Introduction
Osteoarthritis (OA) is a chronic joint disease
characterized by degeneration of the articular cartilage, sclerosis
of subchondral bone and osteophyte formation. The incidence of OA
increases during every decade of life and by the age of 65 years,
almost one-third of the population develops OA of the knee joints
(1). Degeneration of articular
cartilage is accompanied by chronic pain and significant disability
in OA. However, at present no treatment is available which prevents
or cures OA. Various factors, including obesity, previous injury
and lifestyle, have been related to the pathogenesis of OA. Current
concepts of the pathogenic mechanisms of OA suggest a shift in the
homeostatic balance between the destruction and synthesis of bone
and cartilage (2). Notably,
previous studies have indicated the involvement of oxidative stress
in the pathogenesis and progression of OA (3).
Oxidative stress is the disturbance in the state of
equilibrium of pro-oxidant and anti-oxidant systems in cells and
tissues (4,5). Since Harman proposed the promotion of
aging by oxidative stress in 1956 (6), the association between oxidative
stress and various diseases, such as cancer, diabetes mellitus and
hypertension, has been elucidated. Reactive oxygen species (ROS)
are highly reactive transient chemical substances, which include
nitric oxide, superoxide anion, hydrogen peroxide and hydroxy
radical, with the potential to initiate cellular damage by acting
on molecules, such as proteins, lipids and nucleic acids.
Furthermore, lipid peroxides, i.e., polyunsaturated fatty acids
peroxidized by ROS, have also been shown to induce tissue injury
(7). Several studies have
demonstrated that oxidative damage, due to overproduced ROS from
superoxide, may be involved in the pathogenesis of OA (8,9). In
this regard, it has been reported that oxidative stress leads to
structural and functional changes in chondrocytes and extracellular
matrix, when ROS production exceeds the anti-oxidant capacities
(3,10,11).
A number of animal models have been developed to
investigate the pathology of OA. In STR/Ort (STR) mice, ∼85% of
males spontaneously develop the degeneration of knee cartilage from
25 to 35 weeks of age. These pathological changes are closely
similar to those in human OA, such as joint space narrowing,
subchondral bone sclerosis and osteophyte formation. In STR mice,
visceral fat is accumulated in their peritoneal cavities and they
are slightly obese. Moreover, STR mice exhibit human hyperlipidemic
patient-like symptoms, such as hypercholesterolemia,
hypertriglyceridemia, hyperinsulinemia, insulin resistance,
dysregulation of nonesterified fatty acids and low serum
adiponectin (12).
In this study, to investigate the involvement of
oxidative stress in the pathogenesis of OA, we evaluated the
relationship between oxidative stress and articular cartilage
degradation by measuring the serum levels of biomarkers, such as
malondialdehyde (MDA; an oxidative stress marker), CTX-II (a type
II collagen degradation marker) and CPII (a type II collagen
synthesis marker) in osteoarthritic STR and control CBA mice.
Materials and methods
Animals
All procedures were carried out according to the
institutional Animal Care and Committee Guide of Juntendo
University School of Medicine. In this study, 7-week-old
osteoarthritic STR male mice weighing ∼27 g (n=10) and 7-week-old
control CBA male mice weighing ∼26 g (n=10) were purchased from
Charles River Japan (Yokohama, Japan). Five mice per cage were
housed under a specific pathogen-free condition (controlled
temperature of 24±3°C and humidity of 55±15%) and fed standard
laboratory food ad libitum. All mice were measured for body
weight using a scale every week. All mice were sacrificed at 35
weeks of age.
Tissue preparation and histopathological
examination
Histopathological changes were evaluated on the
sagittal sections of cartilage in the weight-bearing area of the
medial tibiofemoral compartment. The whole knee joint samples were
dissected and fixed in 10% formalin for 24 h. They were then
decalcificated by Gooding and Stewart’s fluid (equal volume of 10%
formalin and 10% formic acid solution) and embedded in paraffin.
Sections (5 μm) were stained with Safranin O. Grading of OA
progression in the medial tibiofemoral compartment was performed
according to the procedure previously described (13,14)
(grade 0, no apparent change; grade 1, superficial fibrillation of
articular cartilage; grade 2, defects limited to uncalcified
cartilage; grade 3, defects extending into calcified cartilage;
grade 4, exposure of subchondral bone at the articular surface)
(Fig. 1). In this study,
histopathological changes with grade 1 or more were considered to
be OA.
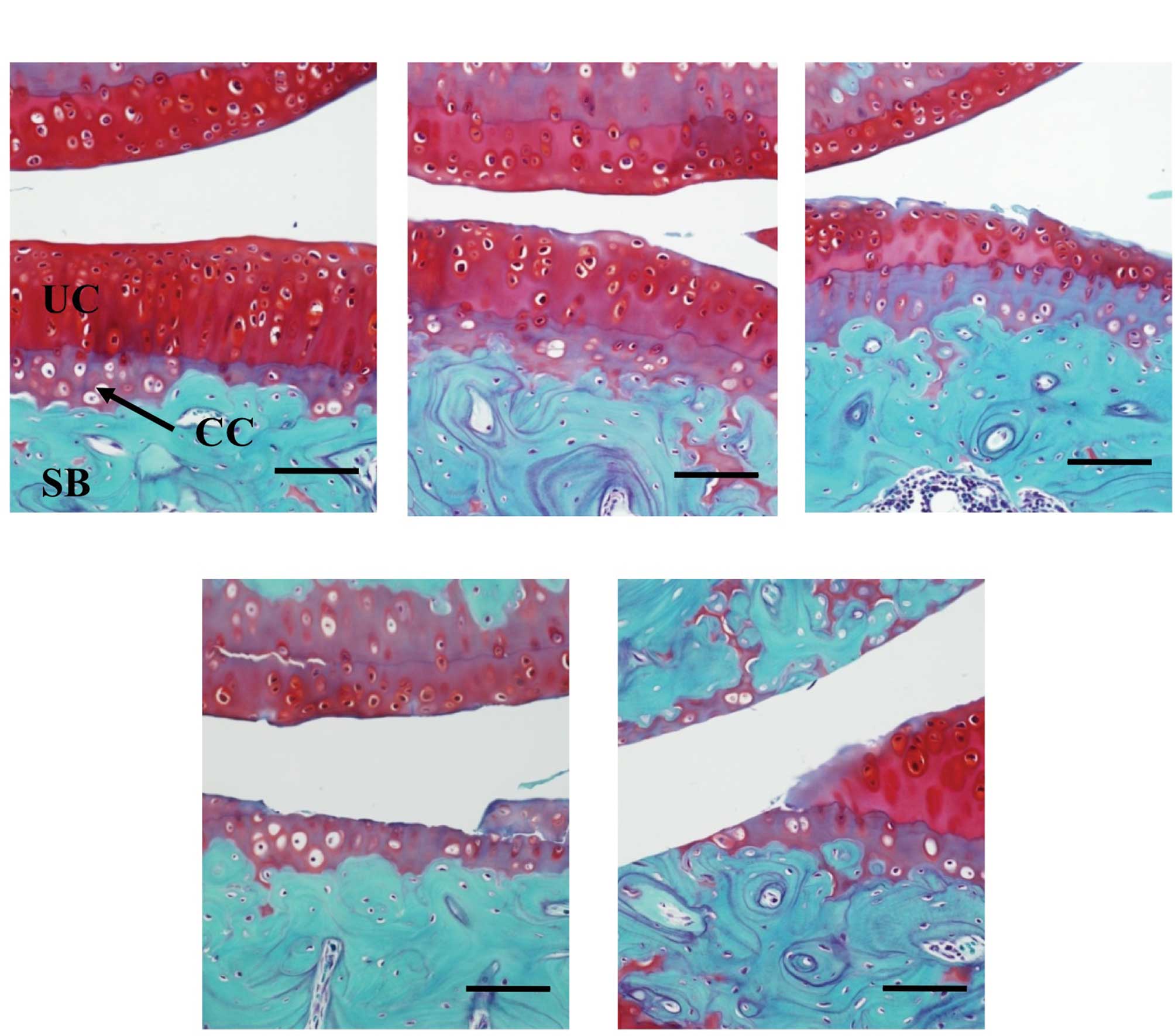 | Figure 1.Histopathological grading of articular
cartilage in STR mice. The sagittal sections of cartilage in the
weight-bearing area of the medial tibiofemoral compartment were
stained with Safranin O. The severity of the OA lesion was graded
on a scale of 0–4 according to the procedure previously described
(13,14). (A) Grade 0, no apparent changes;
(B) grade 1, superficial fibrillation of articular cartilage; (C)
grade 2, defects limited to uncalcified cartilage; (D) grade 3,
defects extending into calcified cartilage; (E) grade 4, exposure
of subchondral bone. Scale bar, 500 μm; CC, calcified cartilage;
UC, uncalcified cartilage; SB, subchondral bone. |
Assays of type II collagen
degradation/synthesis markers and malondialdehyde
Blood was obtained from the heart of the mice at 35
weeks of age, and sera were stored in aliquots at −80°C. Serum
CTX-II (15) was assayed with a
serum Pre-Clinical CartiLaps ELISA kit (Nordic Bioscience
Diagnostic A/S, Herlev, Denmark), which detects C-telopeptide
degradation products of type II collagen (CTX-II) in the sera. By
contrast, serum CPII (15) was
assayed with a Procollagen type II C-propeptide ELISA kit (IBEX
Technologies Inc., Montreal, Canada), which detects carboxy
propeptide of type II collagen (C-propeptide, also referred to as
CPII) in the sera. CPII is cleaved from type II procollagen during
the processing of newly synthesized procollagen and thus can be
used as a type II collagen-synthesis marker.
Serum MDA was assayed with a TBARS Assay kit (Cayman
Chemical Company, USA). MDA is a naturally occurring product of
lipid peroxidation (16,17).
Assay of the serum concentrations of
total cholesterol and triglyceride
The serum concentrations of total cholesterol and
triglyceride were measured using Cholesterol E and L-Type
Triglyceride M (Wako Pure Chemical Industries, Ltd., Osaka, Japan),
respectively.
Statistical analyses
Data are presented as the mean ± SD and were
analyzed for significant differences using the Student’s t-test
(StatView 5.0 program; SAS Institute Inc., NC, USA). Correlation
analysis was also performed with the StatView 5.0 program. A
p-value of <0.05 was considered to be statistically
significant.
Results
Evaluation of body weight and serum
levels of total cholesterol and triglyceride
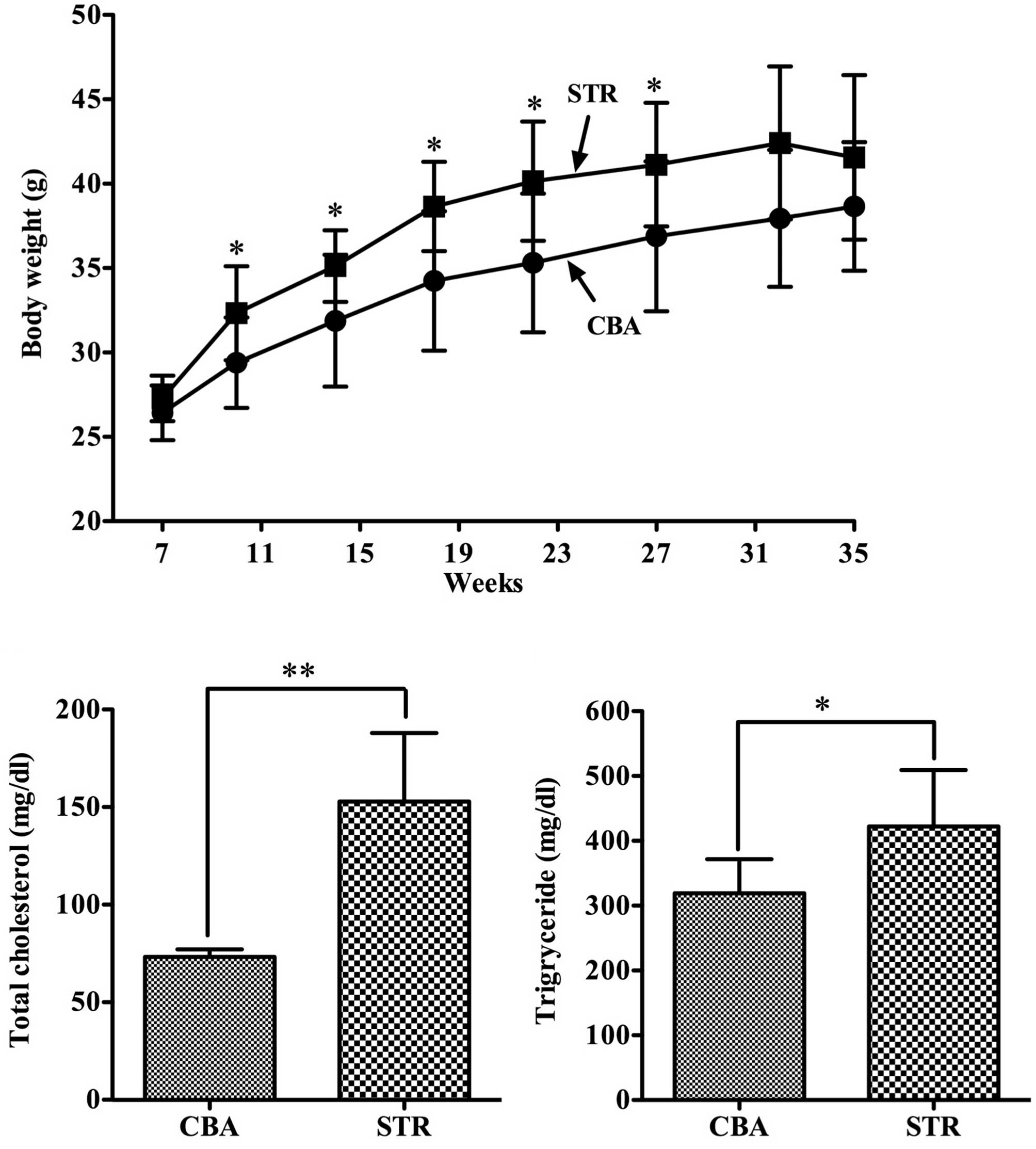
During the experimental periods, the body weight of
STR mice was consistently higher compared to that of the CBA mice
(p<0.05) (Fig. 2A). Moreover,
the serum levels of total cholesterol and triglyceride were
significantly higher in the obese STR mice compared with levels in
the control CBA mice (Fig. 2B and
C).
Evaluation of histopathological changes
in articular cartilage
STR mice spontaneously developed OA in 18 of the 20
knees at 35 weeks (grade 0, 2 knees; grade 1, 6 knees; grade 2, 3
knees; grade 3, 3 knees; grade 4, 6 knees) (Table I). By contrast, control CBA mice
developed OA only in 4 of the 20 knees (grade 0, 16 knees; grade 1,
2 knees; grade 2, 0 knee; grade 3, 1 knee; grade 4, 1 knee).
 | Table I.Histopahological grading of cartilage
degradation in CBA and STR mice. |
Table I.
Histopahological grading of cartilage
degradation in CBA and STR mice.
| Histpathological
grade | CBA mice (20 knees/10
mice) | STR mice (20 knees/10
mice) |
|---|
| 0 | 16 | 2 |
| 1 | 2 | 6 |
| 2 | 0 | 3 |
| 3 | 1 | 3 |
| 4 | 1 | 6 |
Evaluation of type II collagen
degradation/synthesis and oxidative stress markers
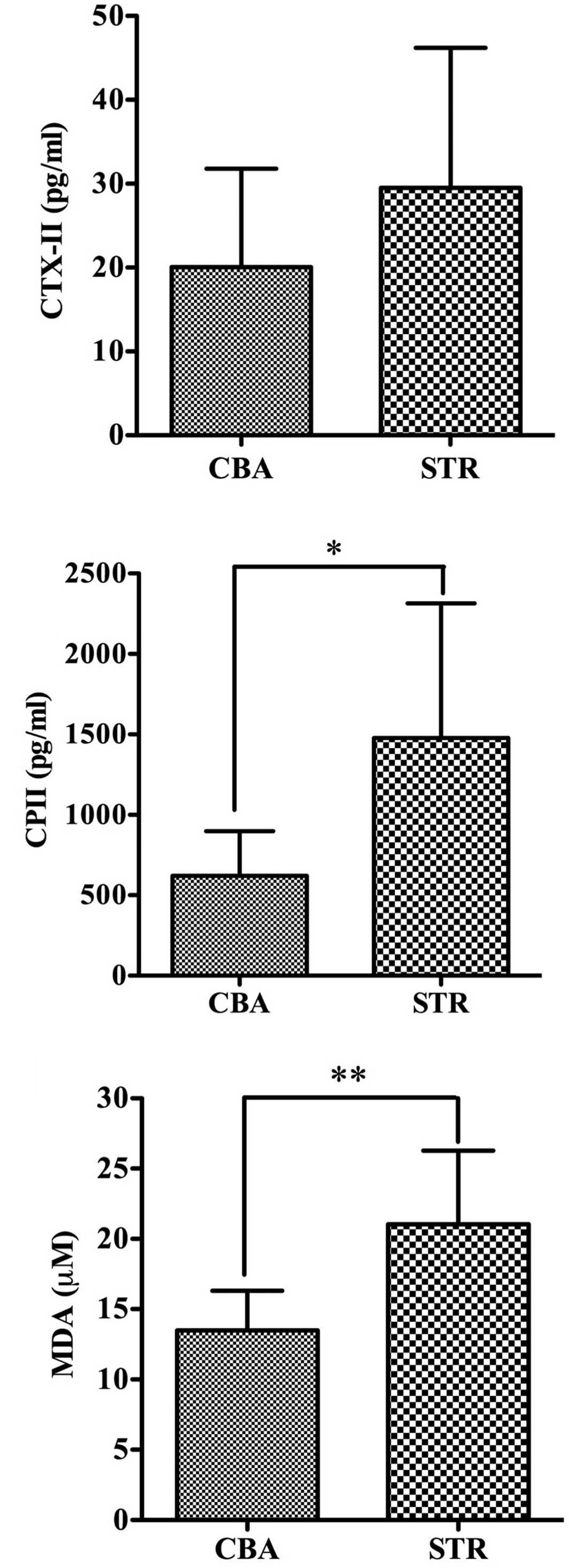
The levels of CTX-II were 29.51±16.98 pg/ml in the
STR mice and 20.04±11.74 pg/ml in the CBA mice at 35 weeks and were
slightly higher in the STR mice than in the CBA mice (p=0.22)
(Fig. 3A). Furthermore, the levels
of CPII were 1478.56±835.75 pg/ml in the STR mice and 621.45±277.7
pg/ml in the CBA mice at 35 weeks, and were significantly elevated
in the STR mice compared to levels in the CBA mice (p<0.05)
(Fig. 3B).
Importantly, the levels of MDA were 21.04±5.23 μM in
the STR/Ort mice and 13.48±5.23 μM in the CBA mice at 35 weeks, and
were significantly higher in the STR mice than levels in the CBA
mice (p<0.01) (Fig. 3C).
Correlation analysis of the biomarkers
for oxidative stress and type II collagen metabolism
To evaluate the effect of oxidative stress on type
II collagen metabolism, correlation analysis was performed between
the levels of serum MDA and CTX-II or CPII at 35 weeks of age in
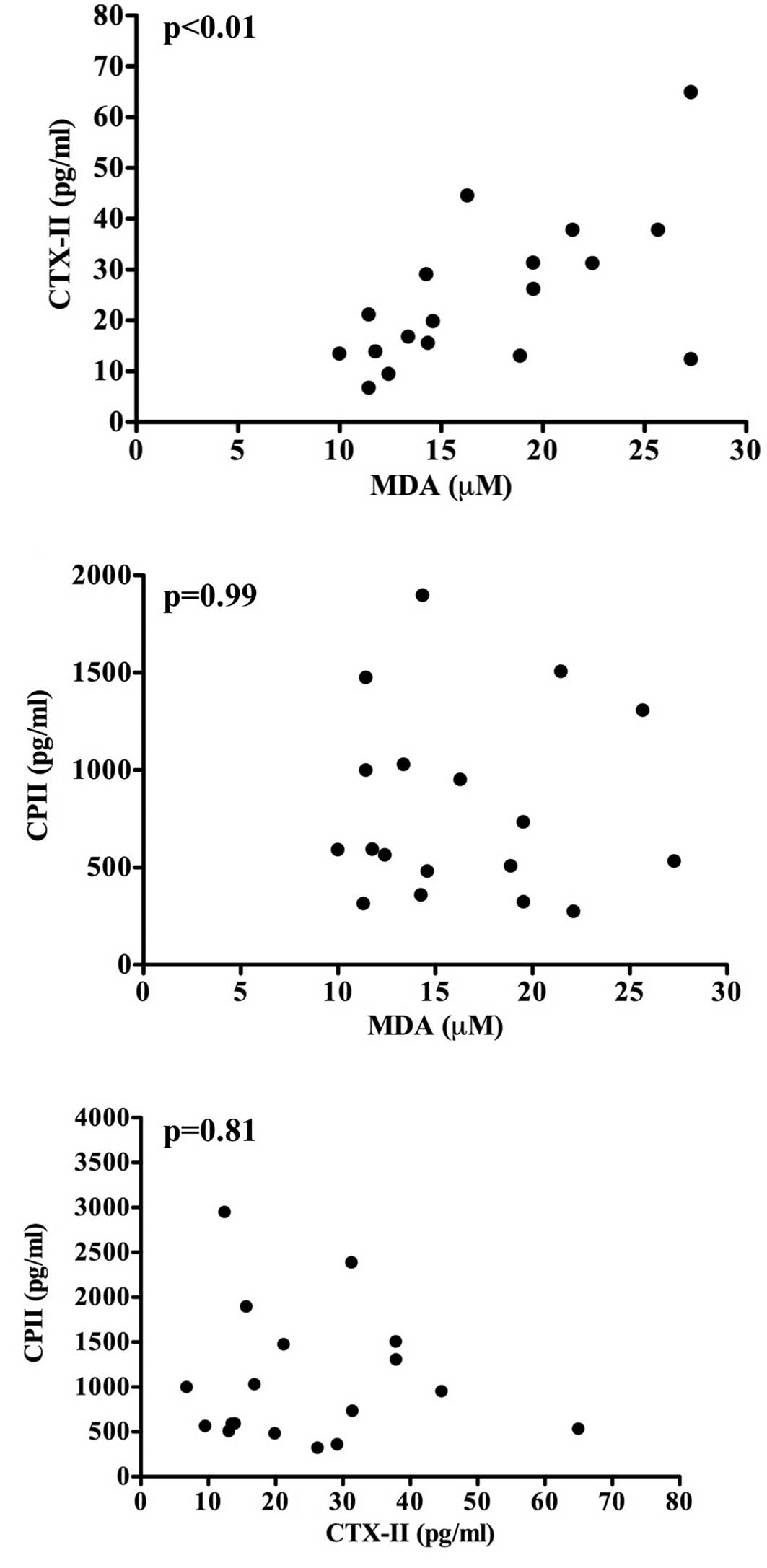
the CBA and STR mice. Notably, the MDA levels were correlated with
the CTX-II levels (r=0.55, p<0.05) (Fig. 4A); however, the MDA levels were not
correlated with the CP-II levels (r=−0.06, p=0.81) (Fig. 4B). These observations indicate that
oxidative stress (the elevation of MDA levels) is associated with
the degradation, but not the synthesis of type II collagen in
articular cartilage, and that the levels of CTX-II and CPII are
unlikely to be changed in parallel within the body, although the
levels of both type II collagen degradation and synthesis markers
(CTX-II and CPII, respectively) are elevated in osteoarthritic STR
mice (Fig. 3A and B). To test the
latter possibility, the correlation between CTX-II and CPII levels
was analyzed. As expected, there was no significant correlation
between the two parameters (r=0.003, p=0.99) (Fig. 4C).
Correlation analysis of oxidative stress
and serum lipids
A higher level of MDA in the obese and
hyperlipidemic STR mice (Fig. 2B and
C) suggests a possibility that the MDA levels may be associated
with serum lipid levels. To test this, we analyzed the correlation
between the serum levels of MDA and total cholesterol or
triglyceride. As expected, there was a significant correlation
between serum MDA and lipid (total cholesterol or triglyceride)
levels (r=0.63, p<0.01 and r=0.53, p<0.05, respectively)
(Fig. 5A and B). Importantly,
there was also a significant correlation between MDA levels and
body weight (r=0.47, p<0.05) (Fig.
5C).
Discussion
Oxidative stress induces tissue injury via the
production of ROS. Among biomolecules, lipids are particularly
susceptible to oxidation and are transformed into lipid peroxides
by oxidation. The accumulated lipid peroxides are involved, not
only in tissue injury, but also in the development and progression
of various diseases, such as lifestyle-related diseases
(atherosclerosis, diabetes mellitus, dementia and cancer) (18,19).
In addition, ROS are capable of inducing apoptotic cell death in
chondrocytes and, more importantly, they induce the degradation of
aggrecan and collagen in articular cartilage (20,21).
In this context, it has been reported that chondrocyte-derived ROS
mediate degradation of aggrecan and that various antioxidants
prevent the degradation process (22). Together these observations suggest
that oxidative stress is involved in the pathogenesis and
progression of OA via the degradation of articular cartilage
components.
MDA is a toxic aldehyde end-product of lipid
peroxidation, which functions as a key molecule for cellular injury
in both plants and animals. Thus, MDA is widely used as an
indicator of oxidative stress in cells and tissues (16,17).
Furthermore, MDA has been demonstrated to mediate the oxidative
degradation of cartilage collagen (23).
Type II collagen is a major constituent of articular
cartilage, representing 90–95% of the total collagen content and
forming the fibrillar structure that gives cartilage its tensile
strength (24). Among several
reported biomarkers (15),
components of type II collagen are recognized as the most important
biomarkers for OA (25). Actually,
it has been reported that CTX-II levels in patients with knee OA
are significantly higher compared to levels in non-OA controls
(26). Moreover, correlations have
been shown between the CTX-II levels and the stage of OA (26–28),
the radiographic changes of OA (29,30),
or the knee pain in symptomatic OA (31). Thus, CTX-II is considered to be a
reliable marker of cartilage degradation. Furthermore, it has been
suggested that abnormalities in the metabolism (degradation and
synthesis) of type II collagen play a key role in the pathogenesis
of OA (32), and that the
C-propeptide of type II procollagen (CPII), released
extracellularly from the newly synthesized molecule, is directly
related to type II collagen synthesis in healthy and osteoarthritic
articular cartilages (33). Thus,
we utilized CPII as a type II collagen synthesis marker.
Male STR mice spontaneously develop knee OA via the
degeneration of cartilage. Their pathological changes are
comparable to those in human OA. STR mice are slightly obese, and
visceral fat is accumulated in their peritoneal cavities. Moreover,
STR mice exhibit hypercholesterolemia, hypertriglyceridemia,
hyperinsulinemia, insulin resistance, dysregulation of
nonesterified fatty acids and low serum adiponectin, as in human
hyperlipidemic patients. Thus, STR mice can be used as a model for
investigating the involvement of dyslipidemia in the underlying
mechanism for OA (12).
In the present study, we revealed that the body
weight was significantly higher in the STR mice compared to that of
the control CBA mice during almost the entire experimental period
(Fig. 2A), and that total
cholesterol and triglyceride levels were also significantly higher
in the STR mice than those in the control CBA mice (Fig. 2B and C). Furthermore, the level of
MDA, an oxidative stress marker, was significantly higher in the
STR mice than that in the CBA mice (Fig. 3C). To date, large scale clinical
studies, such as the Framingham Study, have indicated a correlation
between body mass index and the urinary 8-epi-prostaglandin-F2α
level (a parameter of oxidative stress), an increase in the
oxidative stress level due to obesity (34) and a close association between
visceral fat accumulation (metabolic syndrome) and oxidative stress
(35).
Based on these findings, our observations strongly
suggest that the oxidative stress level is higher in obese and
hyperlipidemic STR mice than in CBA mice. In fact, there was a
significant correlation between serum MDA and total cholesterol,
triglyceride or body weight (Fig.
5A–C). Moreover, the level of CTX-II, a type II collagen
degradation marker, was higher in the STR mice than in the CBA mice
(Fig. 3A), and histopathological
evaluation demonstrated spontaneous development of OA in the STR
mice. In addition, there was a significant correlation between
serum MDA and CTX-II levels (Fig.
4A). Together these observations indicate that oxidative stress
is involved in the degradation of type II collagen in articular
cartilage, thereby possibly promoting the development of OA in
obese and hyperlipidemic STR mice. To note, the level of CPII, a
type II collagen synthesis marker, was significantly increased in
the osteoarthritic STR mice compared to that of the control CBA
mice (Fig. 4B), although oxidative
stress has been reported to suppress collagen synthesis (36). Since the synthesis of type II
collagen has been reported to increase in OA (33), the higher level of CPII in the STR
mice may indicate a compensatory increase in type II collagen
synthesis during the process of cartilage degradation. The present
finding that oxidative stress is possibly associated with type II
collagen degradation provides a novel insight into the pathogenesis
of secondary OA, which is caused by factors associated with
lifestyle-related diseases (obesity, diabetes and
dyslipidemia).
In conclusion, the present study using obese and
hyperlipidemic STR mice suggests that oxidative stress is
associated with the development of OA, possibly via the degradation
of type II collagen in articular cartilage.
Acknowledgements
The authors would like to thank Dr
Katsumi Miyahara and Dr Atsushi Furuhata (Division of Biomedical
Imaging Research, Juntendo University Graduate School of Medicine)
for the technical expertise in the staining of the articular
tissues. This study was partially supported by the High Technology
Research Center Grant from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1.
|
Shah R, Raska K Jr and Tiku ML: The
presence of molecular markers of in vivo lipid peroxidation in
osteoarthritic cartilage. Arthritis Rheum. 52:2799–2807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hamerman D: The biology of osteoarthritis.
N Engl J Med. 320:1322–1330. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Henrotin YE, Bruckner P and Pujol JP: The
role of reactive oxygen species in homeostasis and degradation of
cartilage. Osteoarthritis Cartilage. 11:747–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Khan IM, Gilbert SJ, Caterson B, Sandell
LJ and Archer CW: Oxidative stress induces expression of
osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine
articular cartilage. Osteoarthritis Cartilage. 16:698–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Harman D: Aging: a theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gey KF: On the antioxidant hypothesis with
regard to arteriosclerosis. Bibl Nutr Dieta. 37:53–91.
1986.PubMed/NCBI
|
|
8.
|
Valvason C, Musacchio E, Pozzuoli A,
Ramonda R, Aldegheri R and Punzi L: Influence of glucosamine
sulphate on oxidative stress in human osteoarthritic chondrocytes:
effects on HO-1, p22 (Phox) and iNOS expression. Rheumatology.
47:31–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Melchiorri C, Meliconi R, Frizziero L,
Silvestri T, Pulsatelli L, Mazzetti I, Borzi RM, Uguccioni M and
Facchini A: Enhanced and coordinated in vivo expression of
inflammatory cytokines and nitric oxide synthase by chondrocytes
from patients with osteoarthritis. Arthritis Rheum. 41:2165–2174.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mathy-Hartert M, Hogge L, Sanchez C,
Deby-Dupont G, Crielaard JM and Henrotin Y: Interleukin-1beta and
interleukin-6 disturb the antioxidant enzyme system in bovine
chondrocytes: a possible explanation for oxidative stress
generation. Osteoarthritis Cartilage. 16:756–763. 2008. View Article : Google Scholar
|
|
11.
|
Henrotin Y, Kurz B and Aigner T: Oxygen
and reactive oxygen species in cartilage degradation: friends or
foes? Osteoarthritis Cartilage. 13:643–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Uchida K, Urabe K, Naruse K, Ogawa Z,
Mabuchi K and Itoman M: Hyperlipidemia and hyperinsulinemia in the
spontaneous osteoarthritis mouse model, STR/Ort. Exp Anim.
58:181–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Walton M: Degenerative joint disease in
the mouse knee joint; histological observations. J Pathol.
123:109–122. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Schunke M, Tillmann B, Bruck M and
Muller-Ruckholtz W: Morphologic characteristics of developing
osteoarthritic lesions in the knee cartilage of STR/IN mice.
Arthritis Rheum. 31:898–905. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rousseau JC and Delmas PD: Biological
markers in osteoarthritis. Nat Clin Pract Rheumatol. 3:346–356.
2007. View Article : Google Scholar
|
|
16.
|
Yagi K: Simple assay for the level of
total lipid peroxides in serum or plasma. Methods Mol Biol.
108:101–106. 1998.PubMed/NCBI
|
|
17.
|
Armstrong D and Browne R: The analysis of
free radicals, lipid peroxides, antioxidant enzymes and compounds
to oxidative stress as applied to the clinical chemistry
laboratory. Adv Exp Med Biol. 366:43–58. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Witztum JL and Steinberg D: Role of
oxidized low density lipoprotein in atherogenesis. J Clin Invest.
88:1785–1792. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kinoshita M, Oikawa S, Hayasaka K,
Sekikawa A, Nagashima T, Toyota T and Miyazawa T: Age-related
increases in plasma phosphatidylcholine hydroperoxide
concentrations in control subjects and patients with
hyperlipidemia. Clin Chem. 46:822–828. 2000.
|
|
20.
|
Carlo MD Jr and Loeser RF: Increased
oxidative stress with aging reduces chondrocyte survival:
correlation with intracellular glutathione levels. Arthritis Rheum.
48:3419–3430. 2003. View Article : Google Scholar
|
|
21.
|
Bates EJ, Harper GS, Lowther DA and
Preston BN: Effect of oxygen-derived reactive species on cartilage
proteoglycanhyaluronate aggregates. Biochem Int. 8:629–637.
1984.PubMed/NCBI
|
|
22.
|
Tiku ML, Gupta S and Deshmukh DR: Aggrecan
degradation in chondrocytes is mediated by reactive oxygen species
and protected by antioxidants. Free Radic Res. 30:395–405. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tiku ML, Allison GT, Naik K and Karry SK:
Malondialdehyde oxidation of cartilage collagen by chondrocyte.
Osteoarthritis Cartilage. 11:159–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Garnero P, Rousseau JC and Delmas PD:
Molecular basis and clinical use of biochemical markers of bone,
cartilage, and synovium in joint disease. Arthritis Rheum.
43:953–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Garnero P, Piperno M, Gineyts E, Christgau
S, Delmas PD and Vignon E: Cross sectional evaluation of
biochemical markers of bone, cartilage, and synovial tissue
metabolism in patients with knee osteoarthritis: relations with
disease activity and joint damage. Ann Rheum Dis. 60:619–626. 2001.
View Article : Google Scholar
|
|
26.
|
Garnero P, Ayral X, Rousseau JC, Christgau
S, Sandell LJ, Daugados M and Delmas PD: Uncoupling of type II
collagen synthesis and degradation predicts progression of joint
damage in patients with knee osteoarthritis. Arthritis Rheum.
46:2613–2624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Garnero P, Conrozier T, Christgau S,
Mathieu P, Delmas PD and Vignon E: Urinary type II collagen
C-telopeptide levels are increased in patients with rapidly
destructive hip osteoarthritis. Ann Rheum Dis. 62:939–943. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Reijman M, Hazes JM, Bierma-Zeinstra SM,
Koes BW, Christgau S, Christiansen C, Uitterlinden AG and Pols HA:
A new marker for osteoarthritis: cross-sectional and longitudinal
approach. Arthritis Rheum. 50:2471–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Jordan KM, Syddall HE, Garnero P, Gineyts
E, Dennison EM, Sayer AA, Delmas PD, Cooper C and Arden NK: Urinary
CTX-II and glucosyl-galactosyl-pyridinoline are associated with the
presense and severity of radiographic knee osteoarthritis in men.
Ann Rheum Dis. 65:871–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Meulenbelt I, Kloppenburg M, Kroon HM, et
al: Urinary CTX-II levels are associated with radiographic subtypes
of osteoarthritis in hip, knee, hand, and facet joints in subjects
with familial osteoarthritis at multiple sites: the GARP study. Ann
Rheum Dis. 65:360–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zhai G, Cicuttini F, Ding C, Scott F,
Garnero P and Jones G: Correlates of knee pain in younger subjects.
Clin Rheumatol. 26:75–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Nelson F, Dahlberg L, Laverty S, Reiner A,
Pidoux I, Ionescu M, Fraser GL, Brooks E, Tanzer M, Rosenberg LC,
Dieppe P and Poole AR: Evidence for altered synthesis of type II
collagen in patients with osteoarthritis. J Clin Invest.
102:2115–2125. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Keaney JF Jr, Larson MG, Vasan RS, Wilson
PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA and
Benjamin EJ: Obesity and systemic oxidative stress: clinical
correlates of oxidative stress in the Framingham Study.
Arterioscler Thromb Vasc Biol. 23:434–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Fujita K, Nishizawa H, Funahashi T,
Shimomura I and Shimabukuro M: Systemic oxidative stress is
associated with visceral fat accumulation and the metabolic
syndrome. Circ J. 70:1437–1442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Siwik DA, Pagano PJ and Colucci WS:
Oxidative stress regulates collagen synthesis and matrix
metalloproteinase activity in cardiac fibroblasts. Am J Physiol
Cell Physiol. 280:C53–C60. 2001.PubMed/NCBI
|