Introduction
Worldwide, the 5-year survival rate for patients
with squamous cell carcinoma of the head and neck (HNSCC) has not
significantly increased for many years (1–5).
HNSCC is diagnosed predominantly at the age range of 50–70 years,
but is also observed in younger patients (6–8).
Despite aggressive initial management of the primary tumor,
locoregional recurrence occurs in some 60% of cases, and distant
metastasis is observed in some 25%. Therefore, innovative
therapeutic concepts are urgently required.
Angiogenesis is essential for tumor progression and
metastasis. Tumor angiogenesis is complex and involves crosstalk
between tumor-derived growth factors, the modified extracellular
matrix that develops around tumors, and endothelial receptors for
extracellular matrix and growth factors (9,10).
Inhibition of angiogenesis often suppresses the tumor growth of
model tumors, and the suppression and eradication of malignant
tumors by targeting angiogenetic endothelial cells is a rapidly
evolving approach to cancer therapy (10,11).
Such therapies might influence highly vascularized head and neck
cancers (12–17). Integrin antagonists are good
candidates for such antiangiogenic strategies (9,18–23).
In particular, the integrins, αvβ3, αvβ5 and α5β1, have been
implicated in tumor angiogenesis. Inhibitors of these integrins are
being investigated in clinical trials (9,19–21,24–26),
and we previously reported a signal in an HNSCC patient when using
an αvβ3/αvβ5 inhibitor (27).
Integrin action depends on the presence of
complementary ligands. While αvβ5 and α5β1 are conservative in
their ligand binding, being essentially monospecific for
vitronectin and fibronection, respectively, αvβ3 binds
promiscuously to numerous matrix components. The ligands fibrinogen
and osteopontin rather monospecifically target αvβ3 (28). Vitronectin is a common serum
component activated by conformational change (29); the activated molecule is detected
immunologically (30). In the
present study, we evaluated the expression of integrins, αvβ3, αvβ5
and α5β1, and their ligands, fibrinogen (αvβ3, α5β1), fibronectin
(αvβ3, α5β1), osteopontin (αvβ3) and activated vitronectin (αvβ3,
αvβ5), in head and neck cancer and control tissues.
Materials and methods
Patients
Samples of squamous cell carcinomas from 40 patients
(32 male, 8 female) were obtained during oral or maxillofacial
surgery. Control non-cancerous tissues containing squamous
epithelium were obtained from 20 patients undergoing outpatient
surgical procedures (Tables I and
II). Patients provided informed
consent for the collection of samples, and all tissues examined
were taken from the head and neck area with previous consent of the
patients in our clinic in the context of diagnostics and
therapy.
 | Table I.Characteristics of the 40 patients
with head and neck squamous cell carcinoma (HNSCC), localization
and TNMa
classification of the tumors. |
Table I.
Characteristics of the 40 patients
with head and neck squamous cell carcinoma (HNSCC), localization
and TNMa
classification of the tumors.
| No. | Gender/Agea | Localization | TNMb | Stage | Grade |
|---|
| 1 | M/39 | Floor of mouth | pT3 pN1 | 3 | 3 |
| 2 | M/38 | Floor of mouth | pT4 pN2 | 4 | 2 |
| 3 | M/52 | Floor of mouth | pT4 pN0 | 4a | 3 |
| 4 | M/59 | Floor of mouth | pT1 pN2 | 4 | 2 |
| 5 | M/50 | Floor of mouth | pT2 pN2b | 4a | 2 |
| 6 | M/50 | Floor of mouth | pT4 pN1 | 4 | 2 |
| 7 | M/61 | Floor of mouth | pT2 pN2 | 4a | 3 |
| 8 | M/62 | Floor of mouth | pT4 pN2 | 4a | 2 |
| 9 | M/50 | Floor of mouth | pT4 pN1 | 4a | 3 |
| 10 | M/48 | Floor of mouth | pT4 pN2 | 4a | 2 |
| 11 | M/52 | Floor of mouth | pT4 pN2 | 4a | 1 |
| 12 | M/63 | Floor of mouth | pT2 pN0 | 2 | 2 |
| 13 | M/52 | Floor of mouth | pT1 pN0 | 1 | 2 |
| 14 | M/60 | Floor of mouth | pT3 pN2 | 4a | 3 |
| 15 | M/46 | Floor of mouth | pT4 pN0 | 4a | 3 |
| 16 | M/53 | Floor of mouth | pT2 pN0 | 2 | 2 |
| 17 | M/57 | Floor of mouth | pT4 pN3 | 4b | 2 |
| 18 | F/50 | Floor of mouth | pT4 pN2 | 4a | 2 |
| 19 | M/58 | Floor of
mouth/Tongue | pT3 pN2 | 4a | 3 |
| 20 | M/57 | Floor of
mouth/Tongue | pT4 pN0 | 4a | 2 |
| 21 | F/48 | Floor of
mouth/Tongue | pT4 pN2 | 4a | 2 |
| 22 | F/65 | Floor of
mouth/Tongue | pT2 pN0 | 2 | 2 |
| 23 | M/52 | Oropharynx | pT2 pN2 | 4 | 3 |
| 24 | M/59 | Oropharynx | pT3 pN1 | 3 | 2 |
| 25 | M/57 | Oropharynx | pT2 pN1 | 3 | 2 |
| 26 | F/62 | Planum buccale | pT4 pN3 | 4 | 3 |
| 27 | F/76 | Planum buccale | pT3 pN1 | 3 | 2 |
| 28 | F/71 | Planum buccale | pT3 pN0 | 3 | 1 |
| 29 | M/53 | Processus
alveolaris | pT4 pN2 | 4 | 2 |
| 30 | M/58 | Processus
alveolaris | pT4 pN3 | 4b | 2 |
| 31 | M/59 | Processus
alveolaris | pT4 pN2c | 4a | 2 |
| 32 | F/61 | Processus
alveolaris | pT4 pN0 | 4a | 2 |
| 33 | F/64 | Processus
alveolaris | pT4 pN0 | 4a | 1 |
| 34 | M/56 | Tongue | pT1 pN0 | 1 | 3 |
| 35 | M/58 | Tongue | pT2 pN1 | 3 | 2 |
| 36 | M /49 | Tongue | pT2 pN0 | 2 | 2 |
| 37 | M/53 | Tongue/Floor of
mouth | pT4 pN0 | 4a | 3 |
| 38 | M/55 | Tongue/Floor of
mouth | pT4 pN0 | 4a | 3 |
| 39 | M/55 | Tongue/Floor of
mouth | pT4 pN0 | 4a | 3 |
| 40 | M/56 | Tongue/Floor of
mouth | pT1 pN1 | 3 | 3 |
 | Table II.Characteristics of the 20 patients
without tumors and localization of the control tissues. |
Table II.
Characteristics of the 20 patients
without tumors and localization of the control tissues.
| No. | Gender/Agea | Localization |
|---|
| 1 | M/20 | Gingiva |
| 2 | M/58 | Gingiva |
| 3 | M/23 | Gingiva |
| 4 | M/64 | Gingiva |
| 5 | M/33 | Gingiva |
| 6 | F/56 | Gingiva |
| 7 | M/16 | Oral mucosa |
| 8 | M/36 | Oral mucosa |
| 9 | F/36 | Oral mucosa |
| 10 | F/30 | Oral mucosa |
| 11 | F/61 | Oral mucosa |
| 12 | F/30 | Oral mucosa |
| 13 | F/22 | Oral mucosa |
| 14 | M/58 | Oropharynx |
| 15 | F/64 | Oropharynx |
| 16 | F/1 | Oropharynx |
| 17 | F/48 | Planum buccale |
| 18 | M/61 | Tongue |
| 19 | M/60 | Tongue |
| 20 | F/60 | Tongue |
Tumor samples and sample preparation
The tissue samples were stored in isotonic saline
for 15–30 min immediately following removal from patients. All
tissues were cut into pieces with an edge length of ∼4 mm, embedded
in freezing medium (Leica Instrument, Nussloch) in a plastic tube,
shock-frozen for 2 min in liquid nitrogen, and cryopreserved at
−80°C until sectioning. A cryomicrotome (CM3000; Leica Instrument)
was used to prepare 4- to 6-μm sections, which were placed
on coated slides (SuperFrost Plus, Menzel, Braunschweig or Dako,
Denmark), air-dried for ∼12 h at 20°C, and stored frozen in a dry
atmosphere usually at −80°C (occasionally −20°C).
Frozen sections were thawed, air-dried, and fixed
for 15 min in fresh dry acetone at −20°C. Experience revealed that
this method provides clearer and stronger staining compared to
fixing with methyl alcohol-acetone (9 min methanol and 1 min
acetone at −20°C). All fixed sections were incubated with blocking
buffer X0909 (ready-to-use; Dako) for 20 min to reduce non-specific
staining. Samples were incubated with primary antibodies for 60
min. Table III lists the
antibodies and dilution used. Optimal dilutions of antibodies were
identified in preliminary experiments and were then used throughout
the study.
 | Table III.Antibodies. |
Table III.
Antibodies.
| Antibody | Antibody type | Target antigen | Dilution | Author | Refs. |
|---|
| Clone
LM609a,f | Monoclonal
(IgG1) | αvβ3 integrin | 1:300 | Cheresh and
Spiro | 69 |
| Clone
P1F6a,f | Monoclonal
(IgG3) | αvβ5 integrin | 1:300 | Weinacker et
al | 70 |
| Clone
P1D6a,f | Monoclonal
(IgG3) | α5β1 integrin | 1:30 | Wayner et
al | 71 |
| A0080c,e,g | Polyclonal
(IgG) | Fibrinogen | 1:10.000 | | |
|
RB-9097-P1d,e,g | Polyclonal
(IgG) | Osteopontin | 1:30 | | |
| 153b,f | Monoclonal | Vitronectin | 1:200 | Seiffert et
al | 72 |
| A0245c,e,g | Polyclonal
(Ig) | Fibronectin | 1:30 | | |
| M0823 clone
JC70Ac,f | Monoclonal
(IgG1κ) | CD31 | 1:30 | | |
| N1698c | Negative control
(Ig) | Negative control
mouse | 1:1 | | |
| N1699c | Negative control
(Ig) | Negative control
rabbit | 1:1 | | |
An alkaline phosphatase-anti-alkaline phosphatase
(APAAP) system was used to visualize the bound antibody (31). Slides were rinsed three times with
Tris-wash buffer, pH 7.6, (Dako S3001) and incubated for 40 min
with a bridging antibody diluted 1:40. Sections incubated with
monoclonal antibodies (Table III)
were incubated with polyclonal rabbit anti-mouse bridging antibody
(Dako Z02259), and sections incubated with polyclonal antibodies
were incubated with monoclonal mouse anti-rabbit bridging antibody
(Dako M0737), diluted with the antibody diluent (Dako S2022) plus
5% AB serum (Biotest AG, cat. no. 805135) in each case. Sections
were washed again three times in TBS buffer and then incubated for
40 min with the monoclonal APAAP complex (Dako D0651) diluted 1:100
in antibody diluent plus 5% inactivated fetal calf serum (Biochrom
S0115). After thorough rinsing, the subsequent substrate
development was carried out for over 20 min with the substrate
(Dako 070524) containing two drops of levamisole (Dako K5000).
After further rinsing, counterstaining was carried out using
hemalaun (Dako S2020) for 5 min followed by bluing for 5 min in tap
water.
For optimum recognition of squamous cell carcinoma
in the small frozen sections, we used a monoclonal antibody against
proliferation marker Ki-67 (Dako, M7240, clone MIB-1) and a
monoclonal antibody against the adhesion molecule CD44v6 (Bender
BMS116, clone VFF-7), performing the same immunohistochemical APAAP
method as previously (32–34). Although this was effective, we did
not use the synopsis of score values for the expression of Ki-67
and CD44v6. Vessel densities were routinely assessed using CD31
staining including score values.
Evaluation of expression with
immunoreactivity scores and number of vessels
The evaluation of immunoreactivity scores (IHS) was
carried out using x200 magnification as described (32–35).
Sections were evaluated three times including an evaluation by a
tumor pathologist in a blinded manner. Staining intensity (SI) was
assessed according to a categorical scale: 0, no staining; 1, faint
staining; 2, slight staining; 3, moderate staining; and 4, strong
staining. The percentage of positively stained cells (PP) was
assessed as: 0, no positive cells; 1, 0–25% positive cells; 2,
26–50% positive cells; 3, 51–75% positive cells; and 4, 76–100%
positive cells. An overall IHS was derived by multiplying the
staining intensity (SI) by the percentage of positive staining or
the staining frequency (PP) scores (range of possible scores 0–16).
Staining of glands, muscle, histiocytes and inflammatory cells was
ignored. In no instances were single cells counted in the tumors or
in the squamous epithelium samples.
An additional parameter was used in the third
microscopic evaluation with assessment of the number of vessels.
This involved quantitative estimation of the number of marked
vessels using a lower magnification (x100). Using antibodies
(Table III), we distinguished the
estimated numbers of marked vessels in the tumors (or squamous
epithelium in controls) and stroma: scale 0, no vessels; scale 1,
isolated vessels; scale 2, few vessels; scale 3, numerous vessels;
and scale 4, large quantities of vessels. First, the highest
possible vessel density was visualized using the antibody directed
at the ‘typical’ endothelial marker, CD31, followed by
visualization of other antigens of interest using the antibodies
described in Table III.
Statistics
PASW Statistics for Windows (version 18.0.0) was
used for statistical evaluation, with a cut-off for significance of
p<0.05. The t-test was used when the values were distributed
normally, and most often with the Mann-Whitney U-test for
non-normally distributed data (36).
Results
Samples analyzed
Tumor samples (n=40) (Table I) were from the floor of the mouth
(n=18), the tongue or tongue plus the floor of the mouth (n=11),
the oropharynx (n=3) and the alveolar process, gingiva, or planum
buccale (n=8). According to pathologic TNM tumor staging,
approximately half of the tumors were T4 (n=21) with the remainder
distributed among T3 (n=6), T2 (n=9) and T1 (n=4); in each case
tumors were fairly evenly distributed among N0–N3, and M status was
not available. Overall stage grouping identified 27 samples as S4,
7 as S3, 4 as S2 and 2 as S1; 14 tumors were grade 3, 23 were grade
2 and 3 were grade 1. Control samples (n=20) (Table II) were from the tongue (n=3), the
oropharynx (n=3) and the gingiva, oral mucosa or planum buccale
(n=14).
Expression in tumor and control tissues,
in endothelial cells and in stroma
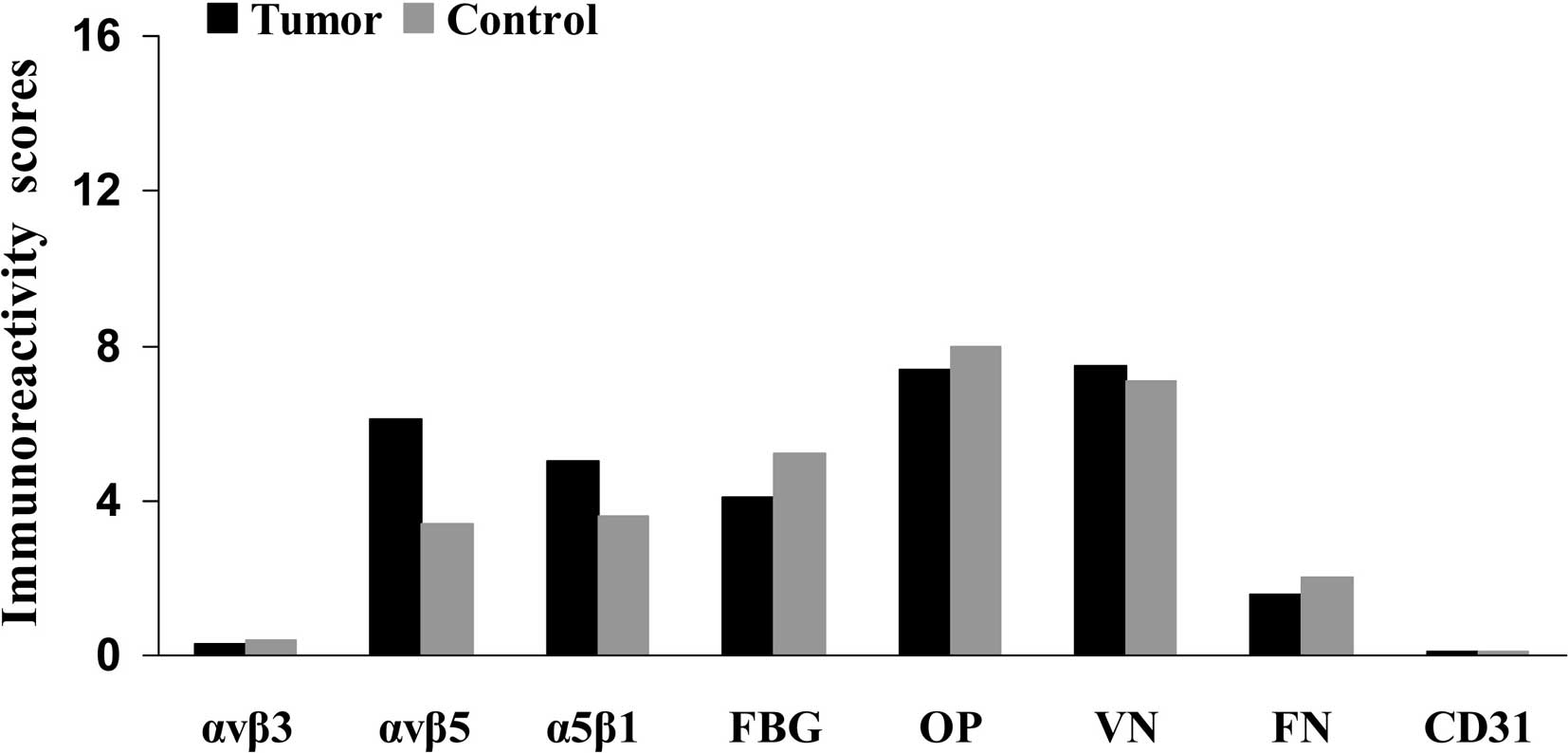
Fig. 1 compares the
IHS (maximum score 16.0) for carcinoma tissue, endothelial cells
and stroma in the samples from patients with oral cancer or from
the control subjects. Table IV
reveals the contributions of frequency (PP) and expression scores
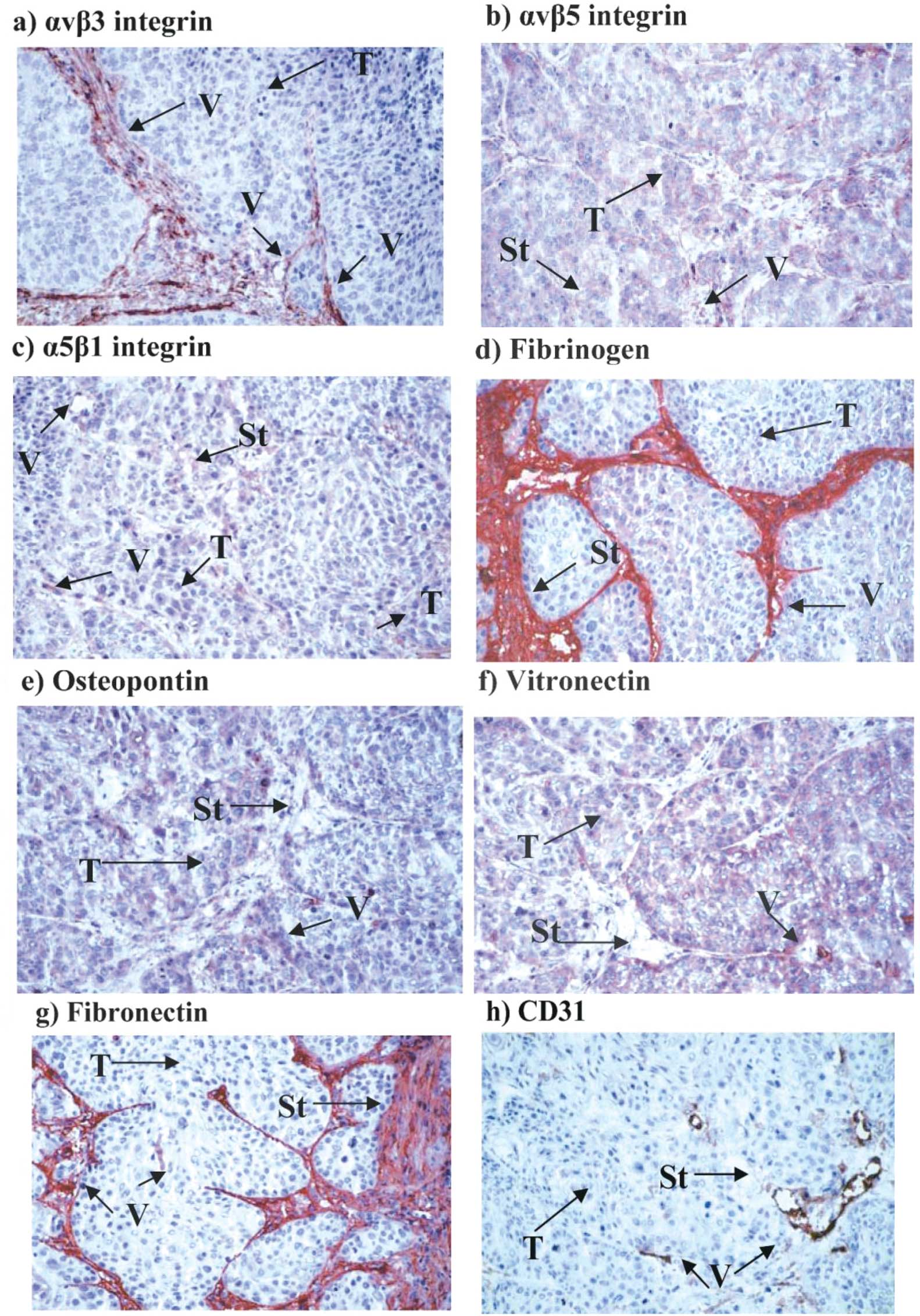
(SI) to the overall IHS. Representative examples of immunostaining
for the integrins and ligands using various sections from a single
patient (no. 30, Table I) are
shown in Fig. 2a–h.
 | Table IV.Contribution of the intensity scores
and frequency scores to the overall immunoreactivity scores for
expression of the integrins and ligands in the tumors and squamous
epithelium of the controls both in the endothelium and stroma,
respectively.a |
Table IV.
Contribution of the intensity scores
and frequency scores to the overall immunoreactivity scores for
expression of the integrins and ligands in the tumors and squamous
epithelium of the controls both in the endothelium and stroma,
respectively.a
| | Squamous cell
carcinoma | Squamous epithelium
controls | Endothelium
| Stroma
|
|---|
| Tumors | Controls | Tumors | Controls |
|---|
| Integrin αvβ3 | Intensity | 0.21±0.57 | 0.30±0.60 | 3.60±0.77 | 3.30±0.80 | 0.80±0.67 | 0.20±0.30 |
| Frequency | 0.72±1.41 | 0.60±1.10 | 3.59±0.84 | 2.70±1.40 | 3.18±1.48 | 1.90±2.00 |
| Immunoreactivity
score | 0.29±0.62 | 0.40±0.60 | 13.18±4.37 | 9.00±5.60 | 3.06±2.60 | 0.70±0.80 |
| Integrin αvβ5 | Intensity | 2.00±0.90 | 1.50±0.90 | 2.70±1.10 | 2.40±1.00 | 2.60±1.40 | 1.30±1.00 |
| Frequency | 3.20±0.90 | 2.60±1.10 | 2.60±1.10 | 2.60±1.30 | 3.80±0.70 | 3.50±1.40 |
| Immunoreactivity
score | 6.10±3.40 | 3.40±2.10 | 7.30±4.90 | 6.60±5.00 | 10.2±5.40 | 4.90±4.00 |
| Integrin α5β1 | Intensity | 1.70±0.90 | 1.30±0.70 | 2.10±0.90 | 1.60±0.90 | 1.80±0.80 | 0.80±0.40 |
| Frequency | 3.00±1.00 | 2.90±0.90 | 2.10±1.20 | 2.20±1.30 | 3.80±0.50 | 3.60±1.10 |
| Immunoreactivity
score | 5.00±2.70 | 3.60±1.90 | 4.40±3.00 | 3.40±2.80 | 6.70±2.90 | 3.00±1.70 |
| Fibrinogen | Intensity | 1.30±0.90 | 2.30±1.40 | 4.00±0.20 | 3.60±0.60 | 4.00±0.00 | 3.80±0.60 |
| Frequency | 3.20±1.10 | 2.40±0.90 | 3.60±0.80 | 2.90±1.20 | 3.90±0.60 | 4.00±0.00 |
| Immunoreactivity
score | 4.10±3.10 | 5.20±3.20 | 14.40±3.30 | 10.1±4.70 | 15.7±2.20 | 15.2±2.20 |
| Osteopontin | Intensity | 2.30±0.80 | 2.90±1.30 | 1.80±1.20 | 1.60±1.50 | 0.80±0.70 | 0.30±0.40 |
| Frequency | 3.20±0.90 | 3.00±0.80 | 1.40±0.80 | 1.50±1.10 | 3.90±0.50 | 2.60±2.00 |
| Immunoreactivity
score | 7.40±3.60 | 8.00±4.20 | 2.60±2.10 | 2.30±2.00 | 3.00±2.80 | 1.10±1.50 |
| Vitronectin | Intensity | 2.30±1.10 | 2.30±1.30 | 2.80±1.40 | 2.80±1.30 | 1.30±0.90 | 1.10±0.90 |
| Frequency | 3.40±0.80 | 3.00±1.10 | 2.60±1.20 | 2.50±1.30 | 3.80±0.60 | 3.90±0.60 |
| Immunoreactivity
score | 7.50±3.60 | 7.10±4.80 | 7.60±5.40 | 7.30±6.00 | 4.90±2.20 | 4.50±3.80 |
| Fibronectin | Intensity | 0.60±0.60 | 1.20±1.30 | 3.80±0.60 | 3.30±0.80 | 3.90±0.30 | 3.40±0.90 |
| Frequency | 1.90±1.60 | 1.60±1.50 | 3.70±0.70 | 2.90±1.10 | 4.00±0.30 | 4.00±0.00 |
| Immunoreactivity
score | 1.60±2.20 | 2.90±4.00 | 14.30±3.70 | 9.50±4.80 | 15.5±1.60 | 13.8±3.80 |
| CD31 | Intensity | 0.01±0.00 | 0.01±0.05 | 4.00±0.00 | 3.90±0.50 | 0.70±0.70 | 0.90±1.40 |
| Frequency | 0.10±0.40 | 0.20±0.90 | 4.00±0.00 | 4.00±0.20 | 3.10±1.50 | 2.10±1.90 |
| Immunoreactivity
score | 0.02±0.09 | 0.04±0.20 | 16.0±0.00 | 15.5±2.20 | 2.40±2.10 | 2.10±2.20 |
The mean IHS for αvβ5 and α5β1 integrins in tumor
cells were significantly higher than those from the control samples
of squamous epithelium (Fig. 1a;
Tables IV and V); this resulted from higher SI and PP
scores for αvβ5 and from a higher SI score for α5β1 (Table IV). Expression of the other
antigens was comparable between the tumor cells and the control
samples, although there was a tendency in the control samples
towards higher expression of fibrinogen (IHS 5.2 in control vs. 4.1
in tumor cells) and fibronectin (IHS 2.9 in control vs. 1.6 in
tumor cells), but not significantly higher (U-test; fibrinogen,
p=0.145 and fibronectin, p=0.416) (Table VI). αvβ3 expression (IHS 0.29) and
CD31 (IHS 0.02) exhibited weak or no staining in the tumor
cells.
 | Table V.Statistical comparison between the
immunoreactivity scores (IHS) in the tumors, endothelia, stroma or
controls (squamous epithelia, endothelia and stroma),
respectively.a |
Table V.
Statistical comparison between the
immunoreactivity scores (IHS) in the tumors, endothelia, stroma or
controls (squamous epithelia, endothelia and stroma),
respectively.a
| IHS in the
carcinoma cells vs. squamous epithelia in the controls | IHS in the
carcinoma cells are not statistically significantly higher | IHS in carcinoma
cells are statistically significantly higher. |
|
| Integrin αvβ3 | 0.568 | |
| Fibrinogen | 0.145 | |
| Osteopontin | 0.487 | |
| Vitronectin | 0.693 | |
| Fibronectin | 0.416 | |
| CD31 | 0.983 | |
| Integrin αvβ5 | | 0.002 |
| Integrin α5β1 | | 0.034 |
|
| IHS in endothelia
of carcinoma tissues vs. the controls | IHS in endothelia
of carcinoma tissues are not statistically significantly
higher | IHS in endothelia
of carcinoma tissues are statistically significantly higher. |
|
| Integrin αvβ5 | 0.490 | |
| Integrin α5β1 | 0.223 | |
| Osteopontin | 0.544 | |
| Vitronectin | 0.634 | |
| CD31 | 0.168 | |
| Integrin αvβ3 | | 0.004 |
| Fibrinogen | | <0.001 |
| Fibronectin | | <0.001 |
|
| IHS in stroma of
carcinoma tissues vs. the controls | IHS in carcinoma
tissues are not statistically significantly higher | IHS in stroma of
carcinoma tissues are statistically significantly higher |
|
| Fibrinogen | 0.082 | |
| Vitronectin | 0.456 | |
| Integrin αvβ3 | | <0.001 |
| Integrin αvβ5 | | <0.001 |
| Integrin α5β1 | | <0.001 |
| Osteopontin | | <0.001 |
| Fibronectin | | 0.001 |
| CD31 | 0.325 | |
 | Table VI.Contribution of the quantitative
estimate of the number of vessels in the tumor tissues or in
squamous epithelium of the controls and in stroma,
respectively.a |
Table VI.
Contribution of the quantitative
estimate of the number of vessels in the tumor tissues or in
squamous epithelium of the controls and in stroma,
respectively.a
| Vessels in
| Vessels in stroma
of
|
|---|
| Tumor tissues | Control
tissues | Tumor tissues | Control
tissues |
|---|
| Integrin αvβ3 | 1.3±0.9 | 0.5±0.4 | 1.7±0.7 | 1.2±0.8 |
| Integrin αvβ5 | 0.7±0.6 | 0.5±0.5 | 1.2±0.6 | 1.0±0.5 |
| Integrin α5β1 | 0.7±0.5 | 0.4±0.4 | 1.0±0.5 | 0.8±0.4 |
| Fibrinogen | 1.5±0.9 | 1.1±0.7 | 2.0±0.7 | 1.4±0.7 |
| Osteopontin | 0.5±0.4 | 0.7±0.6 | 0.9±0.4 | 0.7±0.6 |
| Vitronectin | 0.9±0.7 | 0.8±0.6 | 1.2±0.7 | 1.0±0.5 |
| Fibronectin | 1.7±0.9 | 0.9±0.6 | 1.9±0.8 | 1.5±0.5 |
| CD31 | 1.9±0.9 | 0.8±0.5 | 2.6±0.8 | 2.3±0.9 |
Integrin αvβ3 (IHS 13.2), fibrinogen (IHS 14.4) and
fibronectin (IHS 14.3) were strongly expressed in the endothelia in
the the tumors [along with the endothelial marker CD31 (IHS 16.0),
while IHS for CD31 was significantly higher: CD31 vs. αvβ3,
p<0.001; CD31 vs. fibrinogen, p=0.002; CD31 vs. fibronectin,
p=0.003; U-test]. In tumors, the average IHS of integrin αvβ3,
fibrinogen and fibronectin were significantly higher than those in
the control tissues (p=0.004, p<0.001 and p<0.001,
respectively) (Table IV; Fig. 1b). Higher average SI and PP scores
contributed to these differences in intensity of expression
(Table IV). Lower mean IHS were
observed for integrins αvβ5 and α5β1, and osteopontin and
vitronectin (Table IV and Fig. 1) with no clear differences between
tumor samples and control tissues (αvβ5, p=0.590; α5β1, p=0.223;
osteopontin, p=0.544; vitronectin, p=0.634; U-test) (Table V).
All three integrins were more strongly and
statistically significantly expressed in tumor stroma compared to
stroma of control squamous epithelia (U-test; p<0.001) (Fig. 1c; Table IV and V), mainly as a result of higher SI scores
for αvβ5 and α5β1, and by higher SI and PP scores for αvβ3.
However, αvβ3 was less strongly expressed than αvβ5 and α5β1, as
judged by the overall IHS. Osteopontin was not strongly expressed,
although the IHS was higher in tumor stroma vs. the control (IHS
3.0 vs. 1.1; p<0.001). Activated vitronectin was expressed
weakly at similar levels in the normal and tumor stroma. Fibrinogen
(IHS 15.7 vs. 15.2; p=0.082) and fibronectin (IHS 15.5 vs. 13.8;
p=0.029) were strongly expressed in the tumor and control samples,
while the expression of CD31 was low and similar between the tumors
and controls (IHS 2.4 vs. 2.1; p=0.325).
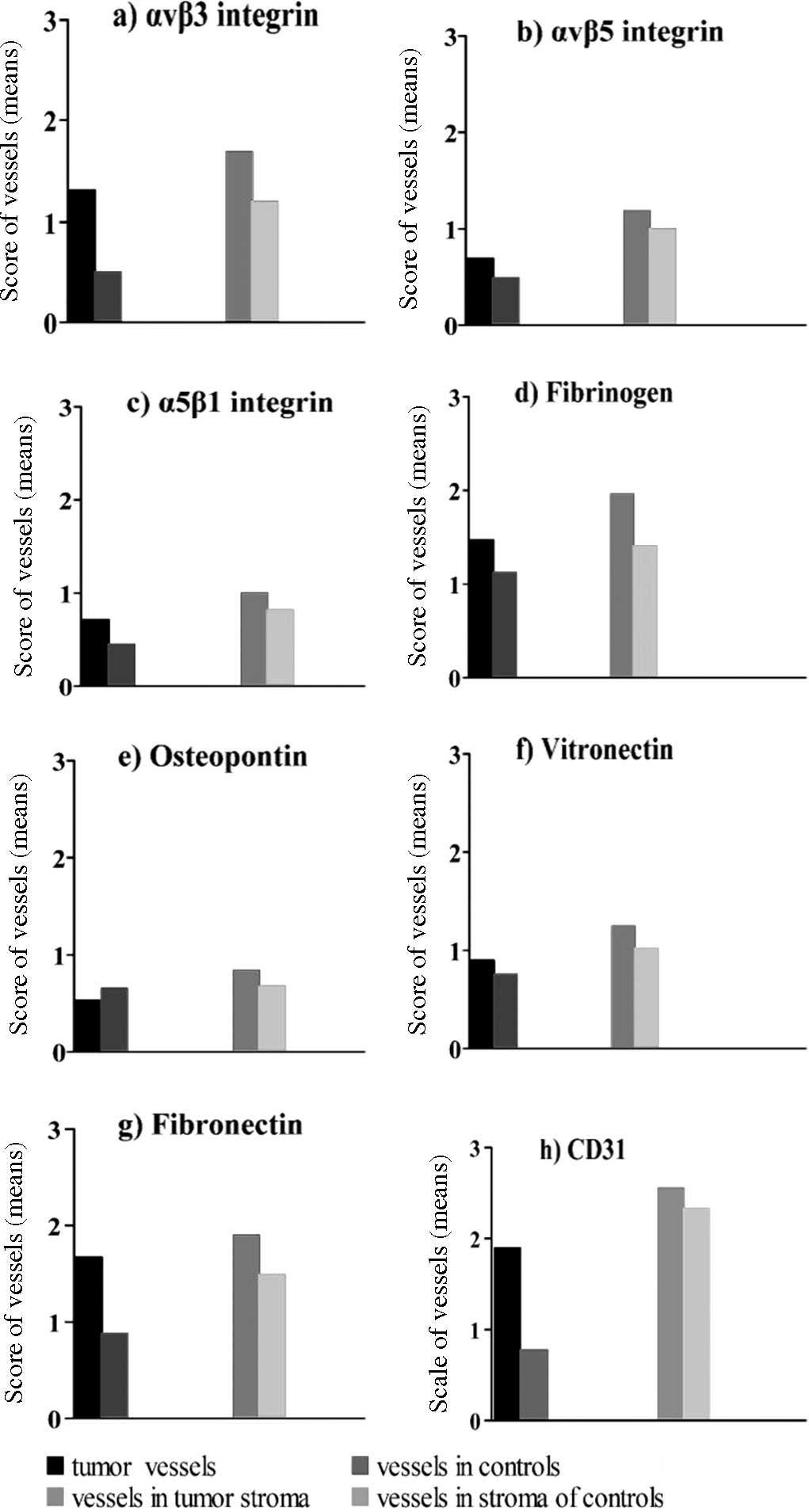
Quantification of blood vessels in the
tumors and control epithelia or stroma in both tissues
Integrin expression in the blood vessels of the
tumor tissues and in stroma were evaluated separately with a
maximal score of 4.0 (Fig. 3).
Using a typical marker of endothelial cells, CD31, immunostaining
revealed a higher density of endothelial cells in the tumors vs.
the control tissues (1.9 vs. 0.8; p=0.099; U-test), with a higher
or similar density of staining in tumor stroma and control samples
(tumor stroma 2.6 vs. stroma in control tissues 2.3; p=0.173;
U-test) (Fig. 3; Tables VI and VII).
 | Table VII.Statistical comparison between the
quantitative estimate of vascularization for squamous cell
carcinomas vs. squamous epithelia of control sections and for
stroma.a |
Table VII.
Statistical comparison between the
quantitative estimate of vascularization for squamous cell
carcinomas vs. squamous epithelia of control sections and for
stroma.a
| Quantitative
estimate of vessels in carcinoma tissues vs. controls | Values assessed in
carcinoma tissues are statistically not significantly higher | Values assessed in
carcinoma tissues are statistically significantly higher |
|
| Integrin αvβ5 | 0.086 | |
| Fibrinogen | 0.145 | |
| Osteopontin | 0.792 | |
| Vitronectin | 0.312 | |
| CD31 | 0.099 | |
| Integrin α5β1 | | 0.034 |
| Fibronectin | | 0.002 |
| Integrin αvβ3 | | <0.001 |
|
| Quantitative
estimate of vessels in stroma of tumor tissues vs. stroma in
controls | Values assessed in
stroma of tumor tissues are statistically not significantly
higher | Values assessed in
stroma of tumor tissues are statistically significantly
higher. |
|
| Integrin αvβ5 | 0.230 | |
| Integrin α5β1 | 0.191 | |
| Osteopontin | 0.117 | |
| Vitronectin | 0.292 | |
| CD31 | 0.173 | |
| Integrin αvβ3 | | 0.012 |
| Fibrinogen | | 0.009 |
| Fibronectin | | 0.025 |
Integrins were more strongly expressed on endothelia
within the tumor tissue than in the control squamous epithelium,
although a clear difference between tumor and control samples was
observed only for integrin αvβ3 (Table
VI; Fig. 3). Endothelial cells
in the stroma expressed integrins more strongly than in the tumor
tissue. The number of vessels, when compared between the tumor and
control samples in the stroma, was greater in the tumor tissues for
αvβ3 and statistically significant (p=0.012, Table VII) compared to the other integrins
(αvβ3, 1.7 vs. 1.2; αvβ5, 1.2 vs. 1.0; α5β1, 1.0 vs. 0.8) (Table VI). Fibrinogen and fibronectin were
expressed strongly in the tumor tissue and tumor stroma and their
respective control tissues, with mean IHS generally comparable with
those for CD31 (tumor tissues vs. controls: fibrinogen, 1.5 vs.
1.1; fibronectin, 1.7 vs. 0.9; CD31, 1.9 vs. 0.8; and in tumor
stroma vs. controls: fibrinogen, 2.0 vs. 1.4; fibronectin, 1.9 vs.
1.5; CD31, 2.6 vs. 2.3) (Table
VI). Osteopontin was expressed less strongly with little
difference in expression between the tumors and control samples for
tumor tissue or stroma (tumor tissues vs. controls: 0.5 vs. 0.7 and
tumor stroma vs. controls: 0.9 vs. 0.7). Tumor endothelia expressed
fibronectin and fibrinogen more strongly than control endothelia,
while staining for vitronectin and osteopontin expression was
unchanged over the control.
Discussion
Integrins interacting with their complementary
extracellular matrix targets regulate normal cellular behavior.
Changes in these interactions are implicated in cancer progression
(23,37–41).
In this study, we used immunohistochemistry to investigate the
expression of integrin-ligand combinations implicated in tumor
angiogenesis within tumor material from 40 HNSSC patients compared
to 20 normal controls. We investigated αvβ3, αvβ5, α5β1 and their
ligands, osteopontin, vitronectin, fibronectin and fibrinogen, and
found that these proteins are disregulated within the tumor
environment. αvβ5 and α5β1 were overexpressed in tumor cells, αvβ3
in endothelia, and each integrin in the tumor stroma. Expression of
the ligands, fibrinogen and fibronectin, was elevated in the tumor
vasculature environment, fibronectin and osteopontin in the stroma,
but none in the tumor cells, while activated vitronectin remained
unchanged in each environment. These results support a role for
αvβ3-osteopontin and fibronectin, α5β1-fibronectin interactions in
influencing HNSCC angiogenesis and α5β1-fibronectin and
αvβ5-vitronectin influencing tumor cell behavior. The elevated
fibrinogen and fibronectin in the vasculature may be related to
defective vascular patency and increased serum leakage within
tumors.
Vitolo et al (39) detected an increasing frequency of
α5β1 expression in oral tissues; expression in 0/7 normal
epithelium, in carcinoma in situ 8/9 and in invasive
carcinoma 8/13, in contrast to lack of expression of αvβ3 in the
same tissues. According to Thomas and Speight (40), the integrin α5β1 was weakly
expressed in oral keratinocytes, while αvβ6 was implicated in HNSCC
progression (42). In the in
vitro study of Reinartz et al (43), αvβ5 was expressed in human
keratinocytic cells (HaCaT). In epithelia of the controls we found
that each of the three integrins, αvβ3, αvβ5 and α5β1, was
expressed; αvβ3 exhibited the weakest expression (Table IV). Expression of αvβ3 remained
weak in normal epithelia, but was significantly higher than in the
tumor tissues (Table V). However,
in our study the epithelia of the controls exhibited weak
expression of α5β1 and significantly lower α5β1 expression than in
the tumor tissues.
Increased or inappropriate expression of integrins
is believed, in coordination with their ligands, to support tumor
growth and metastasis, and to promote tumor angiogenesis in head
and neck carcinomas (37–41,44,45).
These phenomena are of considerable scientific and clinical
interest, as experimental studies indicate that disruption of
integrin function may inhibit the growth, neovascularization and
metastasis of some types of cancers (9,19–23).
Indeed, drugs that block the interaction of integrins with the
extracellular matrix are under development for the management of
several clinically important tumor types. One such drug,
cilengitide, is a selective blocker of ligand interaction with αvβ3
and αvβ5 integrins (9,18,24,25,27):
the integrins assessed in this study.
We demonstrated marked expression of integrins and
their ligands in oral tumor tissues (Table IV), and strong staining for CD31 in
tumor tissues was consistent with angiogenesis and
neovascularization (Table IV and
Fig. 2h), thus confirming
observations in oral cancer by Kurtz et al (15) and Villaret et al (46). In our study we found weak staining
for αvβ3 in tumor or stromal cells (Table IV and Fig. 2a). This is in contrast to
observations noted in malignant gliomas by Schnell et al
(47) and in melanoma by Albelda
et al (48), who found that
tumors expressed higher levels of αvβ3 than normal tissues. A
statistically significant increased staining vs. controls was
demonstrated for αvβ3 in endothelia, but not in stroma (Tables IV and V). In the present study, αvβ5 staining
was statistically significantly increased in tumor samples compared
to the controls (Table V), which
corroborates the findings of Jones et al (37). However, αvβ5 was markedly expressed
in stroma rather than in endothelia. There was some increase in the
expression of α5β1 in tumor samples associated with tumor cells,
endothelia and stroma. Expression of ligands for integrins varied
between the tissue types, with no clear differentiation and no
statistically significant expression between tumor and control
samples, with the notable exception of the αvβ3 ligand osteopontin
and the αvβ3/α5β1 ligand fibronectin, which were significantly
up-regulated in the tumor stroma. This complements the
up-regulation of αvβ3 and α5β1 noted on the tumor vasculature.
Notably, since activated vitronectin was conspicuously uniformly
distributed between the normal and tumor tissues, it appears to be
less involved in tumor-specific integrin-driven behaviors in
HNSCC.
Previous histochemical studies identified the
expression of αvβ3 in various tumors, with a particularly strong
and functional association with tumor invasive blood vessels
consistent with the more detailed analyses of the present study
(49–52). Other studies have found increased
αvβ3 expression to be correlated with greater invasive or
metastatic potential (53–55). Radiotracers specific to αvβ3 have
revealed this integrin in human tumor tissue in situ
(47,56). αvβ5 integrin has also been
implicated in tumor cell invasion and migration (57–59),
and αvβ3 and αvβ5 regulate cellular responses to hypoxia in
glioblastomas (60). α5β1 has also
been implicated in tumor migration and angiogenesis (61–65)
and may control cell migration in concert with αvβ3 (66).
Confirmation of the presence of integrins, αvβ3 and
αvβ5, and their activating ligands in association with HNSCC
tumors, supports a potential role for these integrins in human oral
tumors. Overall, increased expression of integrins within tumors,
particularly expression associated with endothelial cells, supports
the emergent therapeutic concept of selective integrin blockade as
a anticancer strategy (9,23,27,67).
Abbreviations:
|
APAAP,
|
alkaline phosphatase-anti-alkaline
phosphatase;
|
|
FBG,
|
fibrinogen;
|
|
FN,
|
fibronectin;
|
|
HNSCC,
|
squamous cell carcinoma of the head
and neck;
|
|
Ig,
|
immunoglobulin;
|
|
IHS,
|
immunohistochemical score;
|
|
OP,
|
osteopontin;
|
|
PP,
|
staining frequency, percentage of
positive staining;
|
|
SD,
|
standard deviation;
|
|
SI,
|
staining intensity;
|
|
St,
|
stroma;
|
|
TBS,
|
tris buffered saline;
|
|
TNM of malignant tumors: T,
|
tumor;
|
|
N,
|
node;
|
|
M,
|
metastasis;
|
|
V,
|
vessel;
|
|
VN,
|
vitronectin
|
Acknowledgements
This study was supported by a grant
from Merck KGaA. The authors would like to thank Dr Andreas Eilers
(Merck KGaA, Darmstadt) and Dr Mike Gwilt (supported by Merck KGaA)
for editorial assistance. We thank Professor David Loskutoff
(Scippts Research Institute, USA) for the kind gift of monoclonal
antibody 153 and Mr. Franz Hafner (Clinic for Oral and
Maxillofacial Surgery, Campus Virchow Hospital
Charité-Universitätsmedizin, Berlin, Germany) for the micro-photo
scanning.
References
|
1.
|
Rapidis AD, Keramidas T, Panangiotopoulos
H, Andressakis D and Angelopoulos AP: Tumours of the head and neck
in the elderly: analysis of 190 patients. J Craniomaxillofac Surg.
26:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
3.
|
Döbrossy L: Epidemiology of head and neck
cancer: magnitude of the problem. Cancer Metastasis Rev. 24:9–17.
2005.PubMed/NCBI
|
|
4.
|
Pai SI and Westra WH: Molecular pathology
of head and neck cancer: implications for diagnosis, prognosis, and
treatment. Annu Rev Pathol. 4:49–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tejani MA, Cohen RB and Mehra R: The
contribution of cetuximab in the treatment of recurrent and/or
metastatic head and neck cancer. Biologics. 4:173–185.
2010.PubMed/NCBI
|
|
6.
|
Llewellyn CD, Johnson NW and
Warnakulasuriya KA: Risk factors for squamous cell carcinoma of the
oral cavity in young people — a comprehensive literature review.
Oral Oncol. 37:401–418. 2001.
|
|
7.
|
Llewellyn CD, Linklater K, Bell J, Johnson
NW and Warnakulasuriya KA: Squamous cell carcinoma of the oral
cavity in patients aged 45 years and under: a descriptive analysis
of 116 cases diagnosed in the South East of England from 1990 to
1997. Oral Oncol. 39:106–114. 2003.PubMed/NCBI
|
|
8.
|
Rutt AL, Hawkshaw MJ and Sataloff RT:
Laryngeal cancer in patients younger than 30 years: a review of 99
cases. Ear Nose Throat J. 89:189–192. 2010.PubMed/NCBI
|
|
9.
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Stupp R and Ruegg C: Integrin inhibitors
reaching the clinic. J Clin Oncol. 25:1637–1638. 2007.Comment on:
Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher
JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD and
Grossman SA: Phase I and correlative biology study of cilengitide
in patients with recurrent malignant glioma. J Clin Oncol 25:
1651–1657, 2007.
|
|
11.
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hasina R and Lingen MW: Angiogenesis in
oral cancer. J Dent Educ. 65:1282–1290. 2001.
|
|
13.
|
Rüegg C, Dormond O and Foletti A:
Suppression of tumor angiogenesis through the inhibition of
integrin function and signaling in endothelial cells: which side to
target? Endothelium. 9:151–160. 2002.PubMed/NCBI
|
|
14.
|
Erovic BM, Neuchrist C, Berger U,
El-Rabadi K and Burian M: Quantitation of microvessel density in
squamous cell carcinoma of the head and neck by computer-aided
image analysis. Wien Klin Wochenschr. 117:53–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kurtz KA, Hoffman HT, Zimmerman MB and
Robinson RA: Perineural and vascular invasion in oral cavity
squamous carcinoma: increased incidence on re-review of slides and
by using immunohistochemical enhancement. Arch Pathol Lab Med.
29:354–359. 2005.
|
|
16.
|
Walsh JE, Lathers DM, Chi AC, Gillespie
MB, Day TA and Young MR: Mechanisms of tumor growth and metastasis
in head and neck squamous cell carcinoma. Curr Treat Options Oncol.
8:227–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Seiwert TY and Cohen EE: Targeting
angiogenesis in head and neck cancer. Semin Oncol. 35:274–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tucker RW, Sanford KK, Handleman SL and
Jones GM: Alpha v integrin inhibitors and cancer therapy. Curr Opin
Investig Drugs. 4:722–731. 2003.PubMed/NCBI
|
|
19.
|
Cai W and Chen X: Anti-angiogenic cancer
therapy based on integrin alphavbeta3 antagonism. Anticancer Agents
Med Chem. 6:407–428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V
and Chen X: Integrin alpha v beta 3 antagonists for anti-angiogenic
cancer treatment. Recent Pat Anticancer Drug Discov. 2:143–158.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Paolillo M, Russo MA, Serra M, Colombo L
and Schinelli S: Small molecule integrin antagonists in cancer
therapy. Mini Rev Med Chem. 9:1439–1446. 2009. View Article : Google Scholar
|
|
22.
|
Sheldrake HM and Patterson LH: Function
and antagonism of beta3 integrins in the development of cancer
therapy. Curr Cancer Drug Targets. 9:519–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rathinam R and Alahari SK: Important role
of integrins in the cancer biology. Cancer Metastasis Rev.
29:223–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dechantsreiter MA, Planker E, Mathä B,
Lohof E, Hölzemann G, Jonczyk A, Goodman SL and Kessler H:
N-Methylated cyclic RGD peptides as highly active and selective
alpha (V) beta (3) integrin antagonists. J Med Chem. 42:3033–3040.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Reardon DA, Fink KL, Mikkelsen T,
Cloughesy TF, O'Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ,
Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ,
Wittemer SM, Nippgen J, Picard M and Nabors LB: Randomized phase II
study of cilengitide, an integrin-targeting
arginine-glycine-aspartic acid peptide, in recurrent glioblastoma
multiforme. J Clin Oncol. 26:5610–5617. 2008.with comment:
Chamberlain MC: Cilengitide: does it really represent a new
targeted therapy for recurrent glioblastoma? J Clin Oncol 27:
1921–1922, 2009.
|
|
26.
|
Reardon DA, Nabors LB, Stupp R and
Mikkelsen T: Cilengitide: an integrin-targeting
arginine-glycine-aspartic acid peptide with promising activity for
glioblastoma multiforme. Expert Opin Investig Drugs. 17:1225–1235.
2008. View Article : Google Scholar
|
|
27.
|
Raguse JD, Gath HJ, Bier J, Riess H and
Oettle H: Cilengitide (EMD 121974) arrests the growth of a heavily
pretreated highly vascularised head and neck tumour. Oral Oncol.
40:228–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Preissner KT: Structure and biological
role of vitronectin. Annu Rev Cell Biol. 7:275–310. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Seiffert D and Smith JW: The cell adhesion
domain in plasma vitronectin is cryptic. J Biol Chem.
272:13705–13710. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Cordell JL, Falini B, Erber WN, Ghosh AK,
Abdulaziz Z, MacDonald S, Pulford KAF, Stein H and Mason DY:
Immunoenzymatic labeling of monoclonal antibodies using immune
complexes of complexes of alkaline phosphatase and monoclonal
anti-alkaline phosphatase (APAAP Complexes). J Histochem Cytochem.
32:219–229. 1984. View Article : Google Scholar
|
|
32.
|
Fabricius E-M, Langford A, Bier J, Hell B,
Wildner G-P and Blümcke S: Immunohistochemical characterization of
E48 and CD44-v6 expression in head and neck carcinomas. Cancer J.
10:325–330. 1997.
|
|
33.
|
Fabricius E-M, Guschmann M, Wildner G-P,
Langford A, Hell B and Bier J: Divergent immunohistochemical E48
and CD44-v6 antigen expression patterns between lymph node
metastases and primary squamous cell carcinomas in the head and
neck region. Cancer J. 11:153–159. 1998.
|
|
34.
|
Fabricius E-M, Guschmann M, Langford A,
Hell B and Bier J: Immunhistochemical assessment of the
tumour-associated epitopes CD44v6 and E48 in tumour-free lymph
nodes from patients with squamous cell carcinoma in the head-neck
region. Anal Cell Pathol. 20:115–129. 2000. View Article : Google Scholar
|
|
35.
|
Remmele W and Stegner HE: Vorschlag zur
einheitlichen Definition eines immunreaktiven Scores (IRS) für den
immunhistochemischen Östrogenrezeptor-Nachweis (ER-ICA) im
Mammagewebe. Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
36.
|
Sachs L: Angewandte Statistik - Anwendung
statistischer Methoden. 11. Auflage Springer Verlag; Berlin: pp.
8892004
|
|
37.
|
Jones J, Watt FM and Speight PM: Changes
in the expression of alpha v integrins in oral squamous cell
carcinomas. J Oral Pathol Med. 26:63–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Thomas GJ, Jones J and Speight PM:
Integrins and oral cancer. Oral Oncol. 33:381–388. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Vitolo D, Ciocci L, Ferrauti P, Cicerone
E, Gallo A, De Vincentiis M and Baroni CD: Alpha5 integrin
distribution and TGFbeta1 gene expression in supraglottic
carcinoma: their role in neoplastic local invasion and metastasis.
Head Neck. 22:48–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Thomas GJ and Speight PM: Cell adhesion
molecules and oral cancer. Crit Rev Oral Biol Med. 12:479–498.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Kramer RH, Shen X and Zhou H: Tumor cell
invasion and survival in head and neck cancer. Cancer Metastasis
Rev. 24:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Thomas GJ, Nystrom ML and Marshall JF:
Alphavbeta6 integrin in wound healing and cancer of the oral
cavity. J Oral Pathol Med. 35:1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Reinartz J, Schäfer B, Batrla R, Klein CE
and Kramer MD: Plasmin abrogates alpha v beta 5-mediated adhesion
of a human keratinocyte cell line (HaCaT) to vitronectin. Exp Cell
Res. 220:274–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Ziober BL, Silverman SS Jr and Kramer RH:
Adhesive mechanisms regulating invasion and metastasis in oral
cancer. Crit Rev Oral Biol Med. 12:499–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Lyons AJ and Jones J: Cell adhesion
molecules, the extracellular matrix and oral squamous carcinoma.
Int J Oral Maxillofac Surg. 36:671–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Villaret AB, Schreiber A, Facchetti F,
Fisogni S, Lonardi S, Lombardi D, Cocco D, Redaelli de Zinis LO and
Nicolai P: Immunostaining patterns of CD31 and podoplanin in
previously untreated advanced oral/oropharyngeal cancer: prognostic
implications. Head Neck. 32:786–792. 2010.PubMed/NCBI
|
|
47.
|
Schnell O, Krebs B, Carlsen J, Miederer I,
Goetz C, Goldbrunner RH, Wester HJ, Haubner R, Pöpperl G,
Holtmannspötter M, Kretzschmar HA, Kessler H, Tonn JC, Schwaiger M
and Beer AJ: Imaging of integrin {alpha} v{beta}3 expression in
patients with malignant glioma by [18F] galacto-RGD positron
emission tomography. Neuro Oncol. 11:861–870. 2009.
|
|
48.
|
Albelda SM, Mette SA, Elder DE, Stewart R,
Damjanovich L, Herlyn M and Buck CA: Integrin distribution in
malignant melanoma: association of the beta 3 subunit with tumor
progression. Cancer Res. 50:6757–6764. 1990.PubMed/NCBI
|
|
49.
|
Max R, Gerritsen RR, Nooijen PT, Goodman
SL, Sutter A, Keilholz U, Ruiter DJ and DeWaal RM:
Immunohistochemical analysis of integrin alpha vbeta3 expression on
tumor-associated vessels of human carcinomas. Int J Cancer.
71:320–324. 1997.Erratum in: Int J Cancer 72: 706–707, 1997.
|
|
50.
|
Sato T, Konishi K, Kimura H, Maeda K,
Yabushita K, Tsuji M and Miwa A: Vascular integrin beta 3 and its
relation to pulmonary metastasis of colorectal carcinoma.
Anticancer Res. 21:643–647. 2001.PubMed/NCBI
|
|
51.
|
Tang Y, Borgstrom P, Maynard J, Koziol J,
Hu Z, Garen A and Deisseroth A: Mapping of angiogenic markers for
targeting of vectors to tumor vascular endothelial cells. Cancer
Gene Ther. 14:346–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Beer AJ, Niemeyer M, Carlsen J, Sarbia M,
Nährig J, Watzlowik P, Wester HJ, Harbeck N and Schwaiger M:
Patterns of alphavbeta3 expression in primary and metastatic human
breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med.
49:255–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Gasparini G, Brooks PC, Biganzoli E,
Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R and Cheresh
DA: Vascular integrin alpha(v)beta3: a new prognostic indicator in
breast cancer. Clin Cancer Res. 4:2625–2634. 1998.PubMed/NCBI
|
|
54.
|
Vonlaufen A, Wiedle G, Borisch B, Birrer
S, Luder P and Imhof BA: Integrin alpha(v)beta(3) expression in
colon carcinoma correlates with survival. Mod Pathol. 14:1126–1132.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Schnell O, Krebs B, Wagner E, Romagna A,
Beer AJ, Grau SJ, Thon N, Goetz C, Kretzschmar HA, Tonn JC and
Goldbrunner RH: Expression of integrin alphavbeta3 in gliomas
correlates with tumor grade and is not restricted to tumor
vasculature. Brain Pathol. 18:378–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Zannetti A, Del Vecchio S, Iommelli F, Del
Gatto A, De Luca S, Zaccaro L, Papaccioli A, Sommella J, Panico M,
Speranza A, Grieco P, Novellino E, Saviano M, Pedone C and
Salvatore M: Imaging of alphavbeta3 expression by a bifunctional
chimeric RGD peptide not cross-reacting with alphavbeta5. Clin
Cancer Res. 15:5224–5233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Monnier Y, Farmer P, Bieler G, Imaizumi N,
Sengstag T, Alghisi GC, Stehle JC, Ciarloni L, Andrejevic-Blant S,
Moeckli R, Mirimanoff RO, Goodman SL, Delorenzi M and Rüegg C:
CYR61 and alphaVbeta5 integrin cooperate to promote invasion and
metastasis of tumors growing in preirradiated stroma. Cancer Res.
68:7323–7331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Ricono JM, Huang M, Barnes LA, Lau SK,
Weis SM, Schlaepfer DD, Hanks SK and Cheresh DA: Specific
cross-talk between epidermal growth factor receptor and integrin
alphav-beta5 promotes carcinoma cell invasion and metastasis.
Cancer Res. 69:1383–1391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Vocca I, Franco P, Alfano D, Votta G,
Carriero MV, Estrada Y, Caputi M, Netti PA, Ossowski L and
Stoppelli MP: Inhibition of migration and invasion of carcinoma
cells by urokinase-derived antagonists of alphavbeta5 integrin
activation. Int J Cancer. 124:316–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Skuli N, Monferran S, Delmas C, Favre G,
Bonnet J, Toulas C and Cohen-Jonathan Moyal E:
Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for
hypoxia regulation in glioblastoma. Cancer Res. 69:3308–3316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Färber K, Synowitz M, Zahn G, Vossmeyer D,
Stragies R, van Rooijen N and Kettenmann H: An alpha5beta1 integrin
inhibitor attenuates glioma growth. Mol Cell Neurosci. 39:579–585.
2008.PubMed/NCBI
|
|
62.
|
Morozevich GE, Kozlova NI, Cheglakov IB,
Ushakova NA, Preobrazhenskaya ME and Berman AE: Implication of
alpha5-beta1 integrin in invasion of drug-resistant MCF-7/ADR
breast carcinoma cells: a role for MMP-2 collagenase. Biochemistry.
73:791–796. 2008.PubMed/NCBI
|
|
63.
|
Morozevich G, Kozlova N, Cheglakov I,
Ushakova N and Berman A: Integrin alpha5beta1 controls invasion of
human breast carcinoma cells by direct and indirect modulation of
MMP-2 collagenase activity. Cell Cycle. 8:2219–2225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Lee MY, Huang JP, Chen YY, Aplin JD, Wu
YH, Chen CY, Chen PC and Chen CP: Angiogenesis in differentiated
placental multipotent mesenchymal stromal cells is dependent on
integrin alpha5beta1. PLoS One. 4:E69132009. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Zahn G, Vossmeyer D, Stragies R, Wills M,
Wong CG, Löffler KU, Adamis AP and Knolle J: Preclinical evaluation
of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427
in monkey and rabbit models of choroidal neovascularization. Arch
Ophthalmol. 127:1329–1335. 2009. View Article : Google Scholar
|
|
66.
|
Morgan MR, Byron A, Humphries MJ and Bass
MD: Giving off mixed signals-distinct functions of alpha5beta1 and
alphavbeta3 integrins in regulating cell behaviour. IUBMB Life.
61:731–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Carter A: Integrins as target: first phase
III trial launches, but questions remain. J Natl Cancer Inst.
102:675–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Wittekind Ch, Meyer HJ and Bootz F: TNM
Klassifikation Maligner Tumoren. UICC International Union Against
Cancer. 6. Aufl. Korr. Nachdruck. Kopf- und Halstumoren; pp. 19–52.
2005
|
|
69.
|
Cheresh DA and Spiro RC: Biosynthetic and
functional properties of an Arg-Gly-Asp-directed receptor involved
in human melanoma cell attachment to vitronectin, fibrinogen, and
von Willebrand factor. J Biol Chem. 262:17703–17711.
1987.PubMed/NCBI
|
|
70.
|
Weinacker A, Chen A, Agrez M, Cone RI,
Nishimura S, Wayner E, Pytela R and Sheppard D: Role of the
integrin alpha v beta 6 in cell attachment to fibronectin –
heterologous expression of intact and secreted forms of the
receptor. J Biol Chem. 269:6940–6948. 1994.
|
|
71.
|
Wayner EA, Carter WG, Piotrowicz RS and
Kunicki TJ: The function of multiple extracellular matrix receptors
in mediating cell adhesion to extracellaular matrix: preparation of
monoclonal antibodies to the fibronectin receptor that specifically
inhibit cell adhesion to fibronectin and react with platelet
glycoprotein Ic-IIa. J Cell Biol. 107:1881–1891. 1988.
|
|
72.
|
Seiffert D, Ciambrone G, Wagner NV, Binder
BR and Loskutoff DJ: The somatomedin B domain of vitronectin.
Structural requirements for the binding and stabilization of active
type 1 plasminogen activator inhibitor. J Biol Chem. 269:2659–2666.
1994.PubMed/NCBI
|