Introduction
Sepsis is caused by polymicrobial infections
associated with severe systemic inflammatory response syndrome,
which leads to multiple organ failure, such as acute lung injury,
renal and hepatic failure, and septic shock (1–3).
Bacterial endotoxin lipopolysaccharide (LPS) is a major component
of the outer membrane of Gram-negative bacteria and plays a pivotal
role by stimulating mononuclear phagocytes (macrophages and
monocytes) to secrete various inflammatory mediators, such as
cytokines, reactive oxygen species, prostanoid/leukotriens,
proteases and NO (1,3). In addition, the high-mobility group
box-1 (HMGB1), a highly conserved non-histone nuclear protein that
binds with DNA, participates in gene transcription and functions as
a cytokine in the extracellular milieu, up-regulates the expression
of proinflammatory cytokines (e.g., TNF-α, IL-1β and IL-8) in human
mononuclear cells and neutrophils (4,5) and
adhesion molecules, such as the intercellular cell adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)
in endothelial cells (6).
Furthermore, HMGB1 is suggested to play a crucial role in
endotoxin/septic shock as a late phase mediator. This is based on
the findings that serum levels of HMGB1 increase in a late phase
(8–32 h) after LPS exposure, and that administration of an
anti-HMGB1 antibody attenuates endotoxin lethality in mice
(7). Since these pro-inflammatory
substances are overproduced and involved in the pathogenesis of
septic shock, therapeutic strategies have targeted the blockade of
these molecules; however, most of the strategies have been
unsuccessful (8,9). Thus, the development of novel agents
with therapeutic potential for sepsis/endotoxin shock is being
explored.
The resolution phase of inflammation is now
understood as not only a passive dilution of pro-inflammatory
mediators, but also a highly regulated and active process via the
production of various anti-inflammatory and pro-resolving
mediators. Recently, novel ω-3 polyunsaturated fatty acid [e.g.
eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)]-derived
mediators with potent anti-inflammatory and pro-resolving
activities have been discovered and termed resolvins (10,11).
E-series resolvins are derived from EPA, and D-series resolvins are
from DHA. Resolvin D1 (RvD1, 7S,8R,17S-trihydroxy-DHA), a D-series
resolvin, was originally identified in resolving inflammatory
exudates and is produced by sequential oxygenation of DHA by
15-lipoxygenase (LOX) and 5-LOX in vivo (12). RvD1 exerts potent anti-inflammatory
and pro-resolving actions on various cell types. For example, RvD1
was found to suppress the transendothelial migration of human
neutrophils in vitro (12)
and neutrophil infiltration in vivo in a murine peritonitis
model (12), as well as the
release of pro-inflammatory cytokines from LPS-stimulated mouse
macrophages in vitro (13,14).
Based on these potential pro-resolving activities of RvD1, it is
reasonable to speculate that RvD1 may attenuate the pathological
condition of sepsis/endotoxin shock. In this study, we evaluated
the effect of RvD1 on the extracellular release of HMGB1, the
production of inflammatory cytokines, the accumulation of
peritoneal cells and hepatocyte apoptosis in vivo using a
D-galactosamine (D-GalN)-sensitized mouse endotoxin shock
model.
Materials and methods
Reagents
LPS (from E. coli serotype O111:B4) and
D-(+)-galactosamine hydrochloride (D-GalN) were purchased from
Sigma Chemicals (St. Louis, MO, USA). RvD1 was obtained from Cayman
Chemical (Ann Arbor, MI, USA). A mouse inflammation kit (BD™
Cytometric Bead Array system) was purchased from BD Bioscience (San
Jose, CA, USA).
D-GalN-sensitized endotoxin shock
model
A D-GalN-sensitized mouse model (15), which is highly susceptible to LPS,
was utilized to assess the potential of RvD1 to suppress an
inflammatory reaction in vivo. Male C57BL/6 mice aged 7–9
weeks (Sankyo Laboratories, Tokyo, Japan) were intraperitoneally
injected with 400 μl of D-GalN (18 mg, dissolved in saline)
or D-GalN + LPS (25 ng) without or with RvD1 (0.1 or 1 μg).
Thereafter, mice were anesthetized by intraperitoneal injection of
pentobarbital, and blood was collected by cardiac puncture for the
assays of the cytokines (1 h after the challenge) and HMGB1 (5 h
after the challenge). All animal procedures were approved by the
Ethics Committee of Juntendo University, Graduate School of
Medicine and performed according to the institutional
guidelines.
Quantification of serum cytokine
levels
Serum levels of TNF-α, IL-6, IL-10, IL-12p70, MCP-1
and IFN-γ were quantified with the mouse inflammation kit. Sera
were prepared from blood by centrifugation at 1000 g for 10 min and
diluted 10-fold with an assay diluent. Then, 50-μl aliquots
were assayed for cytokines according to the manufacturer’s
instructions. The system includes six fluorescently distinguishable
capture beads coated with antibodies against six analytes (TNF-α,
IL-6, IL-10, IL-12p70, MCP-1 and IFN-γ) and detects these cytokines
by ELISA on microbeads. The detection limits were 7.3 pg/ml for
TNF-α, 5 pg/ml for IL-6, 17.5 pg/ml for IL-10, 10.7 pg/ml for
IL-12p70, 52.7 pg/ml for MCP-1 and 2.5 pg/ml for IFN-γ.
Quantification of serum HMGB1
Serum HMGB1 levels were quantitated with
10-μl aliquots of sera using an HMGB1 ELISA kit II
(Shino-Test Corp., Kanagawa, Japan) according to the manufacturer’s
instructions. The detection limit of HMGB1 was 1 ng/ml.
Peritoneal exudate cell counting
Mice were euthanized by ether anesthesia 5 h after
receiving the peritoneal LPS injection and D-GalN, and peritoneal
exudate cells were recovered by washing with 5 ml ice-cold PBS.
Cells were stained with Turk’s solution for counting the total cell
number. Alternatively, cells were cytocentrifuged (Cytospin 4;
ThermoShandon, Cheshire, UK) and stained with May-Grünwald-Giemsa
solution (Muto Pure Chemicals, Tokyo, Japan) for differential cell
counts (neutrophils and mononuclear cells); at least 200 cells per
slide on duplicate cytospins were examined under a light
microscope.
Evaluation of hepatocyte apoptosis
Mice were euthanized by ether anesthesia 5 h after
the peritoneal LPS injection and D-GalN administration, and the
liver was then resected, trimmed and fixed with 4% paraformaldehyde
for 12 h at 4°C, followed by immersion in a series of PBS/sucrose
solution until reaching the sucrose concentration of 30%. The
tissue was embedded in optimal cutting temperature (OCT) embedding
medium (Sakura Finetechnical, Tokyo, Japan), by freezing in liquid
nitrogen and sectioned at 50 μm with a cryostat (CM3051 S,
Leica, Wetzlar, Germany). Sections were stained with a TUNEL
(terminal deoxynucleotidyltransferase-mediated dUTP nick end
labeling) reagent for detecting apoptosis (In Situ Cell
Death Detection kit, Fluorescein, Roche Diagnostics, Penzberg,
Germany) and mounted with an aqueous medium fluoromount (Diagnostic
Biosystems, Pleasanton, CA, USA). Fluorescent images were captured
with a microscope system Axioplan 2 (Carl Zeiss, Jena, Germany).
The number of TUNEL+ cells was counted in 2 high power
fields/mouse (x200 magnification) and averaged. Sections were also
stained with May-Grünwald-Giemsa; we confirmed that the
inflammatory cells, such as neutrophils and mononuclear cells, were
not infiltrated into the injured liver tissues 5 h after the
injection of LPS/D-GalN (data not shown).
Statistical analysis
Data represent the mean ± standard error of the mean
(SEM). Statistical significance was determined by one-way ANOVA
analysis (Graphpad PRISM, La Jolla, CA, USA). A P-value <0.05
was considered to be significant.
Results
Effects of resolvin D1 on the release and
production of septic mediators in a D-GalN-sensitized endotoxin
shock model
To determine whether RvD1 modulates the levels of
septic mediators in LPS/D-GalN mice, we examined the effect of RvD1
on the release and production of septic mediators in sera. We first
investigated the effect of RvD1 on the serum level of HMGB1, a
non-histone nuclear protein that is extracellularly released from
dying cells and functions as a late-phase mediator in
endotoxin/septic shock (7,16,17).
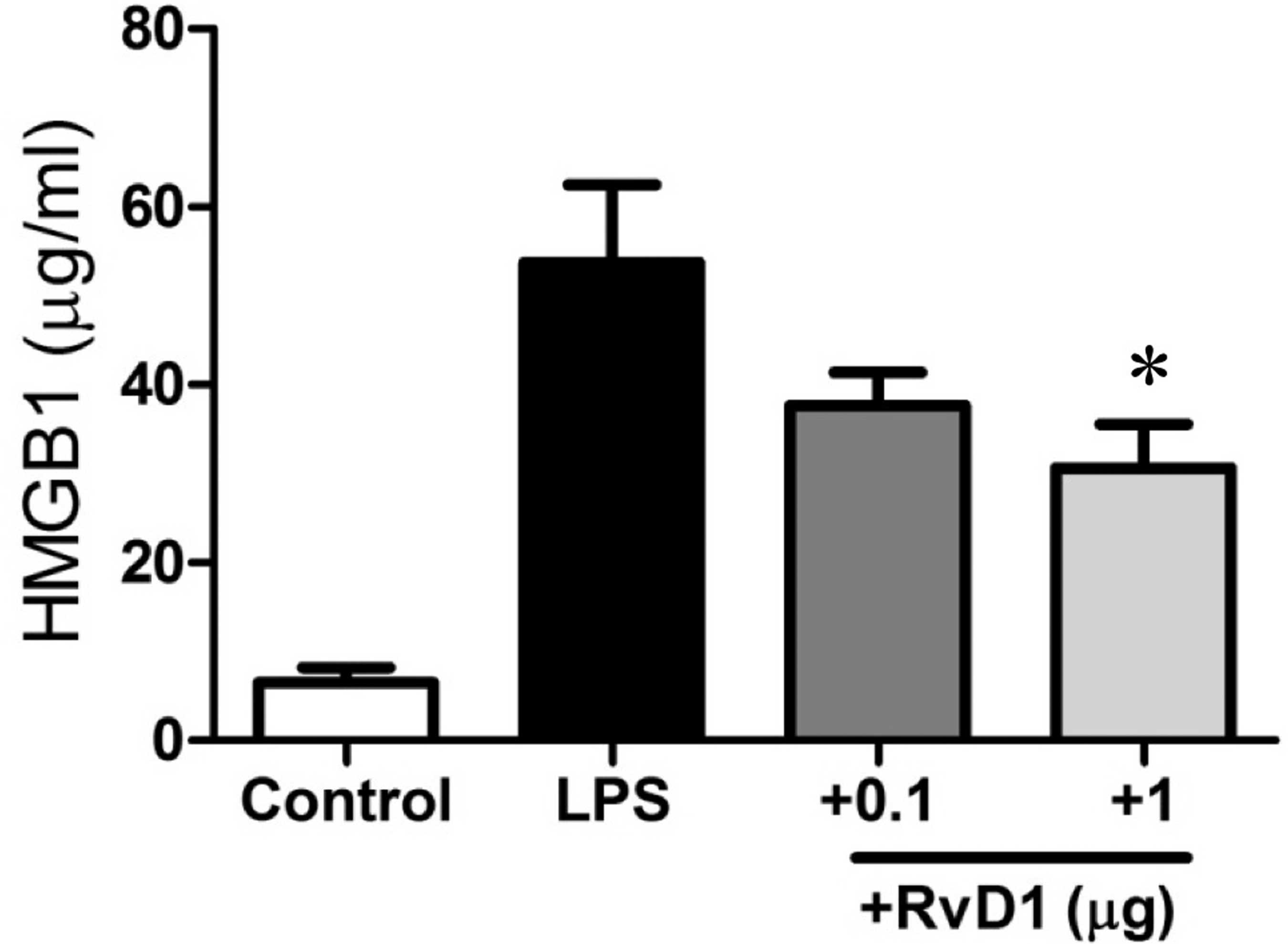
As presented in Fig. 1, the HMGB1
level was strikingly elevated from 6.5±1.7 to 53.7±8.8 ng/ml 5 h
after LPS administration (P<0.001). Notably, the administration
of 1 μg of RvD1 significantly reduced the HMGB1 level to
30.6±5.0 ng/ml (P<0.05). We then examined the effect of RvD1 on
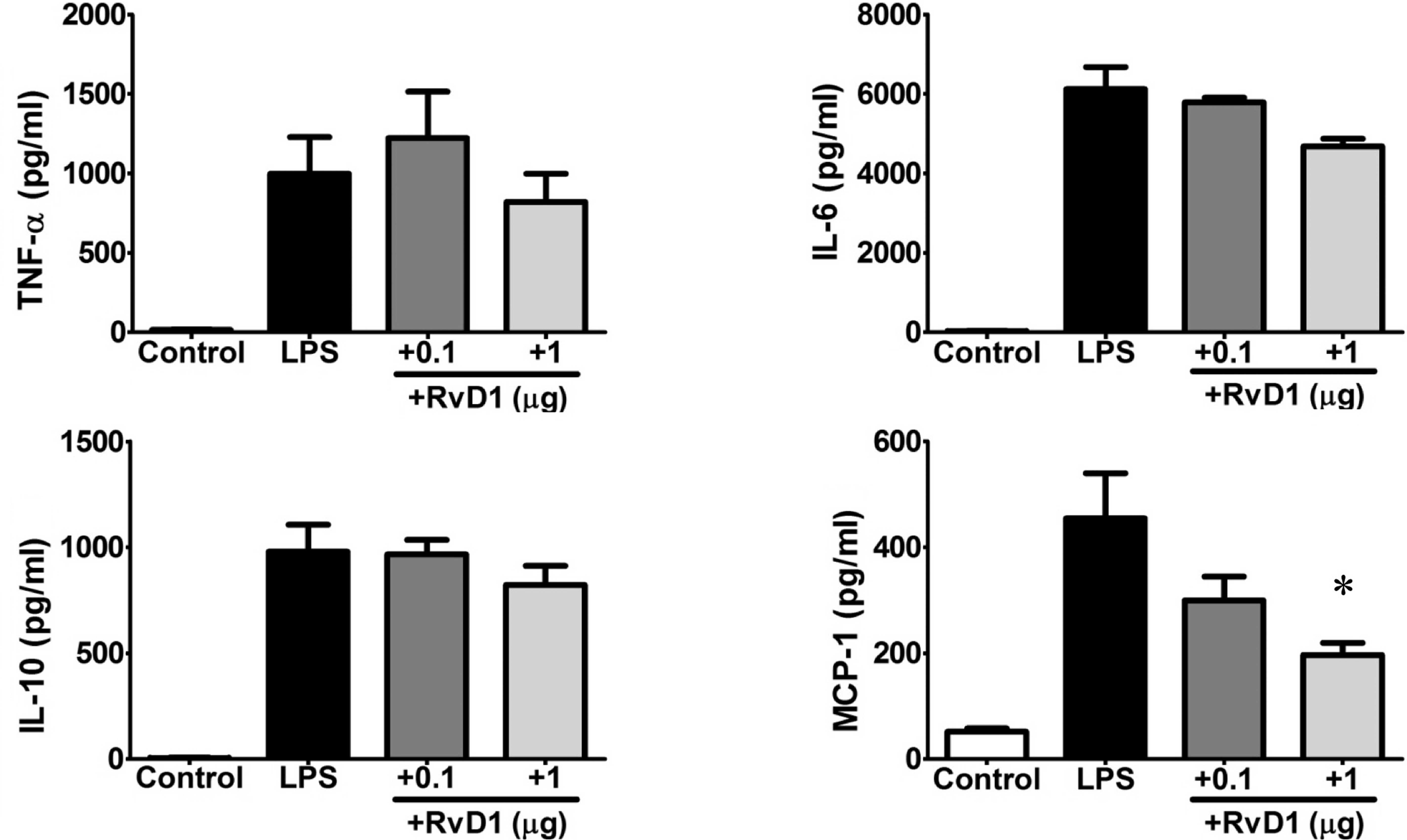
the serum levels of inflammatory cytokines. As presented in
Fig. 2, TNF-α, IL-6, IL-10 and
MCP-1 apparently increased 1 h after the LPS injection
(P<0.001). Notably, RvD1 administration slightly reduced the
levels of TNF-α, IL-6 and IL-10 and significantly suppresed the
level of MCP-1 at 1 μg (P<0.05).
Effect of resolvin D1 on the number of
peritoneal exudate cells
RvD1 was found to suppress the transendothelial
migration of neutrophils in vitro and peritoneal
infiltration of neutrophils in vivo in a murine
zymosan-induced peritonitis model (12). Thus, we examined the effect of RvD1
on the number of peritoneal exudate cells in the LPS/D-GalN mice.
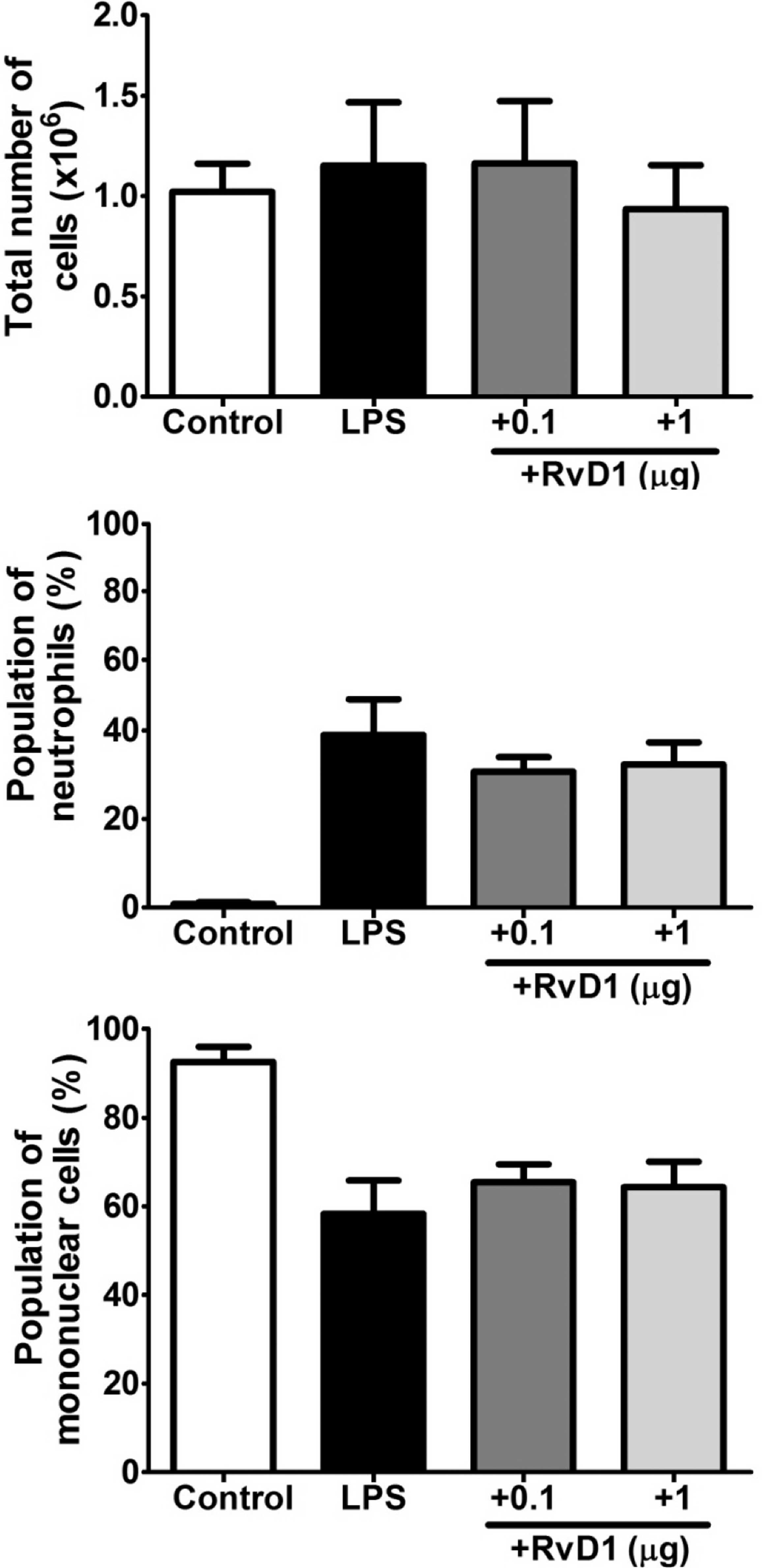
As presented in Fig. 3A, the total
peritoneal cell number was marginally increased at 5 h after the
LPS injection, which was due to the accumulation of neutrophils
(Fig. 3B) and the concomitant
decrease in mononuclear cells (mostly macrophages) in the
peritoneal cavity (Fig. 3C).
Notably, the RvD1 administration slightly decreased the total
peritoneal cell number and neutrophil population, while it
increased the mononuclear cell population, although these changes
were not statistically significant.
Effect of resolvin D1 on hepatocyte
apoptosis
Finally, to examine the effect of RvD1 on organ
failure in endotoxin/septic shock, we evaluated the extent of liver
injury in LPS/D-GalN mice (18).
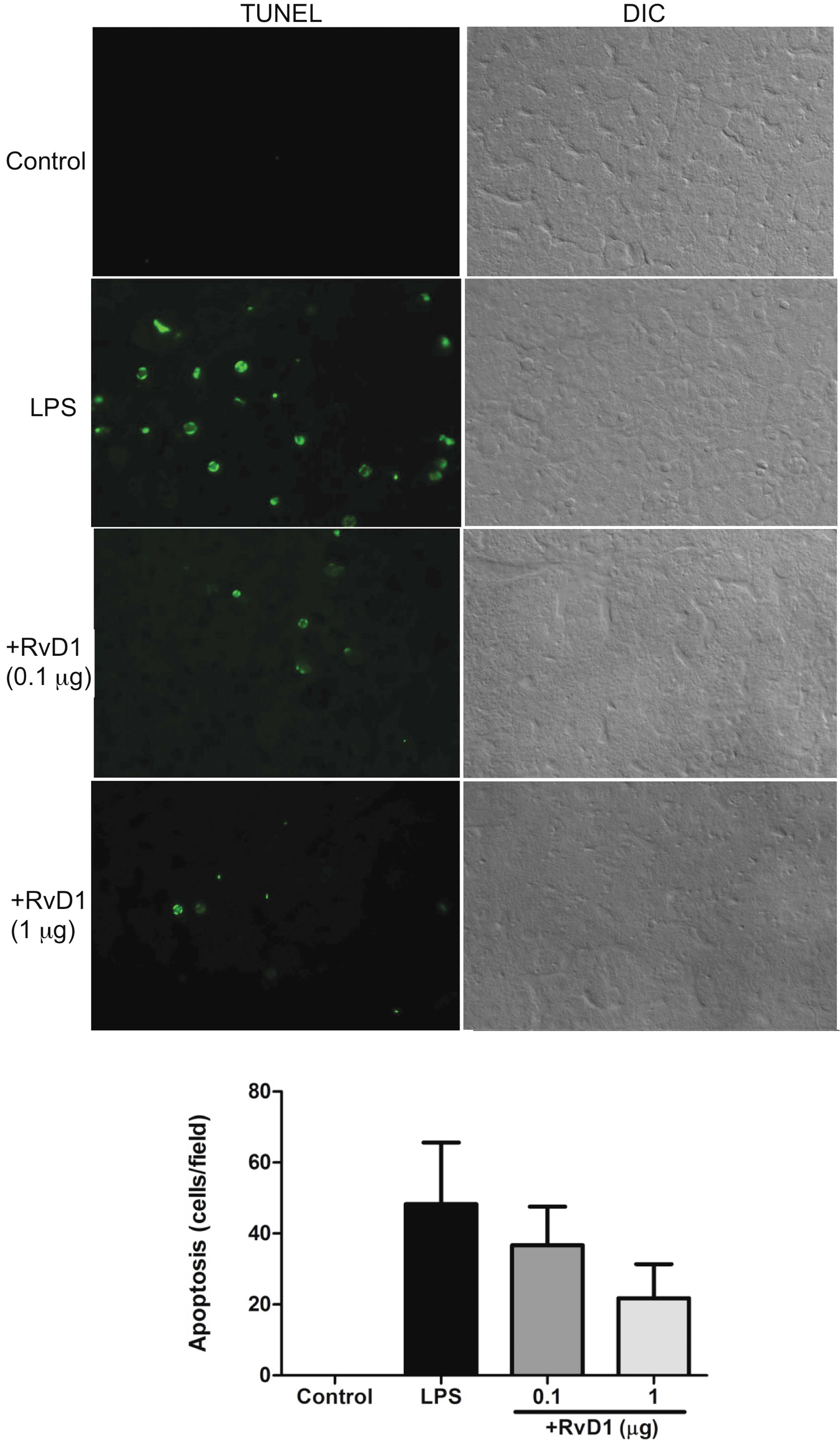
As revealed in Fig. 4A,
TUNEL+ apoptotic cells (mostly hepatocytes) were
apparently increased in the liver of the LPS/D-GalN mice, compared
to the control mice injected with D-GalN alone. To note, RvD1
administration dose-dependently reduced the apoptosis of
parenchymal cells (hepatocytes) in the liver (Fig. 4B).
Discussion
Endotoxin/septic shock is a severe condition that is
induced during serious infections with Gram-negative bacteria
(1–3). Although it is characterized by
systemic inflammatory responses of the host to invading
microorganisms, most anti-inflammatory therapeutic strategies have
failed to improve the prognosis of patients (8,9).
Thus, the development of novel agents with therapeutic potential
for sepsis/endotoxin shock has been explored. In this study, to
evaluate the therapeutic potential of RvD1, a pro-resolving
molecule, on endotoxin/septic shock, we investigated the effect of
RvD1 on the extracellular release of HMGB1, the production of
inflammatory cytokines, the accumulation of peritoneal cells and
hepatocyte apoptosis in vivo using a D-GalN-sensitized mouse
endotoxin shock model.
HMGB1, a 30-kDa non-histone nuclear protein, has
been reported to play a crucial role in endotoxin/septic shock by
functioning as a late phase mediator (16,19,20).
In this context, serum HMGB1 levels are highly elevated in septic
patients who succumb to the disease (non-survivors) compared to
patients with non-lethal infection (survivors); moreover, the
administration of an anti-HMGB1 antibody was found to attenuate
endotoxin lethality in mice (7).
Thus, HMGB1 is recognized as a potential therapeutic target for the
treatment of endotoxin/septic shock (20). In this study, serum HMGB1 levels
were markedly elevated 5 h after the LPS-challenge in LPS/D-GalN
mice. Notably, RvD1 significantly reduced the HMGB1 level.
Inflammatory cytokines such as TNF-α, IL-6, IL-10
and MCP-1 are produced and released by mononuclear phagocytes
(macrophages) shortly after exposure to LPS, and play a pivotal
role in the pathogenesis of lethal systemic inflammation in
endotoxin/septic shock (1). TNF-α
and IL-6 activate neutrophils, lymphocytes and vascular endothelial
cells, up-regulate cellular adhesion molecules and induce the
production of lipid mediators, nitric oxide and reactive oxygen
species, whereas IL-10 negatively regulates these responses. In
addition, MCP-1, as a chemokine, activates inflammatory cells
(particularly macrophages) to migrate into tissues. Notably, RvD1
has been found to suppress the production of inflammatory cytokines
from LPS-stimulated macrophages (13,14).
Moreover, RvD1 apparently down-regulates MCP-1 and IL-8 production
from LPS-stimulated human aortic endothelial cells (14). The present study revealed that the
serum levels of inflammatory cytokines (particularly TNF-α, IL-6,
IL-10 and MCP-1) were elevated in our endotoxin shock model.
Importantly, the RvD1 administration slightly reduced the TNF-α,
IL-6 and IL-10 levels and further lowered the MCP-1 level.
Since RvD1 was found to suppress the peritoneal
infiltration of neutrophils in a murine zymosan-induced peritonitis
model (12), we investigated the
effect of RvD1 on the peritoneal cell count in LPS/D-GalN mice.
RvD1 administration slightly affected the peritoneal cell
accumulation; it decreased the neutrophil population, but increased
the mononuclear cell population.
It has been demonstrated that the death of
LPS/D-GalN mice is mainly a result of injury to the liver, but not
to other organs, such as the lung, as D-GalN is specifically
hepatotoxic (15). Thus, we
evaluated the effect of RvD1 on hepatic apoptosis in the LPS/D-GalN
mice. As previously reported (18), the LPS/D-GalN injection induced
apoptosis in the liver, and TUNEL+ cells were mostly
hepatocytes. Notably, RvD1 administration reduced the apoptosis of
hepatocytes.
The present study revealed the suppressive action of
RvD1 on the release of HMGB1, the production of inflammatory
cytokines, the accumulation of peritoneal neutrophils and hepatic
apoptosis, and suggests that RvD1 may be a therapeutic agent for
sepsis/endotoxin shock, although the molecular mechanisms for the
suppressive actions remain to be elucidated. RvE1, a member of the
resolvin family, has been demonstrated to markedly suppress
leukocyte infiltration/migration and cytokine production by
attenuating NF-κB signaling via the action on specific receptors,
such as ChemR23 and BLT (leukotriene B4 receptor 1)
(21,22). Notably, RvD1 has recently been
suggested to exert pro-resolving actions on neutrophils and
macrophages via the candidate receptors ALX (lipoxin A4
receptor) and GPR32 (G-protein-coupled receptor 32) (23). Moreover, it is known that the
activation of NF-κB is involved in the extracellular release of
HMGB1 and apoptotic cell death (24,25).
Based on these observations, it may be speculated that RvD1, an
analogue of RvE1, also exerts proresolving activities by
attenuating NF-κB signaling, thereby suppressing inflammatory
cytokine production and peritoneal cell infiltration, as well as
HMGB1 release and hepatocyte apoptosis via the action on specific
receptors, such as ALX and GPR32. Nevertheless, the precise
mechanisms for the suppressive actions of RvD1 on LPS/D-GalN mice
observed in this study should be clarified in the future.
Acknowledgements
We thank Dr Noriyoshi Sueyoshi and Dr
Katsumi Miyahara (Division of Biomedical Imaging Research,
BioMedical Research Center, Juntendo University, Graduate School of
Medicne) for support in the histological analysis. This study was
supported in part by a Grant-in-Aid for Scientific Research from
the Japan Society for the Promotion of Science and a Grant-in-Aid
for the 21st Century COE Research from the Ministry of Education,
Culture, Sports, Science and Technology, Japan and Seikagaku
Biobusiness Corporation, Japan.
References
|
1.
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
O’Brien JM Jr, Ali NA, Aberegg SK and
Abraham E: Sepsis. Am J Med. 120:1012–1022. 2007.
|
|
3.
|
Van Amersfoort ES, Van Berkel TJ and
Kuiper J: Receptors, mediators and mechanisms involved in bacterial
sepsis and septic shock. Clin Microbiol Rev. 16:379–414.
2003.PubMed/NCBI
|
|
4.
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Park JS, Arcaroli J, Yum HK, et al:
Activation of gene expression in human neutrophils by high mobility
group box 1 protein. Am J Physiol Cell Physiol. 284:C870–C879.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fiuza C, Bustin M, Talwar S, et al:
Inflammation-promoting activity of HMGB1 on human microvascular
endothelial cells. Blood. 101:2652–2660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Riedemann NC, Guo RF and Ward PA: The
enigma of sepsis. J Clin Invest. 112:460–467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Riedemann NC, Guo RF and Ward PA: Novel
strategies for the treatment of sepsis. Nat Med. 9:517–524. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Serhan CN and Chiang N: Endogenous
pro-resolving and anti-inflammatory lipid mediators: a new
pharmacologic genus. Br J Pharmacol. 153(Suppl 1): S200–S215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Chiang N and Serhan CN: Cell-cell
interaction in the transcellular biosynthesis of novel
omega-3-derived lipid mediators. Methods Mol Biol. 341:227–250.
2006.PubMed/NCBI
|
|
12.
|
Sun YP, Oh SF, Uddin J, et al: Resolvin D1
and its aspirintriggered 17R epimer. Stereochemical assignments,
anti-inflammatory properties, and enzymatic inactivation. J Biol
Chem. 282:9323–9334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Duffield JS, Hong S, Vaidya VS, et al:
Resolvin D series and protectin D1 mitigate acute kidney injury. J
Immunol. 177:5902–5911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Merched AJ, Ko K, Gotlinger KH, Serhan CN
and Chan L: Atherosclerosis: evidence for impairment of resolution
of vascular inflammation governed by specific lipid mediators.
FASEB J. 22:3595–3606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Galanos C, Freudenberg MA and Reutter W:
Galactosamine-induced sensitization to the lethal effects of
endotoxin. Proc Natl Acad Sci USA. 76:5939–5943. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wang H, Yang H and Tracey KJ:
Extracellular role of HMGB1 in inflammation and sepsis. J Intern
Med. 255:320–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang H, Yang H, Czura CJ, Sama AE and
Tracey KJ: HMGB1 as a late mediator of lethal systemic
inflammation. Am J Respir Crit Care Med. 164:1768–1773. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Leist M, Gantner F, Bohlinger I, Tiegs G,
Germann PG and Wendel A: Tumor necrosis factor-induced hepatocyte
apoptosis precedes liver failure in experimental murine shock
models. Am J Pathol. 146:1220–1234. 1995.PubMed/NCBI
|
|
19.
|
Dumitriu IE, Baruah P, Manfredi AA,
Bianchi ME and Rovere-Querini P: HMGB1: guiding immunity from
within. Trends Immunol. 26:381–387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yang H, Wang H, Czura CJ and Tracey KJ:
The cytokine activity of HMGB1. J Leukoc Biol. 78:1–8. 2005.
View Article : Google Scholar
|
|
21.
|
Arita M, Bianchini F, Aliberti J, et al:
Stereochemical assignment, antiinflammatory properties, and
receptor for the omega-3 lipid mediator resolvin E1. J Exp Med.
201:713–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Arita M, Ohira T, Sun YP, Elangovan S,
Chiang N and Serhan CN: Resolvin E1 selectively interacts with
leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation.
J Immunol. 178:3912–3917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Krishnamoorthy S, Recchiuti A, Chiang N,
et al: Resolvin D1 binds human phagocytes with evidence for
proresolving receptors. Proc Natl Acad Sci USA. 107:1660–1665.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bonaldi T, Talamo F, Scaffidi P, et al:
Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect
it towards secretion. EMBO J. 22:5551–5560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hsu H, Huang J, Shu HB, Baichwal V and
Goeddel DV: TNF-dependent recruitment of the protein kinase RIP to
the TNF receptor-1 signaling complex. Immunity. 4:387–396. 1996.
View Article : Google Scholar : PubMed/NCBI
|