Introduction
Melanoma antigens (MAGE) have recently
emerged as promising immunotherapeutic targets for anticancer
therapy, as they are recognized by cytotoxic T lymphocytes (CTLs)
in conjunction with MHC class I molecules of various haplotypes on
the tumor cell surface. Several clinical trials involving
gastrointestinal carcinoma, oesophageal carcinoma, pulmonary
carcinoma, melanoma and lung cancer have utilized proteins and
peptides derived from some of these antigens and have shown
encouraging results (1–3, Vansteenkiste J, et al, J Clin Oncol 25:
abs. 7554, 2007).
MAGE antigens are a large number of
closely-related proteins (4)
classified into type 1 (MAGE-A, MAGE-B, and
MAGE-C) and type 2 (MAGE-D) based on differences in
tissue-specific expression patterns and gene structure (5). While type 2 MAGE antigens are
almost universally expressed, expression of type 1 MAGE
antigens has not been reported in normal adult somatic tissues
apart from the testis (5,6). However, expression of MAGE 1
antigens has been documented in a broad variety of malignancies
(7–13). These observations have resulted in
the general consensus that expression of MAGE 1 antigens is
a tumor-specific phenomenon.
However, our previous research has raised a
significant possibility that certain MAGE 1 antigens are
also expressed in the normal lung tissue of smokers with non-small
cell lung cancer (NSCLC) (8). We
therefore hypothesized that the expression of MAGE-A1, -A3
and -B2 antigens is a process initiated in bronchial
epithelium exposed to tobacco carcinogenic insult even prior to
malignant transformation. To test this hypothesis, we carried out
the present study to assess the effect of smoking on the expression
of MAGE genes in chronic former smokers, with the aim of
determining the potential of these antigens as targets for
immunotherapeutic approaches in lung cancer chemoprevention.
Materials and methods
Study population
Bronchial brush samples before any chemopreventive
intervention were obtained from chronic smokers who had a smoking
history of at least 20 pack-years and had quit smoking for at least
1 year prior to the time of enrollment. The study was reviewed and
approved by the Institutional Review Board, and informed consent
was obtained from each subject. Smoking history was recorded as
packs/day, smoke-years, and pack-years (the product of packs/day
and smoke-years). None of the subjects had any history of lung
cancer, and all were clinically free of any cancer at the time of
enrollment. Bronchial brush samples were collected from carina
through bronchoscopy and placed in culture medium without serum.
Samples were immediately transported to the laboratory, washed
twice with PBS and stored in aliquots at −80°C until use.
RNA extraction, RT-PCR and the sequencing
of PCR products
Bronchial epithelial cells were used for extracting
total RNA using Tri-reagent according to the manufacturer's
instructions (Invitrogen, Carlsbad, CA). Approximately 1 μg
of total RNA from each sample was reverse-transcribed using
Superscript Reverse Transcriptase (Life Technologies, Gaithersburg,
MD) according to the manufacturer's protocol to generate cDNA. To
test cDNA integrity, the ß-actin gene transcript was analyzed for
each sample as cDNA quality controls. A normal lung cDNA library,
NSCLC cell lines (H 1944 for MAGE-A1 and -A3, and H
1792 for MAGE-B1) expressing a high level of MAGE
genes, and genomic DNA from each sample were used as controls in
each experiment. To avoid amplification of genomic DNA of the
MAGE genes, we designed PCR primer sets flanking intron(s):
MG1 forward (5′-TGTGGGCAG GAGCTGGGCAA-3′) and MG1 reverse
(5′-GCCGAA GGAACCTGACCCAG-3′) for MAGE-A1; MG3 forward
(5′-AAGCCGGCCCAGGCTCGGT-3′) and MG3 reverse
(5′-GCTGGGCAATGGAGACCCAC-3′) for MAGE-A3; and MGB2 forward
(5′-CAGCCAGGGGTGAATTCTCAG-3′) and MGB2 reverse
(5′-TTCTCACGGGCACGGAGCTTA-3′) for MAGE-B2. PCR conditions
for each MAGE gene were 95°C for 15 min; 35 cycles of 94°C
for 30 sec, 62°C for 1 min and 72°C for 1 min; and a final
extension at 72°C for 5 min in a volume of 12.5 μl with 0.5
units of Hotstar Taq Polymerase (Qiagen, Chatsworth, CA). RT-PCR
products were separated on 2.5% agarose gels, and the results were
read as positive or negative; the data were interpreted by two
independent observers to eliminate bias. Representative DNA bands
in agarose gels were eluted in 50 μl of sterile water using
a QIAamp spin column (Qiagen, Valencia, CA) and directly sequenced
using an AmpliCycle Sequencing kit (Perkin-Elmer, Foster City, CA)
according to the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using the
χ2 test or Fisher's exact test for associations between
genes and between gene status and other categorical demographic
factors. The Wilcoxon rank-sum test was used to analyze differences
in median age, packs/day, smoke-years, pack-years, and quit-years
between groups. All of the tests were two-sided. A P-value <0.05
was considered statistically significant.
Results
Expression of MAGE antigens in the
bronchial epithelial cells of chronic smokers
The 123 subjects consisted of 67 males and 56
females with a median age of 57 years (range 36–75) and a median of
40 pack-years (range 20–136) smoking history (Table I). MAGE-A1 antigen
expression was detected in 31 (25.2%) of the 123 specimens;
MAGE-B2 expression in 46 (37.4%) of the samples, and
MAGE-A3 expression in 38 (30.9%) of the samples. As reported
previously, expression of alternative spliced forms of the
MAGE genes was observed. RT-PCR products were directly
sequenced and confirmed to be the expected MAGE gene
transcripts (data not shown). The primers used in the study did not
amplify the corresponding genomic DNAs, excluding the possibility
of presenting a pseudogene.
 | Table I.Patient characteristics and MAGE
1 expression. |
Table I.
Patient characteristics and MAGE
1 expression.
| MAGE gene
expression | | n | Age, years median
(range) | PPD median
(range) | Smoke-years median
(range) | Pack-years median
(range) | Quit-years median
(range) |
|---|
| -A1 | − | 92 | 56.41
(38.52–75.47) | 1.5 (0.8–4) | 28 (10–58.39) | 39.05
(19.98–136) | 7.25
(1.01–45.98) |
| + | 31 | 61.83
(36.33–73.61) | 2 (1–4) | 30 (15–50) | 55.5 (20–136) | 8.84
(1.05–29.26) |
| P-value | | | 0.3170 | 0.0676 | 0.2042 | 0.0357 | 0.6132 |
| -B2 | − | 77 | 54.52
(38.52–73.62) | 1.5 (0.8–4) | 27 (10–48) | 38.6
(19.98–102) | 8.61
(1.02–38.18) |
| + | 46 | 62.44
(36.33–75.47) | 1.5 (1–4) | 30 (14–58.39) | 45 (20–136) | 7.23
(1.01–45.98) |
| P-value | | | 0.0066 | 0.4657 | 0.0368 | 0. 0537 | 0.9501 |
| -A3 | − | 85 | 56.87
(36.33–74.51) | 1.5 (0.8–4) | 28 (10–58.39) | 38.6
(19.98–136) | 8.84
(1.02–38.18) |
| + | 38 | 57.21
(40.49–75.47) | 1.5 (1–4) | 29.45 (15–50) | 46 (21.64–125) | 6.93
(1.01–45.98) |
| P-value | | | 0.9935 | 0.2993 | 0.6581 | 0.1718 | 0.4969 |
| -A1/-A3 | − | 108 | 56.74
(36.33–75.47) | 1.5 (0.8–4) | 28 (10–58.39) | 40 (19.98–136) | 7.5
(1.01–45.98) |
| + | 15 | 58.96
(41.1–73.61) | 1.5 (1–3.5) | 27 (15–50) | 55.5 (24–125) | 7.24
(1.27–23.27) |
| P-value | | | 0.5503 | 0.5230 | 0.4639 | 0.3798 | 0.9355 |
| -A1/-B2 | − | 104 | 56.08
(38.52–75.47) | 1.5 (0.8–4) | 27.79
(10–58.39) | 39.7
(19.98–136) | 7.25
(1.01–45.98) |
| + | 19 | 65.11
(36.33–73.61) | 2 (1–4) | 30 (15–50) | 60 (20–136) | 10.06
(1.05–29.26) |
| P-value | | | 0.0375 | 0.0436 | 0.0940 | 0.0135 | 0.3891 |
| -B2/-A3 | − | 108 | 56.74
(36.33–74.51) | 1.5 (0.8–4) | 28 (10–58.39) | 40 (19.98–136) | 8.43
(1.02–38.18) |
| + | 15 | 58.96
(40.49–75.47) | 1.5 (1–3.5) | 30 (15–50) | 55.5
(22.5–125) | 6.62
(1.01–45.98) |
| P-value | | | 0.5149 | 0.3832 | 0.3592 | 0.1486 | 0.7902 |
|
-A1/-B2/-A3 | − | 114 | 56.74
(36.33–75.47) | 1.5 (0.8–4) | 28 (10–58.39) | 40 (19.98–136) | 7.5
(1.01–45.98) |
| + | 9 | 58.96
(41.1–73.61) | 2 (1–3.5) | 30 (22–50) | 60 (27–125) | 7.2
(1.27–23.27) |
| P-value | | | 0.5386 | 0.1202 | 0.3887 | 0.0561 | 0.7750 |
Pattern of coexpression of different MAGE
antigens
Of the 123 samples studied, 31 (25.2%) showed
coexpression of any two and 9 (7.3%) showed coexpression of all
three antigens (Table II).
Coexpression of MAGE-A1 and -B2 was noted in 19
(15.4%) samples, MAGE-A1 and -A3 in 15 (12.2%)
samples, and MAGE-A3 and -B2 in 15 (12.2%) samples.
Importantly, we found a statistically significant pattern of
coexpression between MAGE-A1 and -B2 (P=0.002) and
between MAGE-A1 and -A3 (P=0.01). Notably, the
significance of the association was stronger for lack of
coexpression; i.e., when one gene is not expressed, there is a high
chance that the other gene is not expressed either. There was no
statistical correlation between the expression of MAGE-A3
and -B2.
 | Table II.MAGE 1 coexpression. |
Table II.
MAGE 1 coexpression.
| -A1 | -B2
| Total |
| 0 | 1 |
|
| 0 | 65 | 27 | 92 |
| 1 | 12 | 19 | 31 |
| Total | 77 | 46 | 123 |
|
| χ2 test,
P=0.002 |
|
| -A1 | -A3
| Total |
| 0 | 1 |
|
| 0 | 69 | 23 | 92 |
| 1 | 16 | 15 | 31 |
| Total | 85 | 38 | 123 |
|
| χ2 test,
P=0.01 |
|
| -B2 | -A3
| Total |
| 0 | 1 |
|
| 0 | 54 | 23 | 77 |
| 1 | 31 | 15 | 46 |
| Total | 85 | 38 | 123 |
|
| χ2 test,
P=0.75 |
Correlation of expression of MAGE
antigens with clinical parameters
Expression of the MAGE genes in the bronchial
epithelium was correlated with subject age, gender, and pack-years
smoking history (Table III).
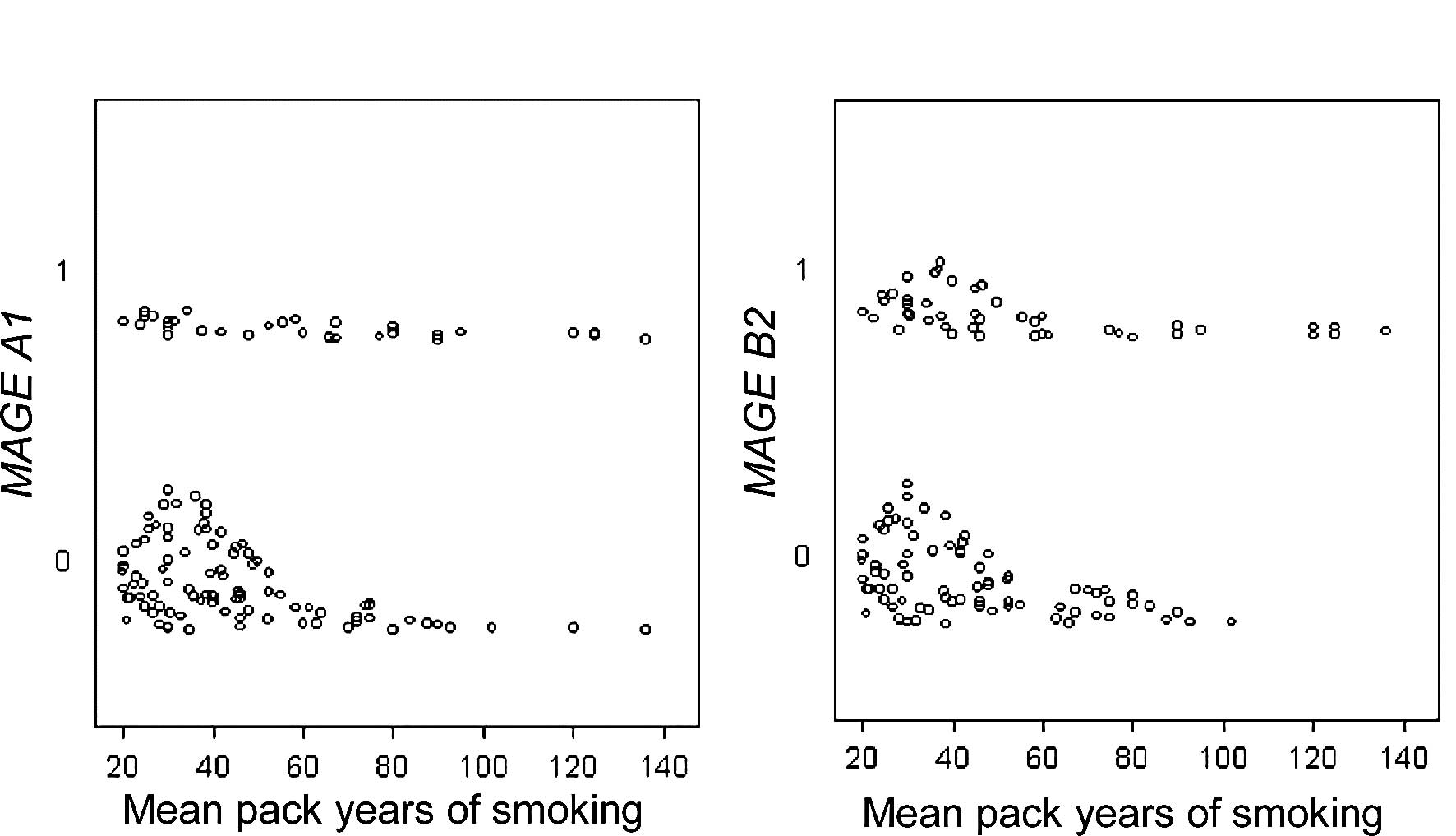
A1 expression was correlated with pack-years of smoking
(55.5 vs. 39.1; P=0.04) (Fig. 1A),
and B2 expression with age (median 62.4 vs. 54.5 years;
P=0.007), years of smoking (30 vs. 27; P=0.04), and pack-years of
smoking (45 vs. 38.6; P=0.05) (Fig.
1B) on univariate analysis. A1 and B2
coexpression was correlated with pack-years of smoking (median 60
vs. 39.7; P=0.01); with packs smoked/day (median 2 vs. 1.5; P=0.04)
and age (median 65 vs. 56 years; P=0.04) on univariate analysis. On
logistic regression multivariate analysis, the expression of
A1 was found to be correlated with pack-years of smoking
(P=0.01) after adjustment for gender (P=0.06), B2 with age
(P=0.046) after adjustment for pack-years, and coexpression of any
two genes with pack-years (P=0.03) after adjustment for gender
(P=0.05). Overall, the expression of A1 and B2
together or independently was found to be correlated with
pack-years.
 | Table III.MAGE 1 expression in logistic
regression analyses. |
Table III.
MAGE 1 expression in logistic
regression analyses.
| MAGE
gene | Parameter | Estimate | SE | Wald 95% CI | P-value |
|---|
| -A1 | Gender (M vs.
F) | 0.8703 | 0.4637 | −0.0386 | 1.7791 | 0.0605 |
| Pack-years | 0.0191 | 0.0076 | 0.0041 | 0.0340 | 0.0123 |
| Quit-years | −0.0086 | 0.0245 | −0.0566 | 0.0393 | 0.7243 |
| -B2 | Age | 0.0568 | 0.0260 | 0.0059 | 0.1077 | 0.0288 |
| Gender (M vs.
F) | −0.1994 | 0.4026 | −0.9885 | 0.5897 | 0.6204 |
| Pack-years | 0.0116 | 0.0080 | −0.0041 | 0.0272 | 0.1472 |
| Quit-years | −0.0228 | 0.0248 | −0.0714 | 0.0258 | 0.3578 |
| -A3 | Gender (M vs.
F) | 0.3706 | 0.4048 | −0.4228 | 1.1641 | 0.3599 |
| Pack-years | 0.0062 | 0.0072 | −0.0079 | 0.0203 | 0.3904 |
| Quit-years | −0.0223 | 0.0229 | −0.0671 | 0.0225 | 0.3286 |
Impact of smoking cessation on the
expression of MAGE antigens
To determine the potential impact of smoking
cessation on the expression of MAGE antigens, the subjects
were catgorized into three groups according to the duration of
smoking cessation: cessation duration <5 years, 5–10 years, and
>10 years. Subsequently, the frequencies of MAGE
expression among the groups were analyzed using a logistic
regression model. Despite a prolonged smoking cessation, no
statistically significant decrease in the frequencies of expression
of MAGE-A1 (P=0.6), -B2 (P=0.8) and -A3
(P=0.4), respectively, were found.
Discussion
Our results demonstrated that MAGE-A1, -A3
and -B2 antigens are expressed in the airway epithelial
cells of a proportion of chronic smokers without lung cancer,
supporting our hypothesis that these antigens are activated even
prior to the malignant transformation of bronchial cells. This
novel finding contradicts the current perception that these
antigens are expressed only in cancer cells, and has important
clinical implications. Since these antigens are not expressed in
normal lung tissue, their activation and expression in heavy
smokers might represent an early carcinogenic state. Thus, for the
first time, this finding draws attention to the novel significance
of MAGE antigens as chemopreventive targets.
To date, type 1 MAGE antigens have been
considered tumor-specific (5,6),
since they are not expressed in adult normal tissues apart from the
testis, (14–16), but are expressed in a wide variety
of histologically distinct tumor types (17), including NSCLC (7,8,10).
These observations, coupled with the fact that these antigens are
immunogenic and can be recognized by autologous cytotoxic T cells
(18), have aroused a great deal
of interest and suggest these antigens are ideal cancer
immunotherapy targets. One clinical trial investigating patients
with malignant melanoma demonstrated anti-tumor activity by
vaccination with a MAGE-A3 peptide (1). In a recently completed randomized
phase II clinical trial for patients with surgically resected early
stage NSCLC, long-term administration of a MAGE-A3 peptide
resulted in an improvement in cancer-free survival (Vansteenkiste
J, et al, J Clin Oncol 25: abs. 7554, 2007). This approach is
associated with minimal side effects since the testis, the only
adult normal tissue expressing MAGE, does not express MHC
I/II molecules and therefore would be immune-exempt, as germ cells
cannot present MAGE proteins. Additionally, the testis has a
blood-testis barrier, and is therefore considered an
immunologically privileged site (13,19).
Therefore, immunization of a subject against these antigens, if
successful, would be expected to be cell-specific with no direct
effects on normal tissues. Thus, immunotherapeutic approaches
against these antigens have emerged as a promising and feasible
anticancer approach.
However, the present study expands the potential of
MAGE antigens from a therapeutic to a prevention setting. We
analyzed 123 bronchial brush samples from 123 unrelated individual
former chronic smokers, which allowed for the determination of the
frequencies of MAGE antigen activation in this type of
cohort, as well as the relationship with smoking history and
cessation. Our observations firmly establish that type 1
MAGE antigens are expressed in non-malignant adult
epithelial cells chronically exposed to tobacco carcinogens. Our
findings differ from other reports, which failed to find
MAGE expression in normal tissues (20,21).
This discrepancy is most likely attributable to the use of
different primer sets and a smaller amplicon size in our study,
which could have affected the PCR amplification efficiency. It is
also possible that the normal lung tissues used in other studies
may have contained a limited amount of lung epithelial cells,
whereas the bronchial brush samples used in our study consisted
mainly of lung epithelial cells.
Considering the malignant potential of these
antigens, there is a possibility that the smokers who express these
antigens are at a higher risk of lung cancer development as
compared to other subjects, since these antigens are known to
function as oncoproteins. MAGE-A1 acts as a potent
transcriptional repressor by binding to Ski interacting protein and
recruiting histone deacetylase 1 (HDAC) (22). Transfection of a human
MAGE-A3 vector into murine myoblast cells was found to
enhance resistance of the cells to apoptosis induced by prolonged
ER stress, possibly through its binding and inhibiting murine
caspase-12 (23). This suggests
that MAGE-A3 may protect malignant or premalignant cells
from apoptosis and, therefore, may provide them with a survival
advantage. In fact, MAGE A3 expression has been found to be
associated with lung tumor progression (11).
Expression of MAGE 1 genes could be
indicative of a carcinogenic process, since they have been
demonstrated to be linked to overall DNA demethylation (24). Global hypomethylation is a hallmark
of most cancer genomes, and is associated with tumor progression
(25,26). Thus, it is conceivable that
MAGE-A expression is linked to progressive disease. This
aberrant activation could confer a growth advantage to these cells
as compared to normally methylated cells. The acquisition of DNA
demethylation by susceptible cells enables them to grow rapidly and
to then progressively overwhelm other cells. Targeting these
precancerous cells by specific CTL is attractive, since the
remaining not-so-transformed cells would probably show less
aggressiveness. Thus, a cancer vaccine preventing the emergence of
demethylated MAGE-expressing precancerous cells could halt
the formation and development of cancer.
Although the mechanisms of tobacco-induced
expression of MAGE antigens are yet to be fully elucidated,
it is possible that tobacco carcinogens deregulate the methylation
pattern of these genes. Alternatively, they may cause aberrant
histone modifications and result in expression of MAGE
antigens. Bollati et al (27) observed that low-dose exposure to
airborne benzene was associated with a significant reduction in
LINE-1 (−2.33% for a 10-fold increase in airborne benzene levels;
P=0.009), AluI methylation (−1.00%; P=0.027) and hypomethylation in
MAGE 1 (−0.49%; P=0.049). In this regard, it is important to
note that we recently reported a new subfamily of DNA
methyltransferase 3B termed ΔDNMT3B (28). We observed expression of at least
seven variants resulting from alternative splicing in bronchial
brush samples from cancer-free chronic smokers (data not shown),
suggesting that ΔDNMT3B variants potentially regulate DNA
methylation in early carcinogenesis. Some of these variants,
particularly ΔDNMT3B5, ΔDNMT3B6 and ΔDNMT3B7, which lack the 3′ C
terminal catalytic domain, may be in competition with other ΔDNMT3B
variants, resulting in DNA hypomethylation on pericentromeric
satellite regions, thus leading to transcriptional activation of
MAGE antigens. However, this hypothesis remains to be
confirmed. As chronic smokers often present with chronic pulmonary
inflammation, such as chronic bronchitis, it is possible that the
MAGE antigens are activated through the reactive oxygen
species and oxidative DNA damage produced by cigarette smoke, which
reduces the binding affinity of the methyl-CpG binding protein 2,
thereby resulting in epigenetic alterations (29). Although it is possible that certain
hematologic stem cells in the inflammation field might express
MAGE antigens, we previously demonstrated the expression of
MAGE antigens in non-cancerous bronchial epithelium. Since
most of the bronchial brush samples we analyzed contained a high
purity of epithelial cells, the expression observed in this study
was likely derived from bronchial epithelial cells.
We further assessed the effect of smoking cessation
on the expression of these antigens. Notably, the frequencies of
the expression were not significantly reduced in those who had quit
smoking for more than 10 years compared with those who had quit
smoking for less than 5 years, suggesting that the activation of
these tumor antigens persists even after long-term smoking
cessation. This finding is not surprising, and supplements previous
observations that molecular alterations observed in the bronchial
epithelium of chronic smokers persist for a long time after the
cessation of smoking (30,31). This might be one of the reasons for
the fact that former smokers remain at high risk for developing
lung cancer (32). Future studies
with a quantitative RT-PCR approach will further elucidate the true
effect of smoking continuance or cessation on MAGE 1
expression.
Nevertheless, our findings do suggest that MAGE
1 antigens are ideal immunotherapeutic targets for lung cancer
chemoprevention studies, as they are not expressed in normal lung
tissue, but are present in a certain subset of heavy smokers,
possibly putting those smokers at a higher risk of lung
carcinogenesis than others who lack expression but have a
comparable smoking history. Although our study was not designed to
determine whether individuals with MAGE antigen expression
carry a high risk for lung cancer development, the oncogenic
properties of these antigens raise the possibility that the
expression of MAGE antigens may be a biomarker for lung
cancer risk. The peptides derived from these antigens may be
utilized for immunotherapeutic approaches to lung cancer
prevention, and the identification of individual MAGE genes
would allow for an individualized chemoprevention approach using
specific tumor antigens for each subject. However, our results also
indicate that the presence of markers is heterogeneous, and a
subject expressing one marker may or may not express another. In
approximately one-third of the cases, more than one of the subtypes
was expressed. The high frequency of coexpression of MAGE
antigens calls for a polyvalent vaccination approach. Use of
multiple immunogens is important, as it increases the probability
of inducing a specific immune response and reduces the risk of
tumor escape from the immune system by selection of antigen-loss
variants.
In conclusion, we demonstrated that MAGE
antigens are frequently expressed in lungs with chronic tobacco
exposure, even prior to overt malignant transformation. Since these
antigens have oncogenic properties, smokers who express these
antigens might be at higher risk of lung carcinogenesis, and must
be considered for a chemoprevention program. The expression of
MAGE antigens in noncancerous cells indicates that the
monitoring of these antigens may be of potential interest for
determining new immunotherapeutic agents, and warrants development
of widely applicable, polyvalent vaccines. Considering the
promising clinical results and the safety profile of MAGE
antigen vaccines, our findings suggest a possibility of applying
the vaccination strategy in a chemoprevention setting for heavy
smokers with positive MAGE antigen expression.
Acknowledgements
The work was supported by National
Cancer Institute grants CA091844, CA136635 and CA16672.
References
|
1.
|
Thurner B, Haendle I, Roder C, Dieckmann
D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von
den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E and
Schuler G: Vaccination with mage-3A1 peptide-pulsed mature,
monocyte-derived dendritic cells expands specific cytotoxic T cells
and induces regression of some metastases in advanced stage IV
melanoma. J Exp Med. 190:1669–1678. 1999. View Article : Google Scholar
|
|
2.
|
Godelaine D, Carrasco J, Lucas S,
Karanikas V, Schuler-Thurner B, Coulie PG, Schuler G, Boon T and
Van Pel A: Polyclonal CTL responses observed in melanoma patients
vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J
Immunol. 171:4893–4897. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Morse MA, Garst J, Osada T, Khan S,
Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A,
Hsu DH, Le Pecq JB and Lyerly HK: A phase I study of dexosome
immunotherapy in patients with advanced non-small cell lung cancer.
J Transl Med. 3:92005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chomez P, De Backer O, Bertrand M, De
Plaen E, Boon T and Lucas S: An overview of the MAGE gene family
with the identification of all human members of the family. Cancer
Res. 61:5544–5551. 2001.PubMed/NCBI
|
|
5.
|
Barker PA and Salehi A: The MAGE proteins:
emerging roles in cell cycle progression, apoptosis, and
neurogenetic disease. J Neurosci Res. 67:705–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Osterlund C, Tohonen V, Forslund KO and
Nordqvist K: Mage-b4, a novel melanoma antigen (MAGE) gene
specifically expressed during germ cell differentiation. Cancer
Res. 60:1054–1061. 2004.PubMed/NCBI
|
|
7.
|
Sakata M: Expression of MAGE gene family
in lung cancers. Kurume Med J. 43:55–61. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jang SJ, Soria JC, Wang L, Hassan KA,
Morice RC, Walsh GL, Hong WK and Mao L: Activation of melanoma
antigen tumor antigens occurs early in lung carcinogenesis. Cancer
Res. 61:7959–7963. 2001.PubMed/NCBI
|
|
9.
|
Atanackovic D, Altorki NK, Stockert E,
Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA,
Matsuo M, Selvakumar A, Dupont B, Chen YT, Hoffman EW, Ritter G,
Old LJ and Gnjatic S: Vaccine-induced CD4+ T cell
responses to MAGE-3 protein in lung cancer patients. J Immunol.
172:3289–3296. 2004.PubMed/NCBI
|
|
10.
|
Fischer C, Gudat F, Stulz P, Noppen C,
Schaefer C, Zajac P, Trutmann M, Kocher T, Zuber M, Harder F,
Heberer M and Spagnoli GC: High expression of MAGE-3 protein in
squamous-cell lung carcinoma. Int J Cancer. 71:1119–1121. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sienel W, Varwerk C, Linder A, Kaiser D,
Teschne M, Delire M, Stamatis G and Passlick B: Melanoma associated
antigen (MAGE)-A3 expression in stages I and II non-small cell lung
cancer: results of a multi-center study. Eur J Cardiothorac Surg.
25:131–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sugita M, Geraci M, Gao B, Powell RL,
Hirsch FR, Johnson G, Lapadat R, Gabrielson E, Bremnes R, Bunn PA
and Franklin WA: Combined use of oligonucleotide and tissue
microarrays identifies cancer/testis antigens as biomarkers in lung
carcinoma. Cancer Res. 62:3971–3979. 2002.PubMed/NCBI
|
|
13.
|
Tajima K, Obata Y, Tamaki H, Yoshida M,
Chen YT, Scanlan MJ, Old LJ, Kuwano H, Takahashi T, Takahashi T and
Mitsudomi T: Expression of cancer/testis (CT) antigens in lung
cancer. Lung Cancer. 42:23–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
De Plaen E, Arden K, Traversari C, et al:
Structure, chromosomal localization, and expression of 12 genes of
the MAGE family. Immunogenetics. 40:360–369. 1994.PubMed/NCBI
|
|
15.
|
Lurquin C, De Smet C, Brasseur F,
Muscatelli F, Martelange V, De Plaen E, Brasseur R, Monaco AP and
Boon T: Two members of the human MAGEB gene family located in
Xp21.3 are expressed in tumors of various histological origins.
Genomics. 46:397–408. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lucas S, De Smet C, Arden KC, Viars CS,
Lethe B, Lurquin C and Boon T: Identification of a new MAGE gene
with tumor-specific expression by representational difference
analysis. Cancer Res. 58:743–752. 1998.PubMed/NCBI
|
|
17.
|
Gillespie AM and Coleman RE: The potential
of melanoma antigen expression in cancer therapy. Cancer Treat Rev.
25:219–227. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Van den Eynde B, Peeters O, De Backer O,
Gaugler B, Lucas S and Boon T: A new family of genes coding for an
antigen recognized by autologous cytolytic T lymphocytes on a human
melanoma. J Exp Med. 182:689–698. 1995.
|
|
19.
|
Van Baren N, Brasseur F, Godelaine D,
Hames G, Ferrant A, Lehmann F, Andre M, Ravoet C, Doyen C, Spagnoli
GC, Bakkus M, Thielemans K and Boon T: Genes encoding
tumor-specific antigens are expressed in human myeloma cells.
Blood. 94:1156–1164. 1999.PubMed/NCBI
|
|
20.
|
Jungbluth AA, Busam KJ, Kolb D, Iversen K,
Coplan K, Chen YT, Spagnoli GC and Old LJ: Expression of
MAGE-antigens in normal tissues and cancer. Int J Cancer.
85:460–465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Peikert T, Specks U, Farver C, Erzurum SC
and Comhair SA: Melanoma antigen A4 is expressed in non-small cell
lung cancers and promotes apoptosis. Cancer Res. 66:4693–4700.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Laduron S, Deplus R, Zhou S, Kholmanskikh
O, Godelaine D, De Smet C, Hayward SD, Fuks F, Boon T and De Plaen
E: MAGE-A1 interacts with adaptor SKIP and the deacetylase HDAC1 to
repress transcription. Nucleic Acids Res. 32:4340–4350. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
De Smet C, De Backer O, Faraoni I, Lurquin
C, Brasseur F and Boon T: The activation of human gene MAGE-1 in
tumor cells is correlated with genome-wide demethylation. Proc Natl
Acad Sci USA. 93:7149–7153. 1996.PubMed/NCBI
|
|
25.
|
Gaudet F, Hodgson JG, Eden A,
Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H and Jaenisch R:
Induction of tumors in mice by genomic hypomethylation. Science.
300:489–492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Eden A, Gaudet F, Waghmare A and Jaenisch
R: Chromosomal instability and tumors promoted by DNA
hypomethylation. Science. 300:4552003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bollati V, Baccarelli A, Hou L, Bonzini M,
Fustinoni S, Cavallo D, Byun HM, Jiang J, Marinelli B, Pesatori AC,
Bertazzi PA and Yang AS: Changes in DNA methylation patterns in
subjects exposed to low-dose benzene. Cancer Res. 67:876–880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wang L, Wang J, Sun S, Rodriguez M, Yue P,
Jang SJ and Mao L: A novel DNMT3B subfamily, ΔDNMT3B, is the
predominant form of DNMT3B in non-small cell lung cancer. Int J
Oncol. 29:201–207. 2006.
|
|
29.
|
Valinluck V, Tsai HH, Rogstad DK, Burdzy
A, Bird A and Sowers LC: Oxidative damage to methyl-CpG sequences
inhibits the binding of the methyl-CpG binding domain (MBD) of
methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res.
32:4100–4108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mao L, Lee JS, Kurie JM, Fan YH, Lippman
SM, Lee JJ, Ro JY, Broxson A, Yu R, Morice RC, Kemp BL, Khuri FR,
Walsh GL, Hittelman WN and Hong WK: Clonal genetic alterations in
the lungs of current and former smokers. J Natl Cancer Inst.
89:857–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wistuba II, Mao L and Gazdar AF: Smoking
molecular damage in bronchial epithelium. Oncogene. 21:7298–7306.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Peto R, Darby S, Deo H, Silcocks P,
Whitley E and Doll R: Smoking, smoking cessation, and lung cancer
in the UK since 1950: combination of national statistics with two
case-control studies. BMJ. 321:323–329. 2000. View Article : Google Scholar : PubMed/NCBI
|