Introduction
Recent advances in cancer therapy have significantly
improved patient prognosis (1).
However, a number of gastrointestinal malignancies, including
pancreatic and esophageal cancer, remain difficult to treat and are
often fatal (2,3). The development of a novel therapeutic
approach against these intractable cancers is therefore urgently
required. Molecular-targeted therapy may provide a breakthrough in
cancer treatment. Although such novel approaches have improved the
clinical outcome in several types of cancer, a definitive target
for pancreatic and esophageal cancer has yet to be identified.
Tumor necrosis factor-like weak inducer of apoptosis
(TWEAK, also known as CD255, TNFSF12 and APO3L) is a member of the
TNF superfamily that was originally identified in 1997. It has been
suggested that it plays a pivotal role in various inflammatory
conditions due to its proinflammatory properties (4,5).
Fibroblast growth factor-inducible 14 (Fn14, also known as CD266,
TWEAKR and TNFRSF12A) was identified in 2001 as a TWEAK receptor
that is linked to several intracellular signaling pathways
(5–7). Previous studies have shown that
TWEAK-Fn14 interaction promotes cell proliferation, migration,
differentiation, apoptosis and angiogenesis as well as inflammation
in various cell types (8,9). Furthermore, it has been revealed that
this pathway play a key role in a variety of diseases, including
rheumatoid arthritis, multiple sclerosis and systemic lupus
erythematosus (10–13). In addition, TWEAK and Fn14 may play
roles in certain malignant tumors. Previous studies have
demonstrated TWEAK expression in human malignancies in various
organs, including the brain, lung, large intestine, liver and
breast (4,14,15).
Fn14 expression has also been found in tumors in a variety of
organs, including the liver, breast, brain, lung, pancreas and
large intestine (16–19). Furthermore, a number of functional
studies have suggested that TWEAK and/or Fn14 mediate tumor cell
apoptosis, proliferation, migration, angiogenesis and survival
(20,21). Therefore, it has been proposed that
TWEAK and Fn14 are potential targets for cancer therapy (5,19,22).
However, this strategy requires careful evaluation using in
vitro and in vivo studies since TWEAK/Fn14 appears to
exert diverse biological activities in humans (5,23,24).
In this study, we evaluated both TWEAK and Fn14 protein expression
in actual human esophageal and pancreatic cancer tissues. We
further examined the therapeutic potential of targeting the
TWEAK/Fn14 pathway against gastrointestinal cancer in vitro
and in vivo.
Materials and methods
Patients
A total of 43 patients with esophageal cancer and 51
with pancreatic cancer who underwent surgery at the Department of
Surgery, Nara Medical University, were examined between 1995 and
2004. The esophageal cancers evaluated in this study were
pathologically diagnosed as squamous cell carcinoma (SCC). The
pancreatic cancers were diagnosed as invasive ductal carcinoma. For
immunohistochemistry, each specimen was fixed in 10%
phosphate-buffered formalin and embedded in paraffin. A serial
section from each specimen was stained with H&E for
histological evaluation. The tumors were classified according to
the TNM staging system. Written informed consent was obtained from
the patients prior to the study according to our institutional
guidelines.
Cell lines
Four human esophageal cancer cell lines, TE3 (highly
differentiated squamous carcinoma), TE7 (adenocarcinoma), TE8
(moderately differentiated squamous carcinoma) and TE13 (poorly
differentiated squamous carcinoma), and four human pancreatic
cancer cell lines (CAPAN2, PK-8, PANC-1 and MIAPaCa-2) were
obtained from the Cell Resource Center for Biomedical Research
Institute of Development, Aging and Cancer, Tohoku University. A
murine colorectal cancer cell line (colon-26) was obtained from the
Riken Cell Bank (Ibaragi, Japan). The cell lines were maintained in
Dulbecco's modified Eagle's medium/F12 medium with 10%
heat-inactivated fetal bovine serum (FBS) at 37°C in a humidified
atmosphere of 5% CO2.
Reagents
For the in vitro and in vivo studies,
anti-TWEAK blocking monoclonal antibodies (mAb) (CARL-1; as
anti-human and MTW-1; as anti-murine) and anti-Fn14 blocking mAb
(ITEM-2; as both anti-human and anti-murine) were prepared as
previously described (9,25,26).
Immunohistochemistry
The sections were stained using a Dako EnVision
system (Dako Cytomation, Kyoto, Japan), according to the manual
provided by the manufacturer. As primary antibodies, rabbit
polyclonal anti-TWEAK (FL-249, 1:100 dilution; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) and mouse monoclonal
anti-Fn14 (Clone ITEM-4, 1:100 dilution; eBioscience, San Diego,
CA, USA) were employed. After neutralization of endogenous
peroxidase, sections were incubated for 90 min with primary
antibodies. After three washes in PBS, the sections were incubated
for 1 h with polymeric conjugate washed three times with
phosphate-buffered saline (PBS). The reaction products were
visualized with 3,3′-diaminobenzidine tetrahydrochloride and the
slides were counterstained with hematoxylin.
RT-PCR analysis
Total RNA was isolated from the cell lines using
guanidine isothiocyanate methods. RNA was transcribed to cDNA using
Omniscript Reverse Transcriptase kit (Qiagen, Hilden, Germany) and
oligo dT primers (Amersham Biosciences, Piscataway, NJ, USA). The
RNA was heated for 5 min at 65°C and 4°C cooled on ice. Reaction
buffer 10X (2 μl), 1 μl of RNase inhibitor (10
U/μl), 2 μl of a mixture of dNTPs, 1 μl of
oligodT primers (10 μM), 1 μl of Omniscript Reverse
Transcriptase (4 U/μl) and RNase-free water were added for a
total volume of 20 μl. The mixture was incubated for 60 min
at 37°C. After incubation, the cDNA was stored at −80°C before
analysis. The cDNA solution (0.5 μl) was amplified in a
total volume of 20 μl that contained 2 μl of 10X PCR
buffer, 0.6 μl of MgCl2 (50 mM), 0.2 μl of
Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA, USA),
sense primer (50 μM), antisense primer (50 μM) and 2
μl of a mixture of dNTPs (Takara, Ohtsu, Japan). The
amplification involved an initial step of denaturation at 94°C for
3 min, followed by cycles consisting of denaturation at 94°C for 1
min, annealing at 63°C for 1 min, and chain elongation at 72°C for
1 min. The sequences of sense and antisense primers, and the
product size for TWEAK, Fn14 and β-actin were: TWEAK, sense
5′-CCCTGCGCTGCCTGGAGGAA-3′ and antisense 5′-AGA
CCAGGGCCCCTCAGTGA-3′ (amplicon size 200 bp); Fn14, sense
5′-CCAAGCTCCTCCAACCACAA-3′ and antisense 5′-TGGGGCCTAGTGTCAAGTCT-3′
(amplicon size 242 bp); β-actin, sense 5′-ATCAAGATCCTGACCGAGCG-3′
and anti-sense 5′-TACTTGCGCTCAGGAGGAGC-3′. The amplified products
were analyzed by electrophoresis of the PCR product on a 2% agarose
gel plus ethidium bromide in TBE buffer.
Cell growth inhibition assay
Human cancer cells were added at a concentration of
1×103 cells in 100 μl to the individual wells of
96-well flat-bottomed microtiter plates. Then, 25 μg/ml of
anti-human TWEAK mAb (CARL-1), anti-Fn14 mAb (ITEM-2) and control
IgG were added to each well every other day and were further
incubated at 37°C in a humidified atmosphere of 5% CO2.
After 3 or 7 days, the cells were rinsed with PBS, fixed with 5%
glutaraldehyde (Nacalai Tesque, Kyoto, Japan) in PBS and then
stained with 0.2% crystal violet (Wako, Osaka, Japan) and 100 mM
CAPS (Wako). The absorbance of each well was measured at 540 nm
with Multiskan MS (Labsystems, Helsinki, Finland). The growth
inhibition rate was calculated using the following formula: % of
growth inhibition rate = absorbance of mAb-treated cells -
spontaneous absorbance/absorbance of control cells - spontaneous
absorbance. The samples were examined in triplicate.
Animal study
Female BALB/c mice (8 weeks old) were obtained from
Clea Japan (Tokyo, Japan). The mice were maintained under specific
pathogen-free conditions in the animal facility at Nara Medical
University. The experiments were conducted under a protocol
approved by our institutional review board. Cells (1×106
murine colorectal adenocarcinoma colon-26) were subcutaneously
inoculated on one side of the ventral surface in the lower flank
region of syngeneic BALB/c mice. Treatment was started 7 days later
when a small palpable lump was evident, ranging from 4 to 6 mm in
diameter. Some mice were intraperitoneally injected with 0.3 mg of
each mAb every other day for 3 weeks. The control mice received
control IgG. The tumor size was determined by caliper measurements.
The tumor volume was calculated according to the formula: V = A ×
B2/2 (mm3), where A is the largest diameter
(mm) and B is the smallest diameter (mm).
Statistical analysis
The experimental data of the growth inhibition assay
were analyzed by the Student's t-test. Differences of P<0.05
were considered to indicate statistical significance.
Results
TWEAK and Fn14 expression in human
esophageal and pancreatic cancer
TWEAK and Fn14 protein expression in actual human
esophageal cancer and pancreatic cancer tissues was examined by
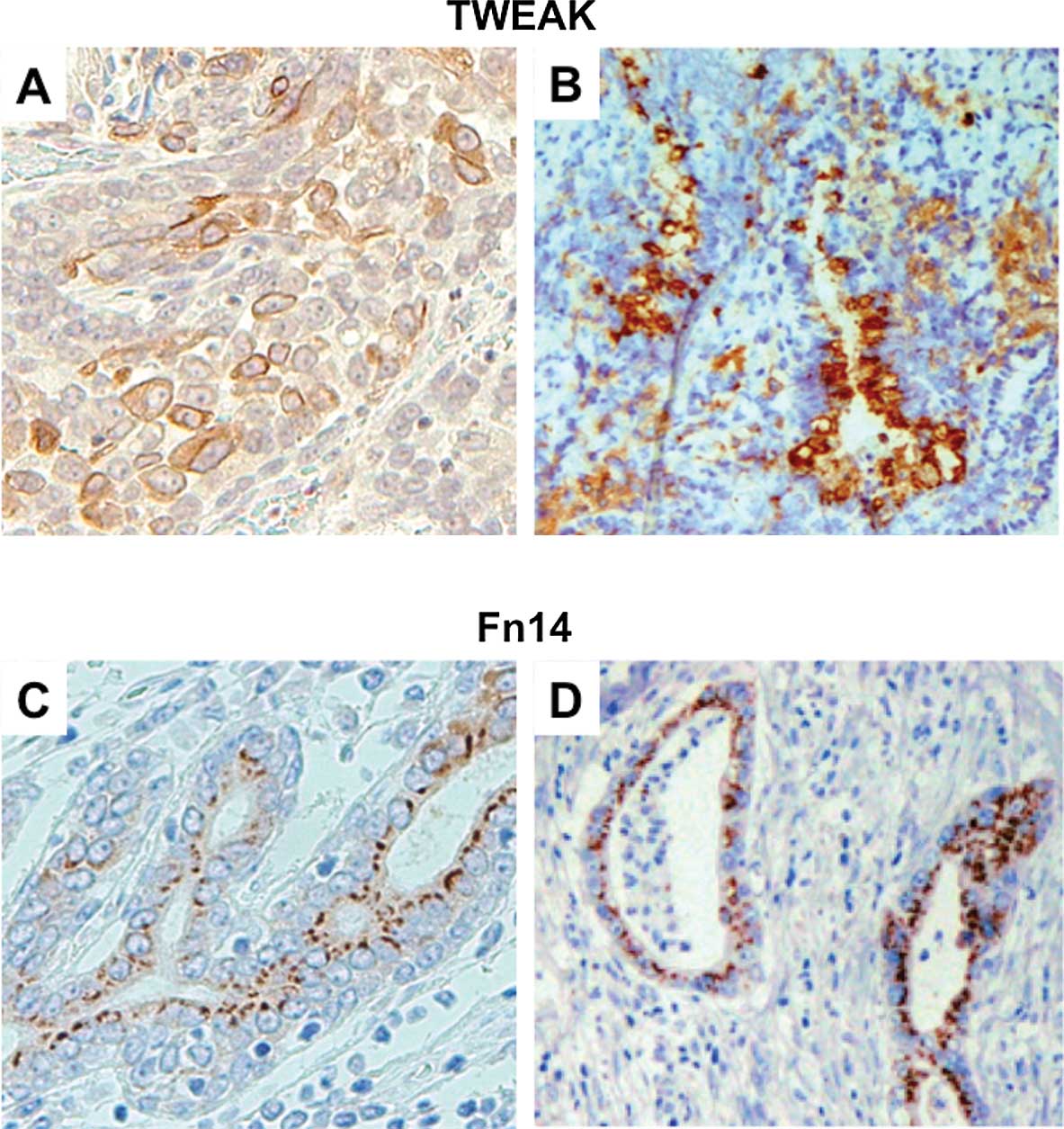
immunohistochemical staining. In both cancer tissues, TWEAK
expression was identified in the cytoplasm and membrane of cancer
cells, and Fn14 was observed mainly in the tumor cellular membrane
(Fig. 1). In esophageal cancer,
20/43 cases (46.5%) stained positively for TWEAK and 10 cases
(23.2%) were positive for Fn14. Overall, either TWEAK or Fn14
expression was observed in 25 cases (58.1%) (Table I). In pancreatic cancer, 18/51
(35.3%) stained positively for TWEAK and 35 (68.6%) were positive
for Fn14. Furthermore, 15 (29.4%) cases showed positive staining
for both TWEAK and Fn14. Overall, either TWEAK or Fn14 expression
was observed in 38 cases (74.5%) (Table II).
 | Table I.Correlation between TWEAK and Fn14
expression in esophageal cancer. |
Table I.
Correlation between TWEAK and Fn14
expression in esophageal cancer.
| TWEAK
|
|---|
| Positive (%) | Negative (%) | Total (%) |
|---|
| Fn14 | | | |
| Positive | 5 (11.6) | 5 (11.6) | 10 (23.2) |
| Negative | 15 (34.9) | 18 (41.9) | 33 (76.8) |
| Total | 20 (46.5) | 23 (53.5) | 43 (100.0) |
 | Table II.Correlation between TWEAK and Fn14
expression in pancreatic cancer. |
Table II.
Correlation between TWEAK and Fn14
expression in pancreatic cancer.
| TWEAK
|
|---|
| Positive (%) | Negative (%) | Total (%) |
|---|
| Fn14 | | | |
| Positive | 15 (29.4) | 20 (39.2) | 35 (68.6) |
| Negative | 3 (5.9) | 13 (25.5) | 16 (31.4) |
| Total | 18 (35.3) | 33 (64.7) | 51 (100.0) |
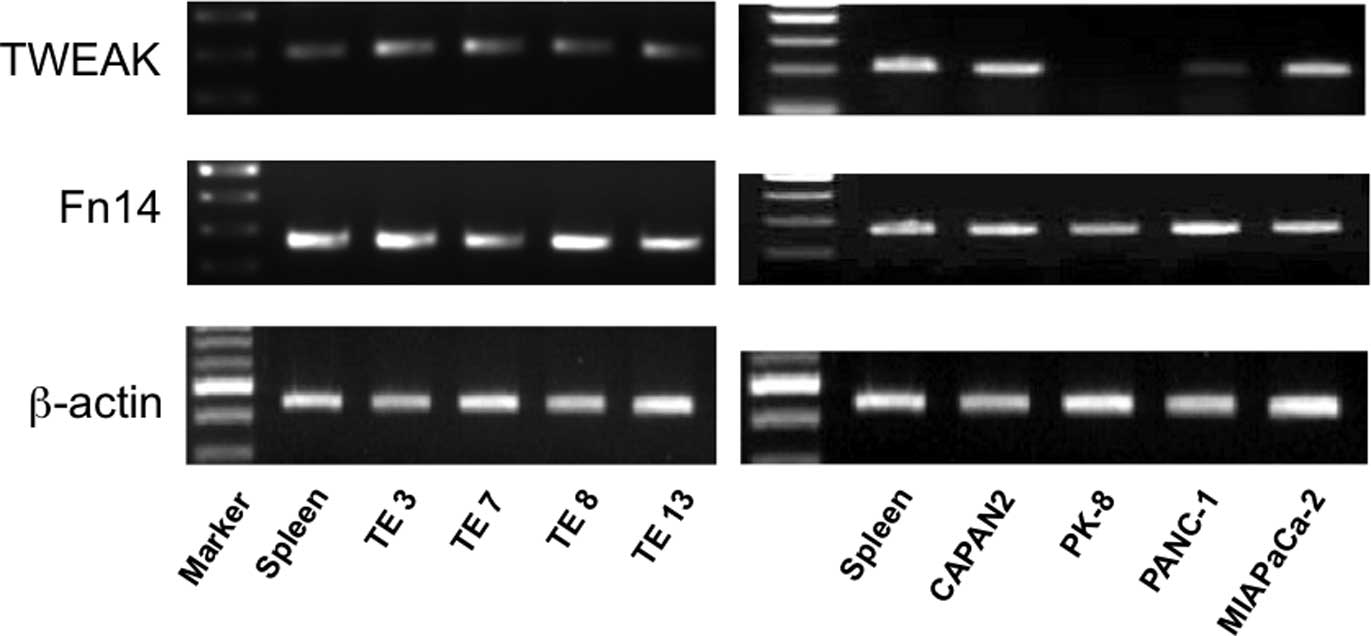
TWEAK and Fn14 mRNA expression in human
esophageal and pancreatic cancer cell lines
The gene expression of TWEAK and Fn14 was examined
in four cell lines of each cancer type. As a result, RT-PCR
analysis indicated that both TWEAK and Fn14 were detected in all
tested esophageal cell lines. In human pancreatic cancer cell
lines, TWEAK was expressed in 3/4 cell lines (CAPAN2, PANC-1 and
MIAPaCa-2), and Fn14 was observed in all the cell lines (Fig. 2).
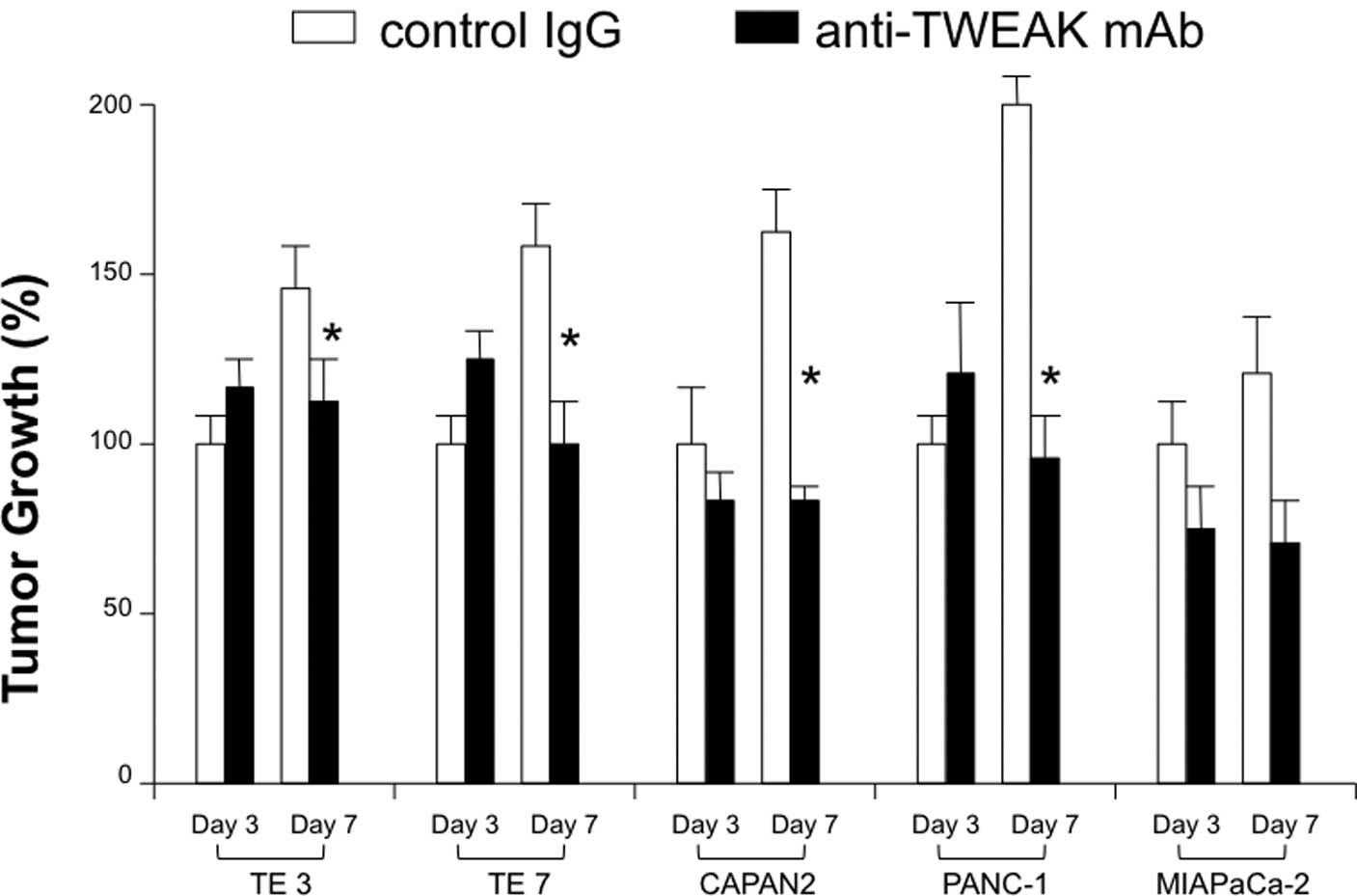
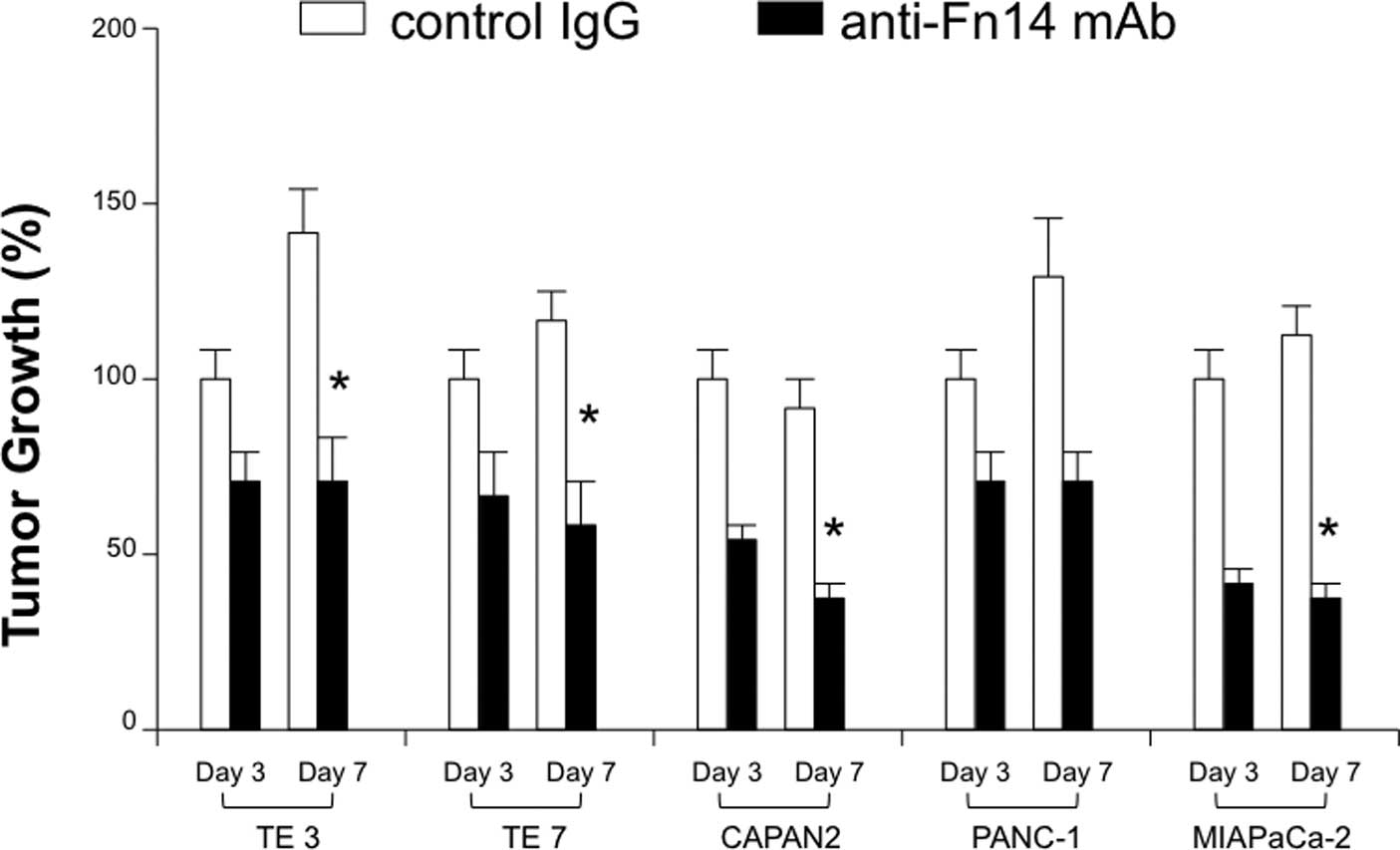
Blockade of the TWEAK/Fn14 pathway
inhibits gastrointestinal tumor growth
For the clinical application of targeting TWEA/Fn14
pathway, the therapeutic efficacy of its blockade on
gastrointestinal cancer cell growth was evaluated using a number of
TWEAK- or Fn14-expressing human cancer cell lines. After co-culture
with mAb, TWEAK blockade had an inhibitory effect on both
esophageal and pancreatic cancer growth (Fig. 3). The reduction rates of cell
growth on the 7th day were: TE3, 22%; TE7, 38%; CAPAN2, 53%;
PANC-1, 47%; and MIAPaCa-2, 47%. Furthermore, the Fn14 blockade
exerted a similar effect on the cell growth of both types of cancer
(reduction rate: TE3, 52%; TE7, 50%; CAPAN2, 55%; PANC-1, 49% and
MIAPaCa-2: 65%) (Fig. 4).
TWEAK/Fn14 blockade inhibits
gastrointestinal cancer growth in vivo
In order to confirm the therapeutic efficacy of
targeting TWEAK/Fn14 in gastrointestinal cancer, a physiological
in vivo model was employed. To this end, using the murine
colorectal adenocarcinoma colon-26 cell line, the efficacy of
TWEAK/Fn14 blockade in wild-type BALB/c mice was evaluated. The
treatment of anti-TWEAK or -Fn14 blocking mAb significantly
inhibited tumor growth in the treated mice (Fig. 5). Thus, the beneficial effect of
either TWEAK or Fn14 blockade on gastrointestinal cancer was
confirmed even in immunocompetent mice.
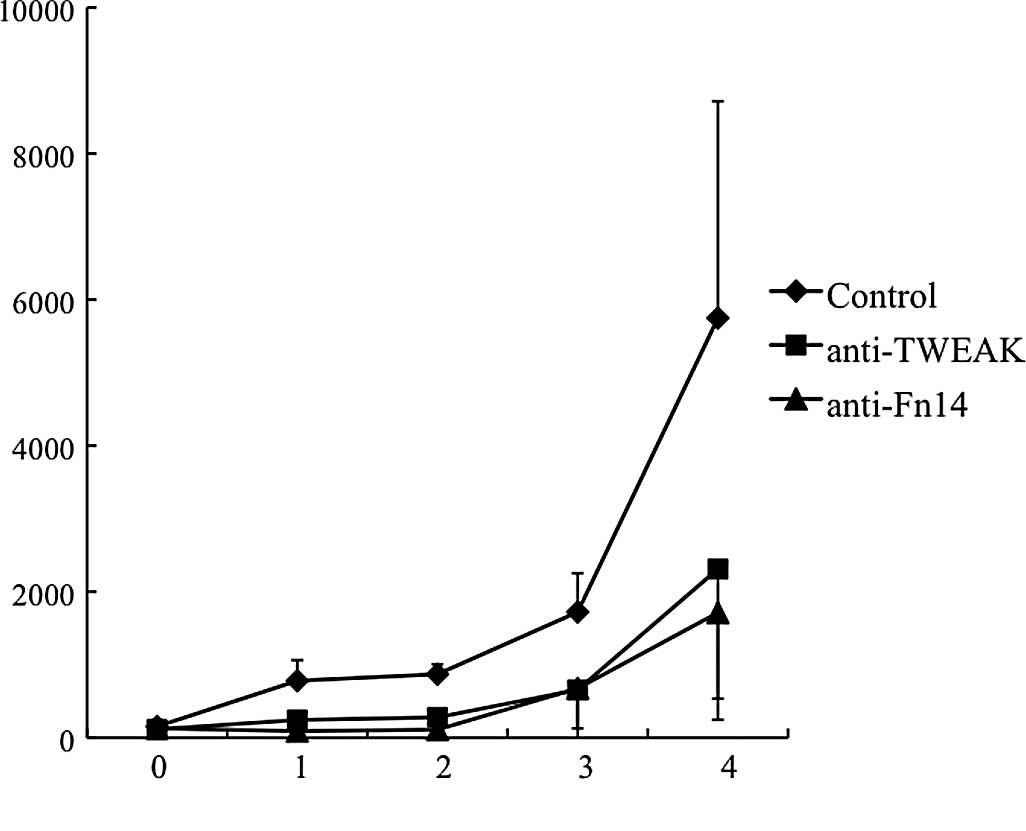 | Figure 5.Therapeutic efficacy of targeting
TWEAK/Fn14 in gastrointestinal cancer. The effect of TWEAK/Fn14
blockade on gastrointestinal cancer was examined in vivo.
Colon-26 was implanted in the flank of the syngeneic BALB/c mice.
The mice were treated with 0.3 mg of each mAb every other day for 3
weeks. The control mice received control IgG. The mean tumor volume
at 1, 2, 3 and 4 weeks after starting treatment was; anti-TWEAK
mAb, 243, 279, 656 and 2,311 mm3, respectively;
anti-Fn14 mAb, 91, 111, 676 and 1,713 mm3, respectively;
control, 781, 870, 1,722 and 5,747 mm3, respectively.
n=6 for each group. Data points, mean tumor size; bars, SD. Either
TWEAK or Fn14 blockade inhibited tumor growth compared to the
controls. The statistical differences were; TWEAK blockade, 0.005,
<0.0001, 0.02 and 0.07 at 1, 2, 3 and 4 weeks, respectively,
compared to the control; Fn14 blockade, 0.0007, <0.0001, 0.06
and 0.02, respectively, compared to the control. |
Discussion
TWEAK is a multifunctional cytokine that controls
various cellular activities. TWEAK functions by binding the
cell-surface receptor, Fn14. Recent cumulative evidence has
revealed that the TWEAK/Fn14 pathway regulates a variety of
physiological and pathological conditions (5). Although it has been suggested that
this pathway is involved in tumor development and progression, the
therapeutic potential of targeting TWEAK/Fn14 has yet to be
elucidated (14-16,19,22,27).
In this study, we evaluated the clinical usefulness of
TWEAK/Fn14-targeted therapy in esophageal and pancreatic cancer in
representative intractable gastrointestinal tumors.
First, we examined the protein expression of
TWEAK/Fn14 in actual human cancer tissues. We confirmed this
expression in approximately 60% of esophageal and 75% of pancreatic
cancer cases. Although TWEAK protein expression has not been
previously evaluated in esophageal and pancreatic cancer, it was
recently reported that Fn14 expression is positive in 27% of
esophageal cancer and 60% of pancreatic cancer cases (19). In our study, it was positive in 23
and 68%, respectively. These data appear similar, even though there
are racial and tumor histological differences. Furthermore, most of
the esophageal and pancreatic cancer cell lines examined in this
study demonstrated both TWEAK and Fn14 expression. Notably, Fn14
mRNA expression was recently reported as one of the best markers
for the early diagnosis of esophageal cancer (28). The TWEAk/Fn14 pathway is therefore
a promising novel target for intractable gastrointestinal
cancer.
Next, we investigated the therapeutic potential of
targeting the TWEAK/Fn14 pathway in gastrointestinal cancer both
in vitro and in vivo. We found that TWEAK and Fn14
blockade had a substantial effect on the inhibition of
gastrointestinal cancer. Our in vitro experiments clearly
demonstrated that TWEAK and Fn14 blockade significantly inhibited
cancer cell growth. Thus, data indicate that both molecules are
directly involved in the tumor proliferation of esophageal and
pancreatic cancer. Furthermore, in vivo data evaluated in
physiological conditions also demonstrate that TWEAK and/or Fn14
are functional and may be potential targets for a novel anti-cancer
therapy. However, due to the diverse functions of TWEAK/Fn14, there
may be a number of caveats for clinical application as a
molecular-targeted therapy. Tumor cell apoptosis is essential for
the induction of therapeutic efficacy in a variety of cancer
treatments. TWEAK/Fn14 has been found to be a critical regulator to
promote the apoptosis of certain cells under various circumstances
(4,5,29).
Therefore, its blockade may result in the promotion of resistance
to treatment-related apoptosis of tumor cells. Furthermore, recent
studies have suggested that TWEAK/Fn14 plays a critical role in
tissue repair and regeneration (18,30–32).
Therefore, inhibition of this pathway may have detrimental effects
or be harmful for cancer patients.
On the other hand, accumulating evidence suggests
the therapeutic potential of TWEAK and/or Fn14 blockade as a novel
anti-cancer therapy. Soluble TWEAK has previously been shown to
promote tumor growth and angiogenesis (21). In addition, Fn14 overexpression has
been demonstrated to induce tumor cell migration (20,22).
Furthermore, it has been shown that tumor invasion was
significantly inhibited by Fn14 blockade using the RNA interference
method (16,31). More recently, newly developed
antibodies for targeting Fn14 have been demonstrated to inhibit
tumor growth through direct inhibition and antibody-dependent
cellular cytotoxicity (19). Our
in vitro and in vivo therapeutic data further support
a potential of blocking the TWEAK/Fn14 pathway in cancer treatment.
A number of potential underlying mechanisms are involved in
anti-TWEAK and anti-Fn14 mAb treatment. These include direct
inhibition of proliferation, migration, apoptosis, angiogenesis and
survival of tumor cells (5,22).
Also, antibody-dependent mechanisms may play a critical part in
vivo, in clinically relevant physiological conditions (19). Further studies are required to
clarify the possible mechanisms of the antitumor effect induced by
targeting TWEAK/Fn14 in intractable gastrointestinal cancer.
It is crucial to evaluate the combination therapy of
targeting TWEAK and Fn14 with conventional antitumor treatments,
including chemotherapy and radiotherapy, since chemotherapy and
radiotherapy influence the multifunction of TWEAK and Fn14, leading
to either a synergistic or detrimental antitumor effect.
Particularly in intractable gastrointestinal tumors including
esophageal and pancreatic cancer, multimodal treatment appears to
be essential for improving patient prognosis. Therefore, further
research is warranted to evaluate the combination therapy with
current standard chemotherapy, since new molecular-targeted
therapies have been reported to enhance the clinical outcome of
conventional chemotherapy even in intractable cancer (33). To date, no studies address these
issues in regard to the TWEAK/Fn14 pathway.
In conclusion, we demonstrated TWEAK/Fn14 expression
in human esophageal and pancreatic cancers representative of
intractable gastrointestinal malignancies. Our data indicate that
the TWEAK/Fn14 pathway is a novel molecular target for anti-cancer
therapy and may provide the rationale for developing this novel
strategy against such diseases.
Acknowledgements
This study was supported by the
following grants: Grants-in-Aid for Scientific Research from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan, nos. 19591491 and 21591648; Research Grant from the Pancreas
Research Foundation of Japan; Research Grant from the Foundation
for Promotion of Cancer Research in Japan; Research Grant from the
Daiwa Securities Health Foundation; and Research Grant from the
Nakayama Cancer Research Institute (to M. Sho).
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
3.
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
4.
|
Chicheportiche Y, Bourdon PR, Xu H, Hsu
YM, Scott H, Hession C, Garcia I and Browning JL: TWEAK, a new
secreted ligand in the tumor necrosis factor family that weakly
induces apoptosis. J Biol Chem. 272:32401–32410. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Winkles JA: The TWEAK-Fn14
cytokine-receptor axis: discovery, biology and therapeutic
targeting. Nat Rev Drug Discov. 7:411–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wiley SR, Cassiano L, Lofton T,
Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA
and Fanslow WC: A novel TNF receptor family member binds TWEAK and
is implicated in angiogenesis. Immunity. 15:837–846. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kumar M, Makonchuk DY, Li H, Mittal A and
Kumar A: TNF-like weak inducer of apoptosis (TWEAK) activates
proinflammatory signaling pathways and gene expression through the
activation of TGF-beta-activated kinase 1. J Immunol.
182:2439–2448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Polek TC, Talpaz M, Darnay BG and
Spivak-Kroizman T: TWEAK mediates signal transduction and
differentiation of RAW264.7 cells in the absence of Fn14/TweakR.
Evidence for a second TWEAK receptor. J Biol Chem. 278:32317–32323.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Harada N, Nakayama M, Nakano H, Fukuchi Y,
Yagita H and Okumura K: Pro-inflammatory effect of TWEAK/Fn14
interaction on human umbilical vein endothelial cells. Biochem
Biophys Res Commun. 299:488–493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhao Z, Burkly LC, Campbell S, Schwartz N,
Molano A, Choudhury A, Eisenberg RA, Michaelson JS and Putterman C:
TWEAK/Fn14 interactions are instrumental in the pathogenesis of
nephritis in the chronic graft-versus-host model of systemic lupus
erythematosus. J Immunol. 179:7949–7958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Desplat-Jego S, Creidy R, Varriale S,
Allaire N, Luo Y, Bernard D, Hahm K, Burkly L and Boucraut J:
Anti-TWEAK monoclonal antibodies reduce immune cell infiltration in
the central nervous system and severity of experimental auto-immune
encephalomyelitis. Clin Immunol. 117:15–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Perper SJ, Browning B, Burkly LC, et al:
TWEAK is a novel arthritogenic mediator. J Immunol. 177:2610–2620.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kamata K, Kamijo S, Nakajima A, Koyanagi
A, Kurosawa H, Yagita H and Okumura K: Involvement of TNF-like weak
inducer of apoptosis in the pathogenesis of collagen-induced
arthritis. J Immunol. 177:6433–6439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kawakita T, Shiraki K, Yamanaka Y,
Yamaguchi Y, Saitou Y, Enokimura N, Yamamoto N, Okano H, Sugimoto
K, Murata K and Nakano T: Functional expression of TWEAK in human
colonic adenocarcinoma cells. Int J Oncol. 26:87–93.
2005.PubMed/NCBI
|
|
15.
|
Kawakita T, Shiraki K, Yamanaka Y,
Yamaguchi Y, Saitou Y, Enokimura N, Yamamoto N, Okano H, Sugimoto
K, Murata K and Nakano T: Functional expression of TWEAK in human
hepatocellular carcinoma: possible implication in cell
proliferation and tumor angiogenesis. Biochem Biophys Res Commun.
318:726–733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tran NL, McDonough WS, Savitch BA, et al:
Increased fibroblast growth factor-inducible 14 expression levels
promote glioma cell invasion via Rac1 and nuclear factor-kappaB and
correlate with poor patient outcome. Cancer Res. 66:9535–9542.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Han H, Bearss DJ, Browne LW, Calaluce R,
Nagle RB and von Hoff DD: Identification of differentially
expressed genes in pancreatic cancer cells using cDNA microarray.
Cancer Res. 62:2890–2896. 2002.PubMed/NCBI
|
|
18.
|
Feng SL, Guo Y, Factor VM, Thorgeirsson
SS, Bell DW, Testa JR, Peifley KA and Winkles JA: The Fn14
immediate-early response gene is induced during liver regeneration
and highly expressed in both human and murine hepatocellular
carcinomas. Am J Pathol. 156:1253–1261. 2000. View Article : Google Scholar
|
|
19.
|
Culp PA, Choi D, Zhang Y, et al:
Antibodies to TWEAK receptor inhibit human tumor growth through
dual mechanisms. Clin Cancer Res. 16:497–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tran NL, McDonough WS, Savitch BA, Sawyer
TF, Winkles JA and Berens ME: The tumor necrosis factor-like weak
inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14
(Fn14) signaling system regulates glioma cell survival via NFkappaB
pathway activation and BCL-XL/BCL-W expression. J Biol Chem.
280:3483–3492. 2005. View Article : Google Scholar
|
|
21.
|
Ho DH, Vu H, Brown SA, Donohue PJ, Hanscom
HN and Winkles JA: Soluble tumor necrosis factor-like weak inducer
of apoptosis overexpression in HEK293 cells promotes tumor growth
and angiogenesis in athymic nude mice. Cancer Res. 64:8968–8972.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Winkles JA, Tran NL and Berens ME: TWEAK
and Fn14: new molecular targets for cancer therapy? Cancer Lett.
235:11–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Polavarapu R, Gongora MC, Winkles JA and
Yepes M: Tumor necrosis factor-like weak inducer of apoptosis
increases the permeability of the neurovascular unit through
nuclear factor-kappa B pathway activation. J Neurosci.
25:10094–10100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Potrovita I, Zhang W, Burkly L, Hahm K,
Lincecum J, Wang MZ, Maurer MH, Rossner M, Schneider A and
Schwaninger M: Tumor necrosis factor-like weak inducer of
apoptosis-induced neurodegeneration. J Neurosci. 24:8237–8244.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Nakayama M, Harada N, Okumura K and Yagita
H: Characterization of murine TWEAK and its receptor (Fn14) by
monoclonal antibodies. Biochem Biophys Res Commun. 306:819–825.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nakayama M, Ishidoh K, Kojima Y, Harada N,
Kominami E, Okumura K and Yagita H: Fibroblast growth
factor-inducible 14 mediates multiple pathways of TWEAK-induced
cell death. J Immunol. 170:341–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Han S, Yoon K, Lee K, Kim K, Jang H, Lee
NK, Hwang K and Young Lee S: TNF-related weak inducer of apoptosis
receptor, a TNF receptor superfamily member, activates NF-kappa B
through TNF receptor-associated factors. Biochem Biophys Res
Commun. 305:789–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Watts GS, Tran NL, Berens ME,
Bhattacharyya AK, Nelson MA, Montgomery EA and Sampliner RE:
Identification of Fn14/TWEAK receptor as a potential therapeutic
target in esophageal adenocarcinoma. Int J Cancer. 121:2132–2139.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wang D, Fung JN, Tuo Y, Hu L and Chen C:
TWEAK/Fn14 promotes apoptosis of human endometrial cancer cells via
caspase pathway. Cancer Lett. 294:91–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Jakubowski A, Ambrose C, Parr M, Lincecum
JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang
B, Bissell DM and Burkly LC: TWEAK induces liver progenitor cell
proliferation. J Clin Invest. 115:2330–2340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Tanabe K, Bonilla I, Winkles JA and
Strittmatter SM: Fibroblast growth factor-inducible-14 is induced
in axotomized neurons and promotes neurite outgrowth. J Neurosci.
23:9675–9686. 2003.PubMed/NCBI
|
|
32.
|
Girgenrath M, Weng S, Kostek CA, et al:
TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal
progenitor cells and skeletal muscle regeneration. EMBO J.
25:5826–5839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|