of the HCC
patients.
| HCC-B | HCC-C | HCC-BC | HCC-nonBC | Total |
|---|
| No. | 248 | 809 | 29 | 165 | 1,251 |
| Gender | | | | | |
| Male | 191 | 566 | 19 | 121 | 897 |
| Female | 57 | 243 | 10 | 44 | 354 |
| Ratio
(male/female) | 3.4 | 2.3 | 1.9 | 2.8 | 2.5 |
| Age (IQR), in
years | 57 (15) | 67 (9) | 65 (12) | 67 (14) | 66 (11) |
| <66 | 190 | 341 | 17 | 71 | 619 |
| ≥66 | 58 | 468 | 12 | 94 | 632 |
| Child-Pugh grade | | | | | |
| A | 95 | 70 | 80 | 67 | 412 |
| B | 111 | 213 | 240 | 292 | 1,134 |
| C | 8 | 8 | 9 | 11 | 46 |
Prevalence of type 2 DM by stratification
according to etiology in patients with HCC
Cohorts of patients with HCC were divided according
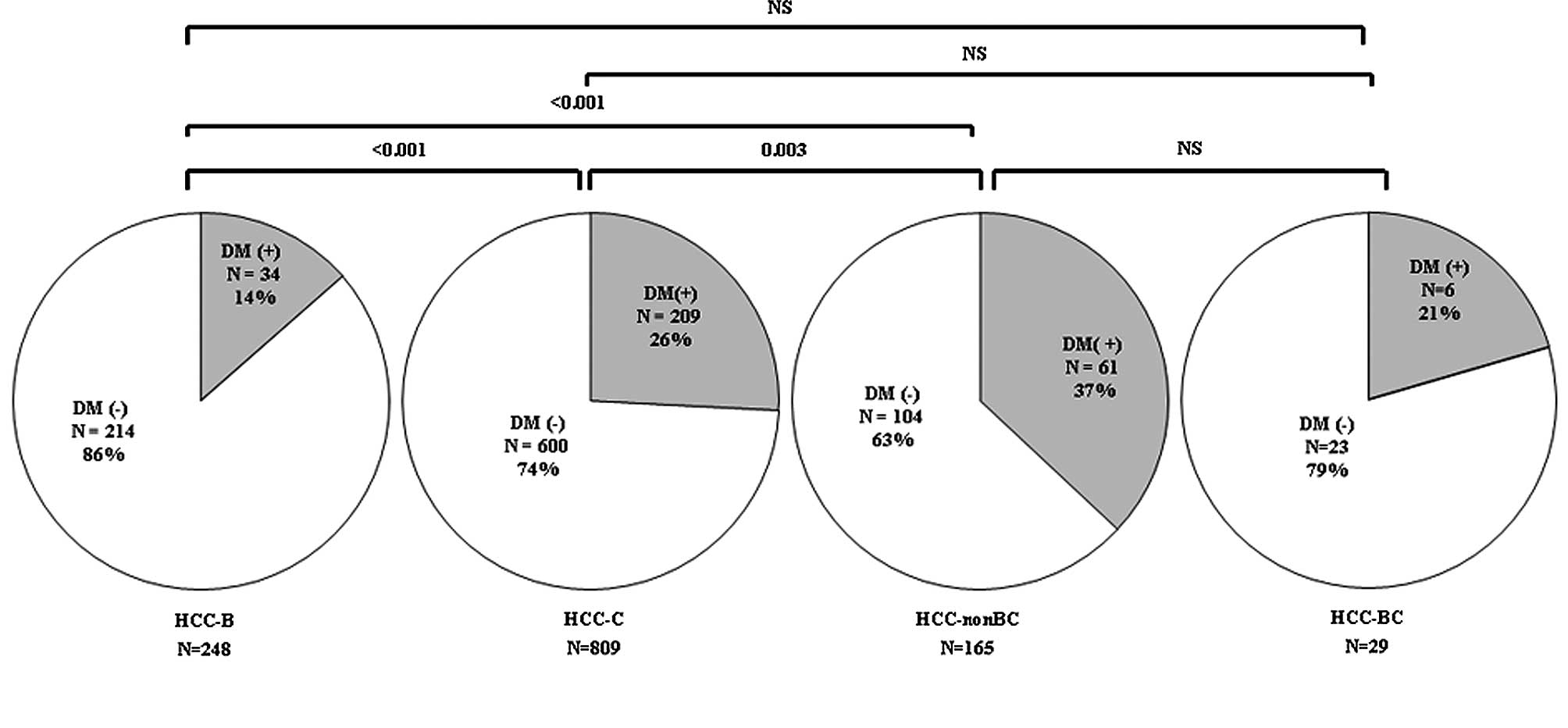
to etiology. Fig. 1 shows that the
prevalence rate of type 2 DM in HCC-B, HCC-C, HCC-BC and HCC-nonBC
was 14% (34/248), 26% (209/809), 37% (61/165) and 21% (6/29),
respectively. The prevalence rate of HCC-nonBC and HCC-C was
significantly higher than that of HCC-B (HCC-B vs. HCC-nonBC,
p≤0.001; HCC-B vs. HCC-C, p≤0.001), while the prevalence rate of
HCC-nonBC was significantly higher than that of HCC-C (HCC-C vs.
HCC-nonBC, p=0.003).
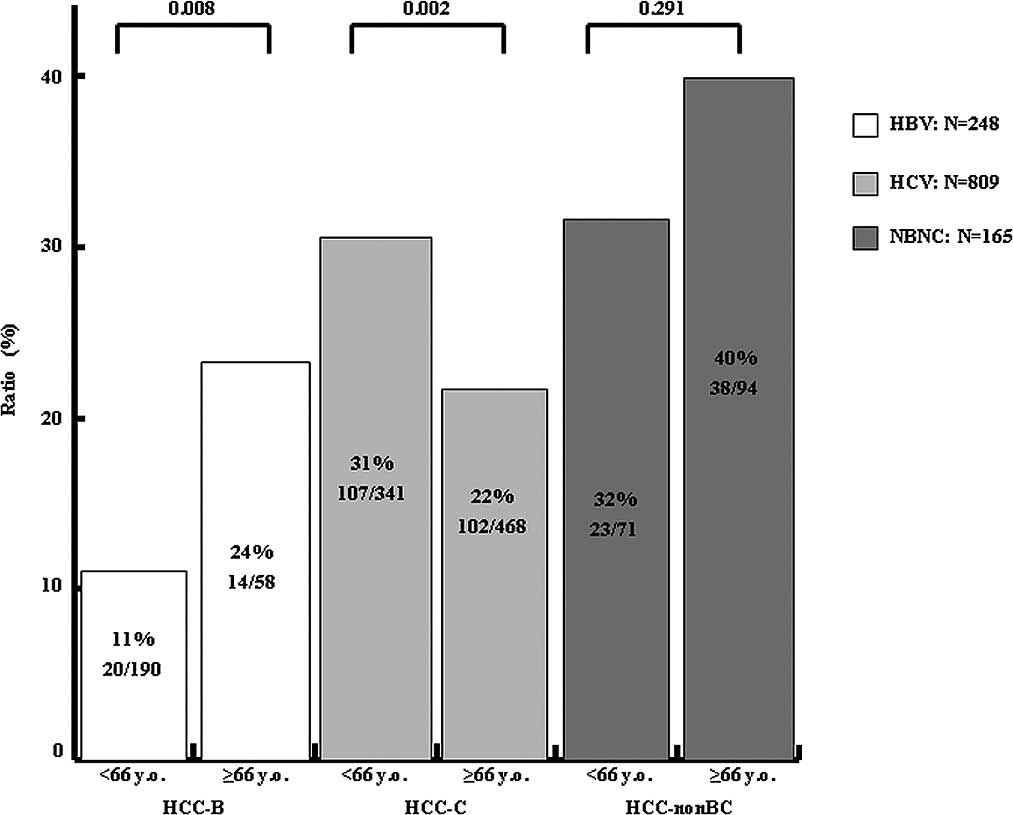
The prevalence rate of type 2 DM was 25% in patients
under 66 years of age (154/619) and 25% in patients over 66 years
of age (156/632). Fig. 2 shows the
age distribution of the prevalence rate for type 2 DM in HCC-B,
HCC-C and HCC-nonBC cases. The prevalence rate of type 2 DM in
HCC-B, HCC-C and HCC-nonBC was 11% (20/190), 31% (107/341) and 32%
(23/71), respectively, in patients under 66 years of age, vs. 24%
(14/58), 22% (102/468) and 40% (38/94), respectively, for those
over 66 years of age. The prevalence rate of type 2 DM in HCC-B and
HCC-nonBC patients over 66 years of age was increased, whereas that
of HCC-C was significantly decreased.
Discussion
A nationwide health survey regarding the prevalence
of DM in the general Japanese population conducted in 2006
indicated that the prevalence of DM in Japan was 12%. However, the
prevalence rate of type 2 DM is higher in patients with HCC than in
the general Japanese population. In this two major liver
center-based cohort study designed to examine the prevalence of
type 2 DM in HCC patients, 25% of patients with HCC had type 2 DM.
Previous studies have suggested that DM is a potential risk factor
for HCC (10–13). Inoue et al prospectively
examined the association between a history of DM and the subsequent
risk of cancer in a Japan Public Health Center-based prospective
study, and found an increased risk of liver cancer in DM patients
(12).
The present study found that the prevalence of type
2 DM was significantly higher in HCC-nonBC than in HCC-B and HCC-C
patients. In particular, type 2 DM persisted in patients without
chronic hepatitis virus infections; type 2 DM in these individuals
may explain a relevant proportion of the observed cases of HCC.
Previous studies have suggested that diabetes and/or non-alcoholic
fatty liver disease account for at least a portion of these
‘idiopathic’ cases (14–16). Findings from the present study
support the hypothesis that the presence of DM alone accounts for
approximately 37% of cases of HCC-nonBC.
Investigations into the possible biological
mechanisms of the association between type 2 DM and HCC-nonBC have
been site-specific. However, these associations may be the result
of metabolic and hormonal aberrations associated with type 2 DM,
and common biological mechanisms may be at least partially
associated with insulin and insulin-like growth factors (IGFs)
(17).
The most obvious change in diabetic patients is
reduced insulin sensitivity with compensatory hyperinsulinemia and
elevated levels of IGF-1, which may in turn stimulate cell
proliferation in the liver (18,19).
At the same time, insulin activates the IGF-1 receptor, which is
known to have a growth-promoting effect, including the modulation
of cell cycle progression. Excess insulin may also indirectly
affect the development of cancer by down-regulating the level of
IGF-binding protein 1, which increases the level and
bioavailability of total circulating IGF-1. Obesity and physical
inactivity also cause hyperinsulinemia, and are thus also
ultimately associated with cancer (17–20).
A survey of HCC-nonBC conducted between 1995 and
2003 in Japan by the Inuyama Hepatitis Research Group found that
individuals with HCC-nonBC accounted for 9.3% of the general
population (2). In the present
study, we found the percentage of HCC-nonBC to be 14.1% in the
Nagasaki area. Furthermore, the number and proportion of HCC-nonBC
cases gradually increased from 1981 to 2005 (4). According to an epidemiological study
on DM by Nakano et al, the number of patients with DM has
been gradually increasing since the development of an automotive
society and the Westernization of the Japanese diet (21). Since the prevalence of DM increases
with age, the proportion of individuals with DM aged 60 or above
has exceeded two-thirds of the estimated total number of patients
in Japan (7.40 million in 2002), which has a rapidly aging society
(21). In other words, the number
of individuals with type 2 DM is increasing in Japan, and these
individuals are at high risk for HCC. Thus, the number of HCC-nonBC
cases will increase in the next decade in Japan.
Approximately 60% of liver cancer cases in Japan are
anti-HCV-positive (4). An
experimental study revealed that HCV infection itself induces
insulin resistance through the disturbance of the insulin
intracellular signaling pathway by the hepatitis virus core protein
(22). Liver fat deposition may
contribute to insulin resistance, which in turn leads to a loss of
the restraining effect of insulin on the production of glucose by
hepatocytes, thereby causing diabetes (23). Steatosis occurs more frequently in
patients with chronic HCV infection than in those with chronic HBV
infection; this may explain the increased risk of DM among HCV
patients (24). Although we
proposed possible explanations for the correlation between HCV
infection and the prevalence rate of type 2 DM in patients in this
study, it is also possible that the mechanism is multifactorial. A
previous study identified chronic hepatitis B as having no
relationship to DM, and on the basis of the results of this study,
we arrive at the same conclusion (25,26).
Several studies have indicated that the progression
from chronic hepatitis to cirrhosis and HCC is accelerated by dual
HCV infection (11,27). The strong effect of DM on HCC in
the absence of hepatitis infection suggests that, in addition to
the hepatitis C causal pathway, HCC is mediated through the
reduction of IGF-1 factors or IGF binding protein-3, caused by
hyperinsulinemia. This in turn stimulates the proliferation of
cancer cells, as demonstrated by Lagiou et al (28). In the present study, the prevalence
rate of DM in patients with HCC-C was significantly higher in
patients older than 66 years of age. Our findings demonstrate that
the effects of the interaction between DM and HCV further the
incidence of HCC.
In conclusion, the prevalence of HCC-nonBC and HCC-C
was significantly higher than that of HCC-B, while the prevalence
of HCC-nonBC was significantly higher than that of HCC-C. In
patients over 66 years of age, the prevalence of type 2 DM in HCC-B
and HCC-nonBC cases was increased, whereas the prevalence of type 2
DM in HCC-C cases was significantly decreased. Our findings
indicate that the interaction between type 2 DM and HCV increases
the prevalence of HCC.
References
|
1.
|
Kiyosawa K and Tanaka E: Characteristics
of hepatocellular carcinoma in Japan. Oncology. 62:5–7. 2002.
View Article : Google Scholar
|
|
2.
|
Umemura T and Kiyosawa K: Epidemiology of
hepatocellular carcinoma in Japan. Hepatol Res. 37(Suppl 2):
95–100. 2007. View Article : Google Scholar
|
|
3.
|
El-Serag HB: Hepatocellular carcinoma: an
epidemiologic view. J Clin Gastroenterol. 35:S72–S78. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Taura N, Yatsuhashi H, Nakao K, Ichikawa T
and Ishibashi H: Long-term trends of the incidence of
hepatocellular carcinoma in the Nagasaki prefecture, Japan. Oncol
Rep. 21:223–227. 2009.PubMed/NCBI
|
|
5.
|
Moore MA, Park CB and Tsuda H:
Implications of the hyperinsulinaemia-diabetes-cancer link for
preventive efforts. Eur J Cancer Prev. 7:89–107. 1998.PubMed/NCBI
|
|
6.
|
El-Serag HB and Everhart JE: Diabetes
increases the risk of acute hepatic failure. Gastroenterology.
122:1822–1828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ajiki W, Tsukuma H and Oshima A: Cancer
incidence and incidence rates in Japan in 1999: estimates based on
data from 11 population-based cancer registries. Jpn J Clin Oncol.
34:352–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shintani Y, Fujie H, Miyoshi H, et al:
Hepatitis C virus infection and diabetes: direct involvement of the
virus in the development of insulin resistance. Gastroenterology.
126:840–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Polesel J, Zucchetto A, Montella M, et al:
The impact of obesity and diabetes mellitus on the risk of
hepatocellular carcinoma. Ann Oncol. 20:353–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
El-Serag HB, Tran T and Everhart JE:
Diabetes increases the risk of chronic liver disease and
hepatocellular carcinoma. Gastroenterology. 126:460–468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Davila JA, Morgan RO, Shaib Y, McGlynn KA
and El-Serag HB: Diabetes increases the risk of hepatocellular
carcinoma in the United States: a population based case control
study. Gut. 54:533–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Inoue M, Iwasaki M, Otani T, Sasazuki S,
Noda M and Tsugane S: Diabetes mellitus and the risk of cancer:
results from a large-scale population-based cohort study in Japan.
Arch Intern Med. 166:1871–1877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fujino Y, Mizoue T, Tokui N and Yoshimura
T: Prospective study of diabetes mellitus and liver cancer in
Japan. Diabetes Metab Res Rev. 17:374–379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wideroff L, Gridley G, Mellemkjaer L, et
al: Cancer incidence in a population-based cohort of patients
hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst.
89:1360–1365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ichikawa T, Yanagi K, Motoyoshi Y, et al:
Two cases of non-alcoholic steatohepatitis with development of
hepatocellular carcinoma without cirrhosis. J Gastroenterol
Hepatol. 21:1865–1866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Adami HO, Chow WH, Nyren O, et al: Excess
risk of primary liver cancer in patients with diabetes mellitus. J
Natl Cancer Inst. 88:1472–1477. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Le Roith D: Seminars in medicine of the
Beth Israel Deaconess Medical Center. Insulin-like growth factors.
N Engl J Med. 336:633–640. 1997.PubMed/NCBI
|
|
18.
|
Stuver SO, Kuper H, Tzonou A, et al:
Insulin-like growth factor 1 in hepatocellular carcinoma and
metastatic liver cancer in men. Int J Cancer. 87:118–121. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Su TS, Liu WY, Han SH, et al: Transcripts
of the insulin-like growth factors I and II in human hepatoma.
Cancer Res. 49:1773–1777. 1989.PubMed/NCBI
|
|
20.
|
Macaulay VM: Insulin-like growth factors
and cancer. Br J Cancer. 65:311–320. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nakano T and Ito H: Epidemiology of
diabetes mellitus in old age in Japan. Diabetes Res Clin Pract.
77(Suppl 1): 76–81. 2007. View Article : Google Scholar
|
|
22.
|
Davila JA, Morgan RO, Shaib Y, McGlynn KA
and El-Serag HB: Hepatitis C infection and the increasing incidence
of hepatocellular carcinoma: a population-based study.
Gastroenterology. 127:1372–1380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Banerji MA, Buckley MC, Chaiken RL, Gordon
D, Lebovitz HE and Kral JG: Liver fat, serum triglycerides and
visceral adipose tissue in insulin-sensitive and insulin-resistant
black men with NIDDM. Int J Obes Relat Metab Disord. 19:846–850.
1995.PubMed/NCBI
|
|
24.
|
Czaja AJ, Carpenter HA, Santrach PJ and
Moore SB: Host- and disease-specific factors affecting steatosis in
chronic hepatitis C. J Hepatol. 29:198–206. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fraser GM, Harman I, Meller N, Niv Y and
Porath A: Diabetes mellitus is associated with chronic hepatitis C
but not chronic hepatitis B infection. Isr J Med Sci. 32:526–530.
1996.PubMed/NCBI
|
|
26.
|
Chang KC, Tsai PS, Hsu MC, Hung SF, Tsai
CC and Lu SN: Chronic hepatitis C increased the mortality rates of
patients with hepatocellular carcinoma and diabetes mellitus in a
triple hepatitis virus endemic community. J Gastroenterol.
45:636–645. 2010. View Article : Google Scholar
|
|
27.
|
Lai MS, Hsieh MS, Chiu YH and Chen TH:
Type 2 diabetes and hepatocellular carcinoma: a cohort study in
high prevalence area of hepatitis virus infection. Hepatology.
43:1295–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lagiou P, Kuper H, Stuver SO, Tzonou A,
Trichopoulos D and Adami HO: Role of diabetes mellitus in the
etiology of hepatocellular carcinoma. J Natl Cancer Inst.
92:1096–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|