Introduction
Hypertension is one of the most prevalent diseases
in humans who live a modern life style. It is a silent disease and
does not markedly affect quality of life when it is moderate and
not serious. However, when left untreated it leads to
life-threatening diseases associated with atherosclerosis,
including myocardial infarction, renal failure and strokes
(1–3). In Japan, where general medical care
should be sufficient to treat hypertension, more than 300,000
patients per year die from diseases that are related to
hypertension (4,5). Clarification of the genetic nature
and more effective care for hypertension are required.
There are a number of methods for investigating the
genetic nature of hypertension. The polygenic nature of human
essential hypertension has made it challenging to isolate the genes
involved in the genesis of the disease. DNA microarrays are a
potentially powerful tool for studying the genetics of
hypertension, as they facilitate the measurement of the expression
of thousands of genes simultaneously (6–8).
Inbred homozygous rodent models of human essential hypertension are
ideal for microarray research, and animal models of essential
hypertension have been studied using microarrays (9).
In this study, we present a comparison of kidney
gene expression in three hypertensive rat models: spontaneously
hypertensive rats (SHRs) (10) and
two substrains derived from SHR – stroke-prone SHRs (SHRSPs)
(11) and malignant-type SHRSPs
(M-SHRSPs) (12). SHR, the current
model for essential hypertension research, were developed in a
breeding program based solely on the selection of elevated blood
pressure (BP) in Wistar Kyoto (WKY) rats (10). Normotensive WKY rats, from which
the SHR strain was derived, were used as controls. SHRSPs were
established from SHRs by selective inbreeding for stroke proneness
(11), and M-SHRSPs were selected
and established through the brother-sister mating of selected
SHRSPs that showed higher BP following treatment with hydralazine
hydrochloride to prevent the development of hypertension and stroke
(12). An inbred strain of
M-SHRSPs shows BPs of 250 mmHg or higher before 14 weeks of age
and, compared to SHRSPs, M-SHRSPs showed more rapid and severe
increases in BP and stroke in almost all animals. Our facility is
one of the original places that bred the three types of SHR
substrains and families. The former director, Professor Kozo
Okamoto, introduced these animals to our school when relocating
from the Kyoto University School of Medicine, the original site of
SHR breeding. Using these three SHR substrains, we previously
reported (13) that a number of
BP-regulating genes, including sparc/osteonectin (Spock2),
kynureninase (Kynu), regulator of G-protein signaling 2
(Rgs2) and gap junction protein α1 (Gja1) were
identified as up-regulated, and urotensin 2 (Uts2),
cytoplasmic epoxide hydrolase 2 (Ephx2), apelin
(Apln), insulin-like growth factor 1 receptor (Igf1r)
and angiotensin II receptor-associated protein (Agtrap) were
identified as down-regulated in the adrenal glands at 6 weeks of
age.
Kidneys are logical candidate organs for studying
hypertension due to their direct influence on body fluids and
endocrine, cardiovascular and sympathetic functions (14,15).
The relationship between kidney function and blood pressure is
known to be influenced by numerous intrinsic and extrinsic factors,
such as the renin-angiotensin system and catecholamine and
aldosterone hormones (16,17). Previously, Sty1 (18), Edg1, Vcam1 (19), C1q, CD24 (20), SPON1 (21), Gstm1 (22), ACE-2 (23), AMPK, APLP2 (24), Ephx2, Ela1 (25) and Egln1 (26) were shown to be hypertension-related
genes in SHR or SHRSP kidneys using a DNA microarray. In contrast
to the reports, this study is the first attempt to compare gene
expression profiles in the kidneys of three SHR substrains, SHR,
SHRSP and M-SHRSP, employing WKY rats as controls and using DNA
microarrays to survey for genes related to hypertension in these
SHR substrains. In addition to analyzing gene expression in these
three types of SHR substrains (Method 1), young rats whose BP
levels were not yet elevated (6 weeks of age) and slightly older
rats that developed hypertension (9 weeks of age) were used to
survey candidate blood pressure elevating genes and to examine the
relationship between blood pressure elevation and gene expression
(Method 2). Next, a hypotensive drug with no known receptor,
hydralazine hydrochloride, was administered to each group of rats
to induce acute hypotension to detect hypertension-associated
genes. Thus, genes induced by acute hypotension were identified
(Method 3). This study aimed to use three analytical methods to
comprehensively identify candidate genes involved in the genesis of
hypertension in the kidneys of SHR substrains.
Materials and methods
Animals
The experiments were performed on rats at 6 and 9
weeks of age. A total of 3 rats were used in each experimental
group. WKY/Izm was used as a wild-type control strain, and SHR/Kpo
(10), SHRSP/Kpo (11) and M-SHRSP/Kpo (12) were hypertensive model rats. WKY/Izm
were purchased from SLC Co. (Shizuoka, Japan) and the other three
substrains were purchased from the Animal Center of Kinki
University School of Medicine. The animals used in this experiment
were handled with due care according to the guidelines established
by the Japanese Association for Laboratory Animal Science, which
complies with international rules and policies. This study was
performed under approval (KAME-19-078 on April 1, 2007) of the
Animal Care and Use Committee of Kinki University. Measures were
taken to minimize the pain and discomfort of the experimental
animals. Hydralazine hydrochloride (30 mg/kg/day) mixed with SP-2
chow (Funahashi Farm, Chiba, Japan) was administered to half of
each rat group (n=3) to induce an acute decline in blood pressure
for 2 days prior to euthanasia.
Systolic blood pressure measurements
Systolic BP (SBP) was measured using the tail-cuff
method with a UR-5000 instrument (Ueda, Tokyo, Japan). Briefly,
three consecutive SBP readings were taken between 09:00–11:00 after
warming the body at 35°C for 5 min in a heater box. The SBP values
were expressed as the mean ± SEM.
Tissue processing and RNA isolation
After the kidneys were harvested under sodium
pentobarbital anesthesia (50 mg/kg i.p.), the organs were
homogenized at a pitch speed of 22 strokes/sec for 2 min (twice) in
a 2-ml plastic tube with 5-mm diameter glass beads with a Qiagen
Tissue Lyser (Retsch GmbH & Co., Haan, Germany). Total RNA was
extracted with an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's protocol. The quality of RNA was
checked with RNA Nano Chips (Agilent Technologies, Waldbornn,
Germany) with an Agilent 2100 Bioanalyzer, and the RNA was then
used in the microarray experiments. For the microarray analysis,
tissues from 3 rats per group of all hypertensive and normotensive
strains were used in each experiment.
Analysis of gene expression profiling
with oligonucleotide arrays
To examine the gene expression profiles of rat
kidneys, cRNA labeled with cyanine 3-CTP (PerkinElmer, Boston, MA,
USA) was synthesized from 1 μg of DNase I-treated total RNA
with a Low RNA Input Amplification kit (Agilent Technologies), and
this was hybridized by incubating with a Whole Rat Genome
Microarray (4×44K formatted) (Agilent Technologies) in a rotor oven
(Agilent Technologies) for 17 h at 65°C, followed by washing. The
hybridized slides were scanned with an Agilent GenPix Scanner 4000
(Agilent Technologies). The data were extracted, and the overall
raw signal intensities on each array were normalized to the median
value of all rat probes with BRB-Array Tool software ver. 3.7.0.
(Biometric Research Branch) (27).
A significance level (P<0.01) for each probe was set using the
univariate Student's t-test.
Annotation of differentially expressed
genes
A BLASTN search of the NCBI RefSeq database was
performed, employing corresponding 60-nucleotide probes (NCBI, GEO
accession: GPL7294) to identify homologous genes with functional
annotations (28). After running
the BLASTN search, clones showing either a score higher than 50 or
an E-value below 5e-05 were defined as annotated clones, and the
remaining clones were defined as non-annotated clones or unknown
genes. The annotated gene and protein symbols are shown in italics
and regular font, respectively.
Strategies to survey candidate genes
related to hypertension
The following three different analyses were adopted
to identify candidate genes related to or causing hypertension as
described above. Method 1: data from the comparison among the SHR
substrains were used to survey candidate genes among the SHRs,
SHRSPs and M-SHRSPs according to the ascending order of
hypertension. Method 2: data from the comparison between the 6- and
9-week-old rats of each SHR substrain were used to survey the
candidate genes in ascending or descending order of expression
between age groups in each substrain. Method 3: data obtained from
the comparison of hypotensive effects, with or without hydralazine
hydrochlo-ride treatment, in the SHR substrains compared to the WKY
rats were used to survey the genes induced by this treatment in
each SHR substrain.
Analyses of genes expressed in
biochemical pathways
Analyses of the role of genes expressed in
biochemical pathways were performed using Skypainter of REACTOME, a
free and open-source database (http://www.reactome.org/), offered on the website of
the Cold Spring Harbor Laboratory, The European Bioinformatics
Institute and The Gene Ontology Consortium.
Statistical analyses
Comparisons between the means of the data in each
group were performed using one-way analysis of variance (ANOVA) and
Scheffe's multiple comparisons test. Differences were considered
significant at P<0.05 and P<0.01 for BP measurements and DNA
array measurements, respectively.
Results
Blood pressure
SBP was measured in the WKY rats and the three SHR
substrains at 6 and 9 weeks of age before and 2 days after
receiving 30 mg/kg/day p.o. hydralazine hydrochloride (Table I). SBP levels in the SHRs and
M-SHRSPs were significantly higher than levels in the WKY rats at 6
weeks of age. At 9 weeks of age, SBP levels increased in the order
WKY rats, SHRs, SHRSPs and M-SHRSPs as compared to those at 6 weeks
of age, and every value was higher than that noted in the WKY rats.
Moreover, hydralazine hydrochloride (30 mg/kg/day p.o.)
significantly decreased SBP levels in every rat at 6 and 9 weeks of
age 2 days after drug administration compared to the values prior
to drug administration
 | Table I.SBP levels (mmHg) of the WKYs, SHRs,
SHRSPs and M-SHRSPs with or without hydralazine administration for
2 days. |
Table I.
SBP levels (mmHg) of the WKYs, SHRs,
SHRSPs and M-SHRSPs with or without hydralazine administration for
2 days.
| Rats | 6 weeks of age
(n=3)
| 9 weeks of age
(n=3)
|
|---|
| No treatment | 30 mg/kg
hydralazine | No treatment | 30 mg/kg
hydralazine |
|---|
| WKY | 130±4.1 | 118±4.9d | 137±5.2 | 122±1.2d |
| SHR | 139±2.5a | 127±6.4d |
158±7.1b,c | 146±4.3d |
| SHRSP | 140±7.6 | 126±1.2d | 180±10b,c | 161±5.8d |
| M-SHRSP | 153±2.1a | 142±3.4d | 217±10b,c | 198±6.0d |
Microarray findings
Since this study aimed to identify the candidate
genes involved in the genesis of hypertension in three SHR
substrains, gene expression profiles were compared to DNA
microchips that contained 41,012 probes using the mRNAs extracted
from the kidneys of the three SHR substrains at 6 and 9 weeks of
age, with or without the hypotensive agent, hydralazine
hydrochloride, which dilates resistant arteries distributed in the
whole body through an unknown receptor. The numbers of up- and
down-regulated genes were less than the number of probes due to
redundancy in the probe sets (i.e., in some cases, two or three
probes represent one gene).
Method 1: Comparison of the spontaneously
hypertensive rat substrains
Significantly increased or decreased DNA array data
from each SHR substrain were obtained and compared to age-matched
WKY rats. In 6-week-old rats, the numbers of significantly
up-regulated genes compared to the WKY rats at the level of a
probability ratio <0.01 were 217, 405 and 224 in SHRs, SHRSPs
and M-SHRSPs, respectively. The numbers of commonly expressed genes
between the SHRs and SHRSPs, SHRSPs and M-SHRSPs, and M-SHRSPs and
SHRs were 135, 123 and 79, respectively. A total of 63 genes were
commonly expressed in the SHRs, SHRSPs and M-SHRSPs Among these
genes, 16 were expressed more than four times higher than in the
WKY rats (Table II). Of these 16
genes, Gc, Sugt 1, Dusp15, Cyp8b1,
Sult1b1, EprE, Armc 3, Serpina3m,
Bri3bp, Ptrh1 and Trps1 were identified as
known functional genes in addition to five previously unidentified
genes. In 9-week-old rats, the numbers of significantly
up-regulated genes compared to the WKY rats at the level of a
probability ratio <0.01 were 5,447, 4,172 and 9,549 in the SHRs,
SHRSPs and M-SHRSPs, respectively. The numbers of commonly
expressed genes between the SHRs and SHRSPs, SHRs and M-SHRSPs, and
SHRSPs and M-SHRSPs were 2,764, 3,647 and 3,018, respectively. In
the SHRs, SHRSPs and M-SHRSPs, 2,103 genes were commonly expressed.
Of these genes, 37 genes were expressed more than four times higher
compared to the WKY rats (Table
III). Of these 37 genes, Dusp15, Armc 3,
Cyp8b1, Acox2, Sugt 1, Rdh2,
Zfp597, Gtpbp4, Serpina3m, Gc,
XR_006738 (similar to nucleolar GTP-binding protein 1),
Tmem14a, XM_347233 (similar to indolethylamine
N-methyltransferase), TC539990 (ATP synthase subunit 8),
TC540923 (phosphatidylinositol 3 kinase regulator),
TC528756 (EprE protein), Gloxd1, Fbxo36,
Ddit4, Sv2a, Cyr61, RGD1560736 (similar
to solute carrier family 9), Dpt, Mett12,
Mapk14, LOC689240 (similar to amyotrophic lateral
sclerosis 2 chromosome region), Bri3bp, Slc11a1 and
Prkar2b were identified as known functional genes in
addition to ten previously unidentified genes. A total of 8 genes
were identified that were commonly expressed at a higher level in
the SHRs, SHRSPs and M-SHRSPs compared to the WKY rats at 6 and 9
weeks of age (Table IV). A total
of 7 known genes, Gc, Dusp15, Sugt 1,
Cyp8b1, Armc 3, Serpina3m and Bri3bp,
and one previously unknown gene were detected.
 | Table II.Genes (16) commonly expressed more than four
times higher in the kidneys of the SHRs, SHRSPs and M-SHRSPs
compared to WKY rats at 6 weeks of age. |
Table II.
Genes (16) commonly expressed more than four
times higher in the kidneys of the SHRs, SHRSPs and M-SHRSPs
compared to WKY rats at 6 weeks of age.
| Fold increases
(/WKY)
| | | |
|---|
| SHR | SHRSP | M-SHRSP | Clones | GB account | Description |
|---|
| 1. | 91.961 | 27.387 | 49.827 |
A_43_P13995 |
Gc | Group-specific
component |
| 2. | 26.341 | 29.378 | 20.778 | A_44_P863709 | AW143870 | RGICB87 5′ end,
mRNA sequence |
| 3. | 18.587 | 17.952 | 17.263 |
A_44_P868694 |
TC538548 | Sugt 1,
kinetochore function |
| 4. | 18.822 | 17.034 | 17.395 |
A_44_P562830 |
Dusp15_predicted | Dual specificity
phosphatase-like 15 (predicted) |
| 5. | 14.245 | 15.181 | 9.883 |
A_44_P653949 |
TC558814 | Unknown |
| 6. | 13.108 | 6.051 | 9.843 |
A_44_P387780 |
Cyp8b1 | Cytochrome P450,
family 8, subfamily b, polypeptide 1 |
| 7. | 8.900 | 7.453 | 9.406 | A_44_P371125 | Sult1b1 | Sulfotransferase
family 1B, member 1 |
| 8. | 6.604 | 7.894 | 7.490 | A_44_P670594 | TC528756 | O87809 EprE
protein |
| 9. | 9.898 | 5.456 | 6.217 |
A_42_P655890 |
XM_225610 | Armc3,
multiple functions in signal transduction |
| 10. | 6.068 | 7.768 | 7.310 |
A_44_P470661 |
Serpina3m | Serine (or
cysteine) proteinase inhibitor, member 3M |
| 11. | 7.768 | 6.944 | 5.705 |
A_42_P732437 |
Bri3bp | Bri3 binding
protein |
| 12. | 7.222 | 6.495 | 6.503 | A_44_P227132 |
RGD1564324_predicted | cDNA clone
UI-R-CV1-bvu-e-07-0-UI 3′ |
| 13. | 6.473 | 6.865 | 5.205 | A_44_P928190 |
RGD1561961_predicted | Similar to IQ motif
and WD repeats 1 (predicted) |
| 14. | 5.301 | 5.194 | 5.294 | A_42_P578953 |
Ptrh1_predicted | Peptidyl-tRNA
hydrolase 1 homolog (predicted) |
| 15. | 5.733 | 5.427 | 4.624 | A_44_P176831 | BG378920 | BG378920
UI-R-CV1-bvu-e-07-0-UI.s1 UI-R-CV1 |
| 16. | 4.841 | 4.507 | 4.438 | A_44_P1033521 |
Trps1_predicted |
Trichorhinophalangeal syndrome I
(predicted) |
 | Table III.Genes (37) commonly expressed more than four
times higher in the kidneys of the SHRs, SHRSPs and M-SHRSPs
compared to the WKY rats at 9 weeks of age. |
Table III.
Genes (37) commonly expressed more than four
times higher in the kidneys of the SHRs, SHRSPs and M-SHRSPs
compared to the WKY rats at 9 weeks of age.
| Fold increases
(/WKY)
| | | |
|---|
| SHR | SHRSP | M-SHRSP | Clones | GB account | Description |
|---|
| 1. | 29.913 | 28.674 | 39.915 | A_44_P863709 | AW143870 | cDNA clone RGICB87
5′ end |
| 2. | 22.900 | 29.660 | 36.970 |
A_44_P562830 |
Dusp15_predicted | Dual specificity
phosphatase-like 15 (predicted) |
| 3. | 40.920 | 14.342 | 25.341 | A_44_P457153 |
RGD1564999_predicted | Similar to
isopentenyl-diphosphate δ isomerase 2 |
| 4. | 26.305 | 15.898 | 14.860 |
A_42_P655890 |
XM_225610 | Armc 3,
multiple functions in signal transduction |
| 5. | 25.413 | 12.309 | 17.761 |
A_44_P387780 |
Cyp8b1 | Cytochrome P450,
family 8, subfamily b, polypeptide 1 |
| 6. | 15.568 | 14.383 | 23.048 |
A_44_P653949 |
TC558814 | Unknown |
| 7. | 13.884 | 9.754 | 15.786 | A_44_P330188 | Acox2 | Acyl-Coenzyme A
oxidase 2 |
| 8. | 9.098 | 10.313 | 16.107 |
A_44_P868694 |
TC538548 | Sugt 1,
kinetochore function |
| 9. | 7.847 | 9.047 | 16.460 | A_44_P970369 | BG664685 | cDNA clone DRABHF03
5′ |
| 10. | 10.039 | 8.030 | 13.229 | A_44_P132729 | Rdh2 | Retinol
dehydrogenase 2 |
| 11. | 9.048 | 8.655 | 11.654 | A_42_P826202 | Zfp597 | Zinc finger protein
597 |
| 12. | 11.879 | 4.934 | 10.385 | A_43_P12900 | Gtpbp4 | GTP binding protein
4 |
| 13. | 6.778 | 7.495 | 10.912 |
A_44_P470661 |
Serpina3m | Serine (or
cysteine) proteinase inhibitor, member 3M |
| 14. | 6.829 | 7.566 | 11.821 | A_44_P746348 |
RGD1562658_predicted | Similar to RIKEN
cDNA
1700009P17 (predicted) |
| 15. | 8.605 | 5.376 | 11.989 |
A_43_P13995 |
Gc | Group-specific
component |
| 16. | 11.364 | 4.291 | 9.047 | A_44_P1036339 | XR_006738 | Similar to
nucleolar GTP-binding protein 1 |
| 17. | 6.639 | 6.330 | 11.704 | A_44_P1029892 |
Tmem14a_predicted | Transmembrane
protein 14A (predicted) |
| 18. | 4.650 | 7.172 | 12.044 | A_43_P23115 | XM_347233 | Similar to
indolethylamine N-methyltransferase |
| 19. | 10.275 | 5.217 | 8.145 | A_44_P792207 | TC539990 | O63614 (O63614) ATP
synthase subunit 8 |
| 20. | 6.619 | 8.338 | 8.469 | A_44_P777328 | TC540923 |
Phosphatidylinositol 3 kinase
regulator |
| 21. | 5.459 | 5.549 | 9.791 | A_44_P670594 | TC528756 | O87809 (O87809)
EprE protein |
| 22. | 6.291 | 5.570 | 8.778 | A_44_P464942 | BF545795 | cDNA clone
UI-R-BT0-qc-d-07-0-UI 5′ |
| 23. | 6.257 | 5.488 | 8.339 | A_44_P117119 | Gloxd1 | Glyoxalase domain
containing 1 |
| 24. | 6.115 | 5.583 | 7.945 | A_42_P645467 |
Fbxo36_predicted | F-box only protein
36 (predicted) |
| 25. | 8.799 | 6.587 | 4.183 | A_43_P16225 | J01879 | Rat brain-specific
identifier sequence RNA, clone p2a120 |
| 26. | 5.900 | 4.490 | 7.362 | A_44_P556895 | Ddit4 |
DNA-damage-inducible transcript 4 |
| 27. | 5.505 | 5.534 | 6.463 | A_44_P928190 |
RGD1561961_predicted | Similar to IQ motif
and WD repeats 1 (predicted) |
| 28. | 4.469 | 4.354 | 8.532 | A_42_P649672 | Sv2a | Synaptic vesicle
glycoprotein 2a |
| 29. | 6.838 | 5.859 | 4.258 | A_44_P218896 | Cyr61 | Cysteine rich
protein 61 |
| 30. | 5.950 | 4.612 | 6.371 | A_44_P333374 |
RGD1560736_predicted | Similar to solute
carrier family 9 (predicted) |
| 31. | 4.734 | 4.573 | 6.306 | A_44_P180259 |
Dpt_predicted | Dermatopontin
(predicted) |
| 32. | 4.471 | 4.216 | 6.773 | A_44_P534791 |
Mettl2_predicted | Methyltransferase
like 2 (predicted) |
| 33. | 4.846 | 4.425 | 5.821 | A_44_P360409 | Mapk14 | Mitogen activated
protein kinase 14 |
| 34. | 5.594 | 4.498 | 4.891 | A_44_P497253 | LOC689240 | Similar to
amyotrophic lateral sclerosis 2 chromosome region |
| 35. | 4.151 | 4.143 | 5.636 | A_42_P732437 | Bri3bp | Bri3 binding
protein |
| 36. | 4.203 | 4.772 | 4.697 | A_44_P466118 | Slc11a1 | Solute carrier
family 11, member 1 |
| 37. | 4.512 | 4.038 | 4.971 | A_44_P316122 | Prkar2b | Protein kinase,
cAMP dependent regulatory, type II β |
 | Table IV.Genes (8) commonly expressed more than four times
in the kidneys of the SHRs, SHRSPs and M-SHRSPs compared to the WKY
rats at 6 and 9 weeks of age. |
Table IV.
Genes (8) commonly expressed more than four times
in the kidneys of the SHRs, SHRSPs and M-SHRSPs compared to the WKY
rats at 6 and 9 weeks of age.
| Fold increases
(/WKY): 6 or 9 weeks
| | | |
|---|
| SHR | SHRSP | M-SHRSP | Clones | GB account | Description |
|---|
| 1. | 91.961 | 27.387 | 49.827: 6W | A_43_P13995 | Gc | Group-specific
component |
| 8.605 | 5.376 | 11.989: 9W | | | |
| 2. | 18.822 | 17.034 | 17.395: 6W | A_44_P562830 |
Dusp15_predicted | Dual specificity
phosphatase-like 15 (predicted) |
| 22.900 | 29.660 | 36.970: 9W | | | |
| 3. | 14.245 | 15.181 | 9.883: 6W | A_44_P653949 | TC558814 | Unknown |
| 15.568 | 14.383 | 23.048: 9W | | | |
| 4. | 18.587 | 17.952 | 17.263: 6W | A_44_P868694 | TC538548 | Sugt 1,
kinetochore function |
| 9.098 | 10.313 | 16.107: 9W | | | |
| 5. | 13.108 | 6.051 | 9.843: 6W | A_44_P387780 | Cyp8b1 | Cytochrome P450,
family 8, subfamily b, polypeptide 1 |
| 25.413 | 12.309 | 17.761: 9W | | |
| 6. | 9.898 | 5.456 | 6.217: 6W | A_42_P655890 | XM_225610 | Armc 3,
multiple functions in signal transduction |
| 26.305 | 15.898 | 14.860: 9W | | | |
| 7. | 6.068 | 7.768 | 7.310: 6W | A_44_P470661 |
Serpina3m | Serine (or
cysteine) proteinase inhibitor, member 3M |
| 6.778 | 7.495 | 10.912: 9W | | |
| 8. | 7.768 | 6.944 | 5.705: 6W | A_42_P732437 | Bri3bp | Bri3 binding
protein |
| 4.151 | 4.143 | 5.636: 9W | | | |
In 6-week-old rats, the numbers of significantly
down-regulated genes compared to the WKY rats at the level of a
probability ratio <0.01 were 1,611, 1,260 and 1,361 in the SHRs,
SHRSPs and M-SHRSPs, respectively. The numbers of commonly
down-regulated genes between the SHRs and SHRSPs, SHRs and
M-SHRSPs, and SHRSPs and M-SHRSPs were 1,094, 1,021 and 851,
respectively. In the SHRs, SHRSPs and M-SHRSPs, 767 genes were
commonly down-regulated. Of these genes, 6 were expressed less than
1/4 the levels noted in the WKY rats (Table V). SclB, Hmmr and
frame 12 were identified as known genes in addition to three
previously unidentified genes. In 9-week-old rats, the numbers of
significantly down-regulated genes compared to those in the WKY
rats at the level of a probability ratio <0.01 were 1,330, 1,465
and 176 in the SHRs, SHRSPs and M-SHRSPs, respectively. The numbers
of commonly down-regulated genes between the SHRs and SHRSPs, SHRs
and M-SHRSPs, and SHRSPs and M-SHRSPs were 844, 121 and 125,
respectively. In the SHRs, SHRSPs and M-SHRSPs, 121 commonly
down-regulated genes were identified. Of these genes, 18 were
expressed less than 1/4 the levels noted in the WKY rats (Table VI) and included Anxa13,
SclB, Olr1455, frame 12, Ephx2,
Kb9, Myr8, Tspan1, Pcdh9 and
CA506853 (HIV-I Nef negative effector of Fas and TNF)
as known genes in addition to 8 previously unidentified genes. A
total of 5 genes were found to be commonly expressed at lower
levels in SHR, SHRSP and M-SHRSP compared to WKY at 6 and 9 weeks
of age (genes shown in bold in Table
V) and included SclB, Hmmr and frame
12.
 | Table V.Genes (6) whose expression was commonly lower in
the kidneys of the SHRs, SHRSPs and M-SHRSPs compared to the WKY
rats at 6 weeks of age. |
Table V.
Genes (6) whose expression was commonly lower in
the kidneys of the SHRs, SHRSPs and M-SHRSPs compared to the WKY
rats at 6 weeks of age.
| Fold changes (/WKY)
| | | |
|---|
| SHR | SHRSP | M-SHRSP | Clones | GB account | Description |
|---|
| 1. | 0.088 | 0.088 | 0.091 |
A_44_P808578 |
TC525845 | SclB,
collagen-like surface protein of Streptococcus |
| 2. | 0.104 | 0.094 | 0.088 |
A_44_P362486 |
BQ780215 | cDNA clone
UI-R-FF0-cpb-k-24-0-UI 3′ |
| 3. | 0.044 | 0.179 | 0.114 | A_44_P292314 | Hmmr | Hyaluronan mediated
motility receptor |
| 4. | 0.127 | 0.141 | 0.102 |
A_44_P402948 |
TC540893 | Unknown |
| 5. | 0.156 | 0.161 | 0.113 |
A_44_P306008 |
AI179380 | Frame 12,
RSPCG42 3′ end |
| 6. | 0.174 | 0.174 | 0.220 |
A_42_P719350 |
RGD1562974_predicted | Similar to
DKFZp434P0316 (predicted) |
 | Table VI.Genes (18) whose expression was commonly lower
in the kidneys of the SHRs, SHRSPs and M-SHRSPs compared to WKY at
9 weeks of age. |
Table VI.
Genes (18) whose expression was commonly lower
in the kidneys of the SHRs, SHRSPs and M-SHRSPs compared to WKY at
9 weeks of age.
| Fold changes (/WKY)
| | | |
|---|
| SHR | SHRSP | M-SHRSP | Clones | GB account | Description |
|---|
| 1. | 0.033 | 0.039 | 0.032 | A_44_P157134 |
Anxa13_predicted | Annexin A13
(predicted) |
| 2. | 0.071 | 0.073 | 0.055 | A_44_P396474 |
RGD1563398_predicted | RGD1563398
(predicted) |
| 3. | 0.062 | 0.054 | 0.093 |
A_44_P362486 |
BQ780215 | cDNA clone
UI-R-FF0-cpb-k-24-0-UI 3′ |
| 4. | 0.077 | 0.080 | 0.077 |
A_44_P808578 |
TC525845 | SclB,
collagen-like surface protein of Streptococcus |
| 5. | 0.044 | 0.045 | 0.158 | A_44_P540755 |
Olr1455_predicted | Olfactory receptor
1455 (predicted) |
| 6. | 0.067 | 0.095 | 0.085 |
A_44_P402948 |
TC540893 | Unknown |
| 7. | 0.086 | 0.056 | 0.157 |
A_44_P306008 |
AI179380 | Frame 12,
RSPCG42 3′ end |
| 8. | 0.100 | 0.083 | 0.121 | A_44_P531870 | Ephx2 | Epoxide hydrolase
2 |
| 9. | 0.073 | 0.112 | 0.146 | A_44_P138838 | Kb9 | Type II keratin
Kb9 |
| 10. | 0.112 | 0.125 | 0.193 | A_44_P850331 | BF554752 | cDNA clone
UI-R-C2p-ol-e-12-0-UI 5′ |
| 11. | 0.150 | 0.108 | 0.179 | A_44_P142391 | LOC360919 | Similar to
α-fetoprotein |
| 12. | 0.158 | 0.163 | 0.127 | A_44_P510436 | Myr8 | Myosin heavy
chain |
| 13. | 0.122 | 0.142 | 0.223 | A_43_P10290 | Tspan1 | Tetraspanin 1 |
| 14. | 0.130 | 0.144 | 0.247 |
A_42_P719350 |
RGD1562974_predicted | Similar to
DKFZp434P0316 (predicted) |
| 15. | 0.217 | 0.128 | 0.206 | A_44_P900530 |
Pcdh9_predicted | Protocadherin 9
(predicted) |
| 16. | 0.198 | 0.135 | 0.249 | A_44_P324806 | AI103119 | cDNA clone REMBX47
3′ end |
| 17. | 0.223 | 0.206 | 0.212 | A_44_P223009 | CA506853 | HIV-I Nef:
negative effector of Fas and TNF |
| 18. | 0.205 | 0.223 | 0.221 | A_44_P780781 | TC564995 | Unknown |
Up-regulated genes in the SHR substrains compared to
the WKY rats were separately analyzed with the Reactome database to
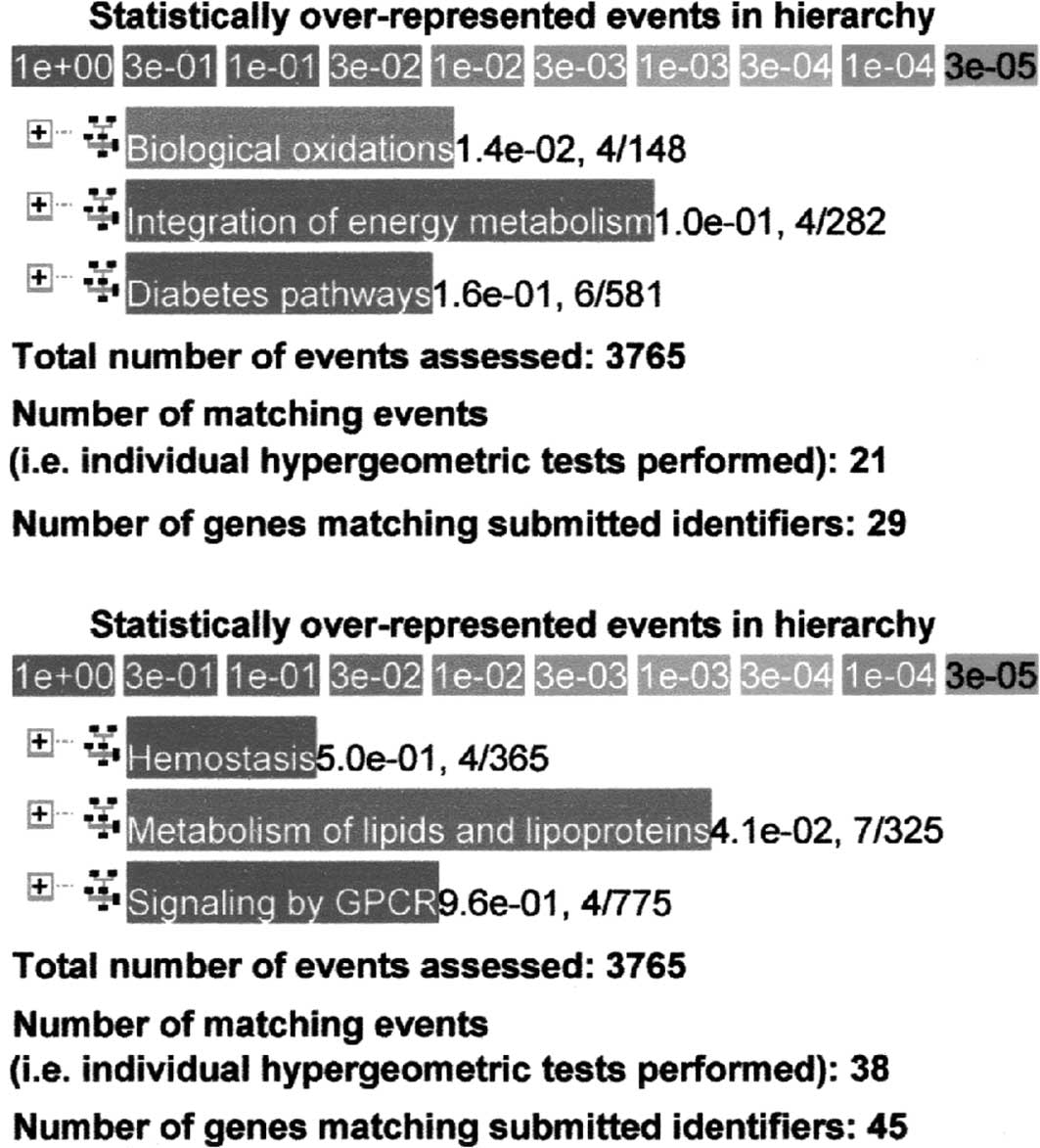
determine the functional relationship in hypertension. An example
of analysis of genes up-regulated more than two times in the
M-SHRSPs compared to the WKY rats at 6 weeks of age (n=193) is
shown in Fig. 1A. Analysis of
genes up-regulated more than four times in the M-SHRSPs compared to
the WKY rats at 9 weeks of age (n=228) is shown in Fig. 1B. A total of 4 genes, including
Yc2, Cyp2c, Gsta3 and Cyp8b1,
participate in biological oxidation with a relatively strong
relationship at the P=1.4×10−2 level at 6 weeks of age.
A total of 7 genes, including RGD1564999, Hmgcs2,
Apob, Aptlc1, Acox2, Angptl4 and
Cyp8b1, participate in the metabolism of lipids and
lipoproteins with a relatively stronger relationship at the
P=4.1×10−2 level at 9 weeks of age.
Method 2: Comparison of two different
ages of each spontaneously hypertensive rat substrain
SBP was elevated in each SHR strain during the
period from 6 to 9 weeks of age as shown in Table I. Therefore, candidate genes that
were up- or down-regulated during this time may be related to
hypertension. Multiple methods were used to survey candidate genes
related to increased blood pressure during the ages of 6 to 9 weeks
in each substrain. The numbers of genes that were up-regulated to a
greater extent in rats at 9 compared to 6 weeks of age were 302,
680, 881 and 1,352 in the WKY rats, SHRs, SHRSPs and M-SHRSPs,
respectively. Of these genes, 79, 124 and 7 genes were more highly
up-regulated in the SHRs, SHRSPs and M-SHRSPs compared to the WKY
rats, respectively. A total of 8 genes were up-regulated >1.5
times between rats 6 to 9 weeks of age in two or more substrains or
in the M-SHRSPs (Table VII). The
known genes were Nef3, Slc26a4, Cyp2C,
Gfra1 and Resp18, and three previously unidentified
genes were noted as well. The numbers of genes showing reduced
expression in rats at 9 weeks of age compared to 6 weeks of age
were 988, 285, 1,285 and 38 in the WKY rats, SHRs, SHRSPs and
M-SHRSPs, respectively. Of these genes, 5, 51 and 0 genes showed
reduced expression in the SHRs, SHRSPs and M-SHRSPs compared to the
WKY rats, respectively. A total of 2 genes, Atp12a and
Hbb, were expressed at less than 1/4 the levels at 6
compared to 9 weeks of age in more than two substrains (Table VIII).
 | Table VII.Genes (8) whose expression was higher in the
kidneys of the SHRs, SHRSPs and M-SHRSPs at 9 compared to 6 weeks
of age and whose expression was higher in the SHR substrains than
in the WKY rats. |
Table VII.
Genes (8) whose expression was higher in the
kidneys of the SHRs, SHRSPs and M-SHRSPs at 9 compared to 6 weeks
of age and whose expression was higher in the SHR substrains than
in the WKY rats.
| Clones | Fold increases (9/6
weeks of age)
| GB account | Description |
|---|
| SHR | SHRSP | M-SHRSP |
|---|
| 1. | A_42_P752336 | 5.281 | 8.974 | 10.278 | Nef3 | Neurofilament 3,
medium |
| 2. | A_44_P669819 | 2.513 | 2.886 | 5.548 | TC543467 | Unknown |
| 3. | A_42_P800771 | | 8.739 | 4.865 |
RGD1563825_predicted | Similar to
ENSANGP00000020885, SOD |
| 4. | A_44_P302221 | | 5.804 | 5.057 | LOC689753 | Similar to
K06A9.1b |
| 5. | A_44_P1017035 | 1.475 | | 2.020 | Slc26a4 | Solute carrier
family 26, member 4, controls the balance of charged ions |
| 6. | A_44_P280786 | 3.749 | 7.594 | | Cyp2C | Cytochrome P450,
subfamily lic |
| 7. | A_43_P11634 | | | 2.117 | Gfra1 | Glial cell line
derived neurotrophic factor family receptor α 1 |
| 8. | A_43_P12005 | | | 2.165 | Resp18 | Regulated
endocrine-specific protein 18 |
 | Table VIII.Genes (2) whose expression was lower in the
kidney of the SHRs and SHRSPs at 9 compared to 6 weeks of age and
was lower compared to the WKYs. |
Table VIII.
Genes (2) whose expression was lower in the
kidney of the SHRs and SHRSPs at 9 compared to 6 weeks of age and
was lower compared to the WKYs.
| Clones | Fold changes (9/6
weeks of age)
| GB account | Description |
|---|
| SHR | SHRSP |
|---|
| 1. | A_42_P684885 | 0.229 | 0.118 | Atp12a | ATPase,
H+/K+ transporting, non-gastric, α
polypeptide |
| 2. | A_44_P306307 | 0.162 | 0.247 | Hbb | Hemoglobin β chain
complex |
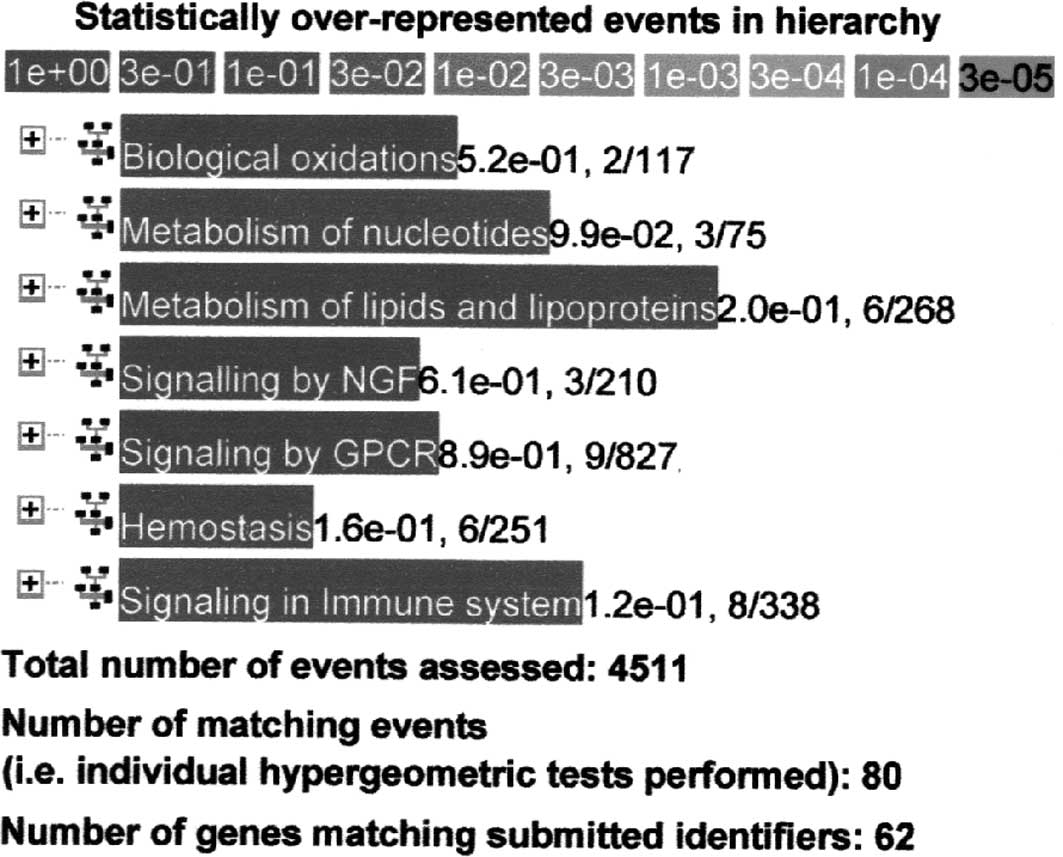
Using the Reactome database as a representative
sample, genes that were up-regulated more than two times (n=391) at
9 compared to 6 weeks of age in M-SHRSP were separately analyzed to
determine the functional relationship in hypertension. Although
three genes (Slc28a1, Xdh and Gda) were
related to the metabolism of nucleotides at a P=9.9×10−2
level, no significant relationship with other processes was
observed (Fig. 2).
Method 3: Comparison between genes
expressed with and without hydralazine hydrochloride-induced
hypotensive effects in the Wistar Kyoto rats and the spontaneously
hypertensive rat substrains
BP was decreased in all rats after 30 mg/kg/day
hydralazine hydrochloride treatment for 2 days. In this paradigm,
candidate genes related to hydralazine hydrochloride-induced
hypotension may be up- or down-regulated, particularly in the SHR
substrains. Following administration of hydralazine hydrochloride,
22, 35, 26 and 66 genes in the WKYs, SHRs, SHRSPs and M-SHRSPs were
up-regulated 4, 2, 2 and 8 times at 6 weeks of age, respectively.
The numbers of genes up-regulated >1.0-fold in the WKY rats and
M-SHRSPs, SHRs and SHRSPs, SHRs and M-SHRSPs, and SHRSPs and
M-SHRSPs at 6 weeks of age were 67, 5, 9 and 23, respectively.
Following administration of hydralazine hydrochloride, 9, 34, 60
and 4 genes in the WKY rats, SHRs, SHRSPs and M-SHRSPs were
up-regulated 1.2, 1.2, 1.5 and 1.2 times at 9 weeks of age,
respectively. The numbers of genes up-regulated >1.0-fold in the
WKY rats and M-SHRSPs, SHRs and SHRSPs, SHRs and M-SHRSPs, and
SHRSPs and M-SHRSPs at 9 weeks of age were 67, 5, 9 and 23,
respectively. On the other hand, following administration of
hydralazine hydrochloride, 13, 20, 11 and 18 genes showed
expression that was reduced 0.6, 0.2, 0.6 and 0.25 times in the WKY
rats, SHRs, SHRSPs and M-SHRSPs at 6 weeks of age, respectively.
The numbers of genes showing expression that was reduced by
<1.0-fold in the WKY rats and M-SHRSPs, SHRs and SHRSPs, SHRs
and M-SHRSPs, and SHRSPs and M-SHRSPs at 6 weeks of age were 3, 0,
21 and 3, respectively. In addition, the numbers of genes showing
expression that was reduced <0.8 times following administration
of hydralazine hydrochloride were 11, 7, 7 and 41 in the WKY rats,
SHRs, SHRSPs and M-SHRSPs, respectively, at 9 weeks of age. The
numbers of genes showing expression that was reduced <1.0-fold
in the WKY rats and M-SHRSPs, SHRs and SHRSPs, SHRs and M-SHRSPs,
and SHRSPs and M-SHRSPs at 9 weeks of age were 3, 0, 21 and 3,
respectively.
Using this method, few genes were identified that
were both up- and down-regulated and commonly expressed between
substrains. Thus, the analyses were modified as follows. Genes
identified with Method 3 and those identified with Method 2 were
combined and considered candidate hypertension-related genes. Using
this method, genes that were up-regulated >1.2 times or
down-regulated <0.8 times in two or more substrains were focused
on. After identifying the genes that were expressed in the WKY rats
and satisfying the criteria of Method 2, ten genes satisfied both
conditions at once. These 10 genes are strongly suggested to be
candidate genes (Table IXA), and
they included TC550463 (farnesyl pyrophosphate synthetase),
Kcnc3, Vnn1 and RGD1561143 (similar to cell
surface receptor FDFACT) in addition to 6 previously unidentified
genes. In addition, 4 genes that were up-regulated >4 times
following administration of hydralazine hydrochloride for 2 days in
6-week-old M-SHRSP and that satisfied the criteria of Method 2 were
identified as possible candidate genes (Table IXB). These genes included
TC560558 (FK506-binding protein 1B), TC564079
(Drosophila melanogaster), XM_343516 (similar to
sulfotransferase K2) and one previously unidentified gene.
 | Table IX.Genes (14) that were up- or down-regulated
following administration of hydralazine, and/or were commonly more
highly expressed in the kidneys of the SHRs, SHRSPs or M-SHRSPs
compared to the WKY rats at 9 compared to 6 weeks of age. |
Table IX.
Genes (14) that were up- or down-regulated
following administration of hydralazine, and/or were commonly more
highly expressed in the kidneys of the SHRs, SHRSPs or M-SHRSPs
compared to the WKY rats at 9 compared to 6 weeks of age.
| A, Genes strongly
suggested to be associated with hypertension |
|
| Fold changes
(hydralazine/none) | Strains | Clones | GB account | Description |
|
| 1.a | 9.646 | 6W M-SHRSP | A_44_P566390 | TC550463 | Farnesyl
pyrophosphate synthetase |
| 2.a | 9.551 | 6W M-SHRSP | A_44_P623610 | TC525804 | Unknown |
| 3.a | 8.615 | 6W M-SHRSP | A_43_P10474 | TC527985 | Unknown |
| 4.a | 8.419 | 6W M-SHRSP | A_44_P808679 | TC541828 | Unknown |
| 5.a | 8.387 | 6W M-SHRSP | A_44_P104687 | Kcnc3 | Potassium
voltage-gate channel protein |
| 6.a | 2.044 | 6W SHRSP | A_44_P871211 | TC566645 | Unknown |
| 7.a | 0.664 | 9W SHR | A_44_P732488 | TC567669 | Unknown |
| 8. | 2.367 | 6W SHR | | | |
| 2.529 | 6W SHRSP | A_42_P811256 | Vnn1 | Participate in an
oxidative-stress response |
| 9. | 2.205 | 6W SHRSP | | | |
| 8.872 | 6W M-SHRSP | A_44_P854454 | TC543180 | Unknown |
| 10. | 1.260 | 9W SHR | | | |
| 2.115 | 6W SHRSP | A_44_P435955 |
RGD1561143_predicted | Similar to cell
surface receptor FDFACT |
|
| B, Genes possibly
suggested to be associated with hypertension |
|
| 1.b | 9.245 | 6M M-SHRSP | A_44_P610417 | TC560558 | FK506-binding
protein 1B |
| 2.c | 8.997 | 6W M-SHRSP | A_44_P841829 | TC564079 | CG4187-PA,
Drosophila melanogaster |
| 3.b | 8.969 | 6W M-SHRSP | A_44_P917243 | TC533180 | Unknown |
| 4.b | 8.533 | 6W M-SHRSP | A_44_P408114 | XM_343516 | Similar to
sulfotransferase K2 |
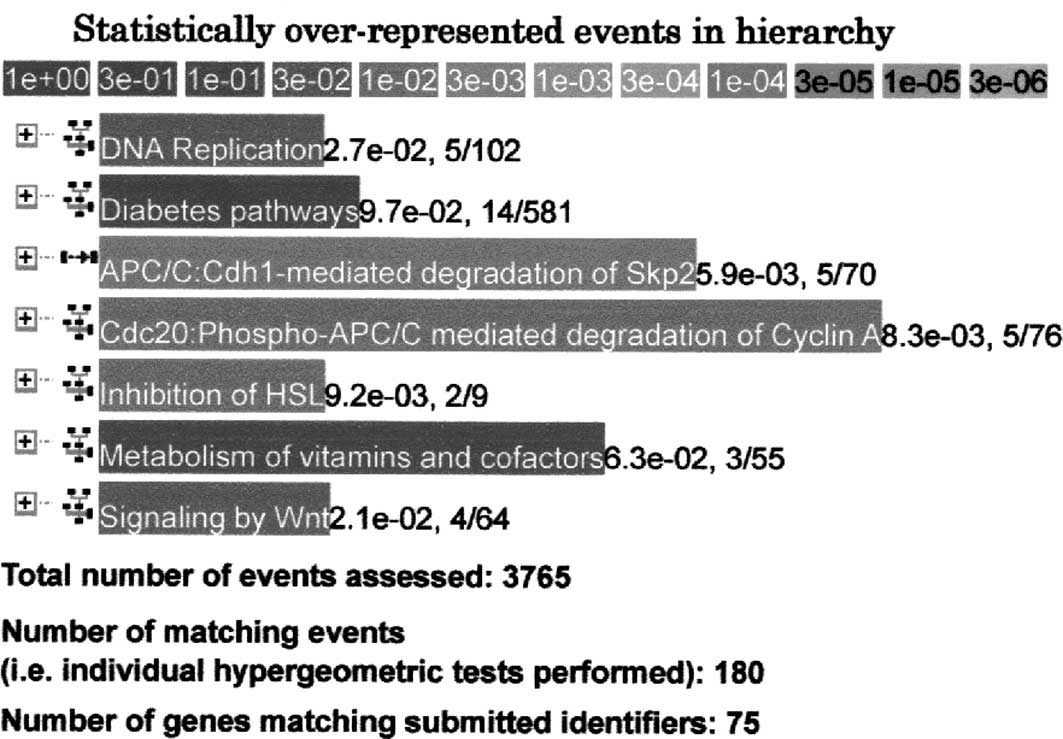
When genes that were up-regulated 1.5 times (n=505)
following hydralazine hydrochloride administration i n 6-week-old
SHRSPs were analyzed further with the Reactome database, 5 genes
(P=2.7×10−2) involved in DNA replication, 14 genes
(P=9.7×10−2) involved in diabetes pathways, 5 genes
(P=5.9×10−3) involved in APC/C:Cdh1-mediated degradation
of Skp2, 5 genes (P=8.3×10−3) involved in Cdc20:
phospho-APC/C-mediated degradation of cyclin A, 2 genes
(P=9.2×10−3) involved in the inhibition of hormone
sensitive lipase (HSL), 3 genes (P=6.3×10−2) involved in
the metabolism of vitamins and cofactors, and 4 genes
(P=2.1×10−2) involved in Wnt signaling were detected
with high significance (Fig. 3).
Thus, expression of numerous genes related to DNA replication and
cell proliferation, including Psmc6, Psma2,
Psma6 and LOC311078 [proteasome (prosome, macropain)
subunits], was modified by hydralazine hydrochloride treatment of
the SHRSPs with high significance.
As shown in Table
X, the expression of three possibly hypertension-related genes
was found to be down-regulated 0.25 times by hydralazine
hydrochloride administration for 2 days in either 6-week-old SHR or
M-SHRSP, including Gabrq (γ-aminobutyric acid A receptor)
and two previously unidentified genes.
 | Table X.Genes (3) down-regulated following administration
of hydralazine in the SHRs and M-SHRSPs at 6 weeks of age. |
Table X.
Genes (3) down-regulated following administration
of hydralazine in the SHRs and M-SHRSPs at 6 weeks of age.
| Fold changes
(hydralazine/none) | Strains | Clones | GB account | Description |
|---|
| 1. | 0.090 | 6W SHR | | | |
| 0.168 | 6W M-SHRSP | A_44_P541438 | RGD1308699 | Similar to
1700060H10Rik protein |
| 2. | 0.132 | 6W SHR | | | |
| 0.129 | 6W M-SHRSP | A_43_P15862 | Gabrq | γ-aminobutyric acid
A receptor |
| 3. | 0.113 | 6W SHR | | | |
| 0.204 | 6W M-SHRSP | A_44_P281788 | BQ196033 | cDNA clone
UI-R-CN1-cmn-f-01-0-UI 3′ |
Discussion
SHRs (10) were
created at the Kyoto University School of Medicine, Japan, around
1963 through continuous brother-sister mating of six generations of
normotensive WKY rats, and they have a slightly higher BP. Using
this strain with high blood pressure, numerous studies concerning
essential hypertension, including pathophysiology and the effects
of food, have been carried out (29,30).
Descendants of SHRs, SHRSPs (11)
were created through continuous brother-sister mating in a closed
colony of SHRs after approximately one decade of mating. M-SHRSPs
(12) were established in 1985
through the brother-sister mating of the SHRSP strain under
hypotensive treatment with hydralazine hydrochloride to avoid
stroke during mating and litter care. An inbred strain of M-SHRSP
shows SBPs of 250 mmHg or higher before 14 weeks of age, and shows
more rapid and severe increases in BP and stroke in almost all
animals (31). These three types
of hypertensive rat substrains, SHR, SHRSP and M-SHRSP, have been
used in multiple investigations of pathophysiology, preventive
medicine, pharmacology and drug development worldwide (29–32).
Our facility is one of the original places that bred the three
types of SHR substrains and families. For our present study, we
decided to use DNA array methodology and these three hypertensive
substrains of rats and the normotensive WKY to identify genes
related to hypertension and, when possible, to identify candidate
genes that cause hypertension.
For theoretical reasons, we should have investigated
hereditary subjects using congenic or consomic animal models of
disease (33,34). We considered the three substrains
of hypertensive rats, SHR, SHRSP and M-SHRSP, to belong to one
family, although several decades have passed since the inbred
strains were established. SHRs are derived from WKY rats, SHRSPs
are derived from SHRs and M-SHRSPs are derived from SHRSPs on the
basis of higher blood pressure and/or high incidences of stroke as
a selection criterion. M-SHRSPs show a higher and earlier elevation
of blood pressure that is accompanied by a higher and earlier
incidence of stroke compared to SHRSPs. This is also true for
SHRSPs compared to SHRs. Therefore, candidate genes for
hypertension must be condensed, increased or expressed at
higher/lower levels to a greater extent in M-SHRSP than in SHRSP
and SHR substrains. On this basis, we searched for candidate genes
for hypertension with these three hypertensive SHR substrains
compared to normotensive WKY rats.
The kidneys were thought to be the most appropriate
tissue for studying hypertension due to their direct influence on
body fluids and endocrine, cardiovascular and sympathetic functions
(14). There are numerous
intrinsic and extrinsic factors, including the renin-angiotensin
system and catecholamine and aldosterone hormones, that control the
relationship between kidney function and blood pressure (14,15).
This study is the first attempt to use DNA microarrays to compare
the gene expression profiles of the kidneys of SHRs, SHRSPs and
M-SHRSPs employing WKY rats as a control. In addition to analyzing
the genes expressed in these three types of SHR substrains (Method
1), young rats (6 weeks old), whose blood pressure was not yet
elevated, and slightly older rats (9 weeks old) that had developed
hypertension were employed to detect candidates genes related to
blood pressure elevation (Method 2). Furthermore, a hypotensive
drug, hydralazine hydrochloride, which acts through an unknown
receptor, was administered to each group of rats to induce acute
hypotension to detect hypertension-associated genes (Method 3).
This study aimed to identify candidate genes for hypertension in
the kidneys of the SHR substrains using these three analytical
methods.
BP was elevated in 9-week-old SHR substrains
compared to 6-week-old rats, and tended to increase from WKY to
SHR, SHRSP and M-SHRSP in this order at 6 and 9 weeks of age.
Hydralazine hydrochloride administration for 2 days decreased blood
pressure in all the rats (Table
I). Therefore, Methods 1, 2 and 3 may be appropriate for
surveying candidate genes related to BP.
In the Method 1 analysis at 6 weeks of age, 16 genes
were significantly more highly expressed in SHR, SHRSP and M-SHRSP
(Table II). Of these genes,
Sugt 1 (kinetochore function), Dusp15 (dual
specificity phosphatase-like 15), Armc 3 (multiple functions
in signal transduction) and Serpina3m (serine or cysteine
protease inhibitor, member 3M) were related to cell proliferation,
protein modification or signal transduction. At 9 weeks of age,
Cyp8b1 (cytochrome P450, family 8, subfamily b, polypeptide
1), Zfp597 (zinc finger protein 597), Gtpbp4 (GTP
binding protein 4), Tmem14a (transmembrane protein 14A),
TC540923 (phosphatidylinositol 3 kinase regulator),
Sv2a (synaptic vesicle glycoprotein 2a), Mapk14
(mitogen activated protein kinase 14) and Prkar2b (protein
kinase, cAMP dependent regulatory type II β) were involved in cell
proliferation, protein modification or signal transduction
(Table III). At 6 and 9 weeks of
age, Gc (group specific component), Dusp15,
TC558814 (unknown), Sugt 1, Cyp8b1, Armc
3, Serpina3m and Bri3bp (Bri3 binding protein)
were significantly more highly expressed (Table IV). A number of genes were
significantly expressed at lower levels and included SclB
(collagen-like surface protein of Streptococcus), Hmmr
(hyaluronan mediated motility receptor) and frame 12
(RSPCG42 3′ end) at 6 weeks of age (Table V), and Anxa13 (annexin A13),
Ephx2 (epoxide hydrolase 2), Kb9 (type II keratin
Kb9), Myr8 (myosin heavy chain), Tspan1 (tetraspanin
1) and Pcdh9 (protocadherin 9) at 9 weeks of age (Table VI). A number of previously
unidentified genes were also found.
The genes that were identified by Method 1 as being
more highly expressed were investigated using the Reactome database
to determine how and where they work in biochemical processes. As
shown in Fig. 1A and B, these
genes were highly related to biological oxidation at 6 weeks of age
and metabolism of lipids and lipoproteins at 9 weeks of age.
However, these genes were not involved in signal transduction or
muscle function in M-SHRSPs compared to WKY rats. Therefore, the
data derived from the Method 1 analysis may show differences in
metabolic characteristics mainly between SHR substrains and WKY
rats, although further study is required.
In the Method 2 analysis, which compared two
different ages of each SHR substrain, Nef3 (neurofilament
3), Slc26a4 (solute carrier family 26, member 4, controls
the balance of ions), Cyp2C (cytochrome P450, subfamily
lic), Gfra1 (glial cell line-derived neurotrophic factor
family receptor α1) and Resp18 (regulated endocrine-specific
protein 18) were identified as known genes that were up-regulated
in addition to three previously unidentified genes (Table VII). In addition, Atp12a
(ATPase, H+/K+ transporting, nongastric, α
polypeptide) and Hbb (hemoglobin β chain complex) were identified
as genes that were down-regulated with age in the SHR substrains
(Table VIII). Functional
relationship analysis with the Reactome database of genes from
6-week-old M-SHRSP showed three genes involved in the metabolism of
nucleotides, including Slc28a1, Xdh and Gda.
Therefore, the genes indentified with this method, including
Slc26a4, Cyp2C, Gfra1, Resp18 and
Atp12a, may be closely related to hypertension as they are
related to energy production and consumption or ion exchange.
Further investigation is required to determine the importance of
these identified genes.
From analysis with Method 3 which compared genes
expressed with or without hydralazine hydrochloride-induced
hypotensive effects in the SHR substrains to WKY rats, Vnn1
(participates in the oxidative-stress response) was identified as a
gene that was more highly expressed, and Gabrq
(γ-aminobutyric acid A receptor) was identified as a suppressed
gene. These two genes were commonly expressed in more than two SHR
substrains. Numerous other genes were uniquely expressed in one SHR
substrain, but not expressed commonly among the three substrains.
Therefore, other candidate genes were identified by combining
Methods 2 and 3. From this process, TC550463 (farnesyl
pyrophosphate synthetase), Kcnc3 (potassium voltage-gate
channel protein), TC560558 (FK506-binding protein 1B) and
XM_343516 (sulfotransferase K2) were selected in addition to
a number of previously unidentified genes. When genes that were
up-regulated more than 1.5 times (n=505) by hydralazine
hydrochloride administration in the 6-week-old SHRSP were analyzed
with the Reactome database software, five genes involved in DNA
replication, five genes involved in the APC/C:Cdh1-mediated
degradation of Skp2, five genes involved in the
Cdc20:phospho-APC/C-mediated degradation of cyclin A, two genes
involved in the inhibition of HSL and four genes involved in Wnt
signaling were detected with high significances (Fig. 3). A number of genes related to DNA
replication and cell proliferation, including Psmc6,
Psma2, Psma6 and LOC311078 [proteasome
(prosome, macropain) subunits], were indentified in 6-week-old
SHRSP. Therefore, genes identified with Methods 2 and 3 may be the
candidate genes closely related to hypertension for which we are
searching.
Although current gene expression arrays permit the
simultaneous analysis of thousands of rat genes, this method is not
yet capable of addressing all functional genes in the genome.
However, as rat genome annotation progresses and arrays continue to
improve in their extent of genomic coverage, a more complete
analysis will be possible. Our present approach identified dozens
of genes, including Dusp15, Cyp8b1, Armc 3,
Gtpbp4, Mettl2, Mapk14, Prkar2b,
frame 12, Anxa13, Ephx2, Myr8 and
Pcdh9 from Method 1; Cyp2C and Atp12a from
Method 2; and Kcnc3, Vnn1, TC560558 and
Gabrq from Methods 2 and 3, in addition to a number of
previously unidentified genes, as probable candidate genes that
cause hypertension in SHR substrains, as determined by common
biochemical knowledge. Of these genes, only Ephx2 has been
previously reported as a strongly related gene in SHRs (24). A key question has arisen regarding
Ephx2. Ephx2 was reported to be a significantly up-regulated
gene in SHRs by 3.39- and 4.30-fold at 3 and 9 weeks of age,
respectively, compared to WKY rats (24). However, we identified Ephx2
as a significantly down-regulated gene at 9 weeks of age (but not
at 6) in the SHRs, SHRSPs and M-SHRSPs by 0.100-, 0.083- and
0.121-fold, respectively (Table
VI). Fornage et al reported lower expression of this
gene in the kidney of 4- to 5-week-old SHRs compared to WKY rats
(35), and Corenblum et al
observed lower expression of Ephx2 in the brain of SHRSPs
compared to stroke-resistant SHRs (36). Although a number of studies have
reported that soluble epoxide hydrolase elevates blood pressure by
degrading vasodilative epoxyeicosatrienoic acids by means of an
inhibitor (37–39), the role of Ephx2 in
hypertension remains controversial. Considering our data and
several other reports, Ephx2 may not be a candidate gene for
hypertension as the expression of Ephx2 was elevated or
decreased in some SHR substrains at some ages and, thus, results
were not consistent. Ephx2 possibly controls the adaptation
of BP changes.
Why the surveyed genes related to hypertension vary
among reports has yet to be elucidated. In addition, we believe
that discovering the roles of unknown genes is crucial, as genes
that are strongly related to hypertension may exist among these
candidates, and these genes may be related to the cause of stroke
in SHRSPs and M-SHRSPs. Since the majority of these genes have not
yet been demonstrated to be responsible for hypertension in SHRs,
we must continue to search for true candidate genes that
participate in the genesis of hypertension in SHR substrains using
current technology.
Acknowledgements
This study was supported by a
Japanese Grant-in-Aid for Scientific Research (no. 19500699), an
Aid Grant to private universities to cover current expenses from
the MEXT in Japan, the Fund of Mishima-Kaiun Memorial Foundation, a
donation from Ueshima Coffee Co., Ltd., and the Kinki University
School of Medicine. We thank the National Center for Biotechnology
Information, USA, and the DNA Data Bank of Japan for access to the
network servers.
References
|
1.
|
Acelajado MC and Oparil S: Hypertension in
the elderly. Clin Geriatr Med. 25:391–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ghiadoni L, Bruno RM, Stea F, Virdis A and
Taddei S: Central blood pressure, arterial stiffness, and wave
reflection: new targets of treatment in essential hypertension.
Curr Hypertens Rep. 11:190–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kannel WB: Table of contents.
Hypertension: reflections on risks and prognostication. Med Clin
North Am. 93:541–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Imaizumi Y: Mortality in the elderly
population aged over 40 in Japan, 1947–1988. Jinko Mondai Kenkyu
(in Japanese). 47:40–57. 1991.
|
|
5.
|
Kesteloot H, Yuan XY and Joossens JV:
Changing mortality patterns in men. Acta Cardiol. 43:133–139.
1988.
|
|
6.
|
Drmanac R, Drmanac S, Strezoska Z, et al:
DNA sequence determination by hybridization: a strategy for
efficient large-scale sequencing. Science. 260:1649–1652. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schena M, Heller RA, Theriault TP, Konrad
K, Lachenmeier E and Davis RW: Microarrays: biotechnology's
discovery platform for functional genomics. Trends Biotechnol.
16:301–306. 1998.
|
|
8.
|
Hacia JG, Brody LC and Collins FS:
Applications of DNA chips for genomic analysis. Mol Psychiatry.
3:483–492. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
McBride MW, Charchar FJ, Graham D, et al:
Functional genomics in rodent models of hypertension. J Physiol.
554:56–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Okamoto K and Aoki K: Development of a
strain of spontaneously hypertensive rat. Jpn Circ J. 27:282–293.
1963. View Article : Google Scholar
|
|
11.
|
Okamoto K, Yamori Y and Nagaoka A:
Establishment of the stroke-prone spontaneously hypertensive rats
(SHR). Circ Res. 34(Suppl 1): 143–153. 1974.
|
|
12.
|
Okamoto K, Yamamoto K, Morita N and Ohta
Y: Establishment and characteristics of rat with precocious and
severe hypertension (M-SHRSP). Acta Med Kinki Univ. 10:73–95.
1985.
|
|
13.
|
Ashenagar MS, Tabuchi M, Kinoshita K, et
al: Gene expression in the adrenal glands of three spontaneously
hypertensive rat substrains. Mol Med Rep. 3:213–222.
2010.PubMed/NCBI
|
|
14.
|
Guyton AC: Dominant role of the kidneys
and accessory role of whole-body autoregulation in the pathogenesis
of hypertension. Am J Hypertens. 2:575–585. 1989.PubMed/NCBI
|
|
15.
|
Guyton AC: Abnormal renal function and
autoregulation in essential hypertension. Hypertension. 18(Suppl
5): 49–53. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gavras H, Brunner HR and Laragh JH: Renin
and aldosterone and the pathogenesis of hypertensive vascular
damage. Prog Cardiovasc Dis. 17:39–49. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Satoh T, Owada S and Ishida M: Recent
aspects in the genetic renal mechanisms involved in hypertension.
Intern Med. 38:919–926. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ely D, Milsted A, Dunphy G, et al:
Delivery of sry1, but not sry2, to the kidney increases blood
pressure and SNS indices in normotensive WKY rats. BMC Physiol.
9:102009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Graham D, McBride MW, Gaasenbeek M, et al:
Candidate genes that determine response to salt in the stroke-prone
spontaneously hypertensive rat: congenic analysis. Hypertension.
50:1134–1141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kreutz R, Schulz A, Sietmann A, Stoll M,
Daha MR, de Heer E and Wehland M: Induction of C1q expression in
glomerular endothelium in a rat model with arterial hypertension
and albuminuria. J Hypertens. 25:2308–2316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Clemitson JR, Dixon RJ, Haines S, et al:
Genetic dissection of a blood pressure quantitative trait locus on
rat chromosome 1 and gene expression analysis identifies SPON1 as a
novel candidate hypertension gene. Circ Res. 100:992–999. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
McBride MW, Brosnan MJ, Mathers J, et al:
Reduction of Gstm1 expression in the stroke-prone spontaneously
hypertension rat contributes to increased oxidative stress.
Hypertension. 45:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhong JC, Huang DY, Yang YM, Li YF, Liu
GF, Song XH and Du K: Upregulation of angiotensin-converting enzyme
2 by all-trans retinoic acid in spontaneously hypertensive rats.
Hypertension. 44:907–912. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kurdi M, Cerutti C, Randon J, McGregor L
and Bricca G: Macroarray analysis in the hypertrophic left
ventricle of renin-dependent hypertensive rats: identification of
target genes for renin. Renin Angiotensin Aldosterone Syst.
5:72–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Seubert JM, Xu F, Graves JP, et al:
Differential renal gene expression in prehypertensive and
hypertensive spontaneously hypertensive rats. Am J Renal Physiol.
289:F552–F561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Charchar FJ, Kaiser M, Bingham AJ,
Fotinatos N, Ahmady F, Tomaszewski M and Samani NJ: Whole genome
survey of copy number variation in the spontaneously hypertensive
rat: relationship to quantitative trait loci, gene expression, and
blood pressure. Hypertension. 55:1231–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Simon R, Lam A, Li M-C, et al: Analysis of
gene expression data using BRB-array tools. Cancer Informatics.
3:11–17. 2007.PubMed/NCBI
|
|
28.
|
Altschul SF, Thomas LM, Alejandro AS, et
al: Gapped BLAST and PSI-BLAST: a new generation of protein
database search programs. Nucleic Acids Res. 25:3389–3402. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Toal C and Leenen FH: Dietary sodium
restriction and development of hypertension in spontaneously
hypertensive rats. Am J Physiol. 245:H1081–H1084. 1983.PubMed/NCBI
|
|
30.
|
Wexler BC: Inhibition of the pathogenesis
of spontaneous hypertension in spontaneously hypertensive rats by
feeding a high fat diet. Endocrinology. 108:981–989. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Okamoto K, Ohta Y, Chikugo T, Shiokawa H
and Morita N: Chronic treatment with captopril, SQ29,852,
hydralazine and a 33% fish meal diet in malignant stroke-prone
spontaneously hypertensive rats. J Hypertens. 9:1105–1117.
1991.PubMed/NCBI
|
|
32.
|
Yamori Y: Implication of hypertensive rat
models for primordial nutritional prevention of cardiovascular
diseases. Clin Exp Pharmacol Physiol. 26:568–572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pravenec M and Kurtz TW: Recent advances
in genetics of the spontaneously hypertensive rat. Curr Hypertens
Rep. 12:5–9. 2010. View Article : Google Scholar
|
|
34.
|
Cowley AW Jr, Roman RJ and Jacob HJ:
Application of chromosomal substitution techniques in gene-function
discovery. J Physiol. 554:46–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Fornage M, Hinojos CA, Nurowska BW,
Boerwinkle E, Hammock BD, Morisseau CHP and Doris PA: Polymorphism
in soluble epoxide hydrolase and blood pressure in spontaneously
hypertensive rats. Hypertension. 40:485–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Corenblum MJ, Wise VE, Georgi K, Hammock
BD, Doris PA and Fornage M: Altered soluble epoxide hydrolase gene
expression and function and vascular disease risk in the
stroke-prone spontaneously hypertensive rat. Hypertension.
51:567–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chiamvimonvat N, Ho CM, Tsai HJ and
Hammock BD: The soluble epoxide hydrolase as a pharmaceutical
target for hypertension. J Cardiovasc Pharmacol. 50:225–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Li J, Carroll MA, Chander PN, Falck JR,
Sangras B and Stier CT: Soluble epoxide hydrolase inhibitor, AUDA,
prevents early salt-sensitive hypertension. Front Biosci.
13:3480–3487. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Yousif MH and Benter IF: Role of
20-hydroxyeicosatetraenoic and epoxyeicosatrienoic acids in the
regulation of vascular function in a model of hypertension and
endothelial dysfunction. Pharmacology. 86:149–156. 2010. View Article : Google Scholar : PubMed/NCBI
|