Introduction
Glioblastoma is one of the most malignant forms of
brain tumors and is associated with a poor median patient survival
of approximately 12 months in unselected series (1). In selected patients, multimodal
treatment with radiochemotherapy achieves prolonged survival times
of more than 2 years in 25% of the patients (2). However, extended use of chemotherapy
may lead to myelosuppression, including anemia, which may require
the application of growth factors. Moreover, cognitive impairment
may result from glioma treatment, raising the question whether
neuroprotective agents are useful during combined radiochemotherapy
(3).
Erythropoietin (EPO) is a glycoprotein hormone
primarily known as a regulator of erythropoiesis. EPO-mRNA codes
for a pre-pro-protein with 193 amino acids. During
post-translational modification, 27 amino acids are cut at the
N-terminal and arginin at the C-terminal residue through an
intracellular carboxypeptidase, which results in the 165-amino acid
EPO protein (4,5).
EPO-receptor (EPOR) is a cytokine receptor. Its
extra-cellular domain is similar to the receptors for other
cytokines, such as G-CSF or GM-CSF (6). In erythropoietic precursor cells,
three different splice variants have been identified: full-length
EPOR (f-EPOR), truncated EPOR (t-EPOR) and soluble EPOR (s-EPOR)
(7). All forms have the same
extracellular binding domain, but f-EPOR and s-EPOR have shortened
cytoplasmic and transmembranous components, respectively. The
distinct role of these various forms has yet to be elucidated.
The role of EPO in cancer and its possible
therapeutic use in the management of side effects of neoplastic
disease itself and the effects of anticancer treatment is being
debated. EPO is used to treat anemia associated with neoplastic
disease itself or with antineoplastic chemotherapy (8). Moreover, EPO has been shown to exert
neuroprotective potential during radiotherapy through EPOR
expression on cerebral neurons (3,9). On
the other hand, EPOR has also been detected on glioma cells
(10), raising concern about the
safety of EPO treatment in glioma patients (11).
In the present study, we assessed the expression of
EPO and EPOR in human glioblastomas. The results were correlated
with overall patient survival with respect to age, extent of
resection and antineoplastic treatment. In selected cases, the
expression of both proteins in primary and recurrent tumors was
compared.
Patients and methods
Tumor bank
Cases of glioblastoma, for which the complete
clinical course was documented and enough paraffin-embedded tissue
was available for immunohistochemistry were collected. Material was
available in the archives of the Department of Neuropathology from
patients that underwent surgery between 1998 and 2005 at the
Department of Neurosurgery of the University of Göttingen.
The patients were assigned to 5 treatment groups.
The first group of 36 patients underwent tumor resection only; 10
patients received radiotherapy and adjuvant sequential chemotherapy
with ACNU and temozolomide (150–200 mg/m2, days 1–5/28);
8 patients received temozolomide concomitantly to radiotherapy (75
mg/m2) followed by six cycles of adjuvant temozolomide
(150–200 mg/m2, days 1–5/28); 14 patients received
adjuvant ACNU after radiotherapy; and 22 patients were treated with
adjuvant temozolomide only (150–200 mg/m2, days 1–5/28)
after radiotherapy.
Immunohistochemistry
For immunohistochemical staining, the following
antibodies were used: rabbit anti-ErythropoetinR (C-20) sc-695 and
goat anti-Erythropoetin (N-19) sc-1310, (both from Santa Cruz
Biotechnology, Inc., USA). The bridge antibody was AffiniPure
rabbit anti-goat, lot 77186 (Dianova). EnVision-reagent included in
the Dako Real™ EnVision™ Detection system,
Peroxidase/DAB+, rabbit/mouse, code K5007 (Dako,
Denmark).
Paraffin-embedded tissue blocks were cut into 2- to
4-μm slices and dried overnight at 37°C. Sections were
deparaffinized in xylol and ethanol and washed in demineralized
water. Subsequently, endogenous peroxidase was blocked with
H2O2 for 20 min. Following application of
Tris to buffer pH unspecific binding was blocked with BSA for EPOR
only, since cross-reaction with the bridge antibody was observed
with the EPO protein.
The primary antibody was applied at a concentration
of 2 μg/ml for 60 min. The EPOR antibody was diluted with
Tris-buffer and EPO protein antibody with Tris-buffer plus Tween
reagent 1:200 in order to reduce surface tension and improve
distribution of the antibody. Following 60 min of incubation, the
sections were washed twice with Tris buffer.
Since the Dako Real EnVision™ detection system only
reacts with mouse or rabbit antibodies, an additional rabbit-goat
bridging antibody was applied at 4.5 μl/ml to enable binding
of the goat anti-EPO antibody and incubated for 30 min.
EnVision reagent from the Dako Real EnVision
detection system was used at 100 μl for 30 min as a
secondary antibody to specifically bind to the Fc-fragments of the
EPOR antibody or the bridging antibody for the EPO protein.
To visualise the marked antigen, the color reagent
was synthesised with a mixture of Dako Real™ Substrate Buffer and
Dako Real™ DAB+ Chromogen (concentrated x50) at 50:1.
The solution was applied at 100 μl per slice in a dark wet
chamber for 20 min and then washed four times with demineralized
water. The slices were then stained with hematoxylin for 2 min and
running water for 1 min and then fixed with Cytoseal 60 (Richard
Allen Scientific).
For the positive controls, sections of normal human
kidney tissue were stained using the protocol described. Sections
of glioblastomas and kidney were treated as described, without
primary antibody in the Tris-buffer, to serve as negative controls
using the same staining procedure.
Morphometry
The stained sections were assessed with a regular
light microscope at x100 and x400 magnification, with the help of a
counting net. For each section, the frequency of stained cells was
assessed in 6–8 high-power fields depending on the size of the
probe, and a semiquantitative score was determined: score 1, <5%
positive cells; score 2, 5–20% positivity; score 3, 21–50%
positivity; score 4, >50% positivity. For statistical analysis,
the mean value of all single scores multiplied by 10 was calculated
as the cumulative score, resulting in values from 0 to 40.
Statistical analysis
The data were analyzed with the statistic computer
program Statistica v8.1 based on a significance of p≤0.05. The
Gehan's Wilcoxon test was used to analyze overall survival in
groups divided by age, gender and expression scores. The Chi-square
test was used to assess the survival in therapy groups and groups
of high or low protein and receptor expression. A probable
correlation between gender and expression scores was assessed using
the Mann-Whitney U test. A probable correlation between age and
expression scores and between the expression of EPO and EPOR was
analyzed with the Spearman rank correlation test. Multivariate
analysis, stratified analysis and comparison of sum scores EPO +
EPOR were carried out with the Cox regression model. The comparison
between expression scores of initial and recurrent tumors was
performed with the Wilcoxon signed-rank test.
Results
For immunohistochemical staining, 107
paraffin-embedded probes were collected: 89 primary tumors from 52
male and 37 female patients, of which 13 first and 5 secondary
relapses were available. The cases were diagnosed
neuropathologically as glioblastomas WHO-grade IV, except 2 cases
of gliosarcoma. The median age of patients at diagnosis was 63
years (range 30–81). Only 1 patient was alive at 115 months from
initial diagnosis at the time of analysis. The median survival of
the unselected patients was 9 months (± SD 18.2). Patients ≤63
years old had a mean survival of 13 months, while patients at an
older age were associated with a shorter survival of only 5 months
(p<0.0001)(Table I). Patients
in the surgery only group (n=36) had an average survival of 3.6
months. Patients receiving adjuvant temozolomide after radiotherapy
(n=21) had a mean survival of 16.1 months, while patients receiving
concomitant and adjuvant temozolomide (n=8) had a mean survival of
22.1 months. Patients receiving adjuvant ACNU (n=14) were
associated with an average patient survival of 23.1 months, and
patients receiving concomitant plus adjuvant temozolomide followed
by adjuvant ACNU in second line therapy (n=10) achieved a mean
survival of 25.9 months. The difference in survival times between
the surgery only group and the chemotherapy groups in this
retrospective, not randomized series was statistically different
(p<0.0001). The analysis of the extent of resection showed a
clear benefit for patients with gross total resection (n=45) who
achieved a median survival of 13 months (± SD 23.2), while partial
resection (n=33) was only associated with a survival of 6 months (±
SD 8.6) and biopsy only with 3 months (± SD 6.2).
 | Table I.Median survival in patients with high
or low expression levels of EPO or EPOR. |
Table I.
Median survival in patients with high
or low expression levels of EPO or EPOR.
| p-value | Median survival
advantage with high EPO or EPOR |
|---|
| EPO high vs. low | 0.08 | 6 months, not
significant |
| EPOR high vs.
low | <0.01 | 7 months |
| EPO
(radiochemotherapy) | Not significant | No advantage |
| EPOR
(radiochemotherapy) | 0.04 | 6.5 months |
| EPO (younger
age) | Not significant | No advantage |
| EPOR (younger
age) | 0.02 | 9 months |
| Epo multivariate
analysis | Not significant | No advantage |
| EpoR multivariate
analysis | Not significant | No advantage |
| Cumulative EPO + EpoR
multivariate analysis | 0.07 | No advantage |
| Cumulative EPO +
EpoR | 0.06 | 6.5 months, not
significant |
EPO and EPOR
In some of the probes, there was insufficient tumor
tissue for a statistical analysis of EPO and EPOR. Therefore, 9
probes had to be excluded. For the analysis presented here, 80
probes of first and 13 probes of secondary resections were
included.
Staining with both EPO and EPOR exhibited an
inhomogeneous pattern with preponderance to perinecrotic areas,
mitotic or multinucleated giant cells and microvascular endothelial
proliferates (data not shown).
The median overall survival of the patients in the
EPO and EPOR group was 9 months, the percentage of long-term
survivors over 2 years was 14%. A total of 11 patients (14%)
survived less than 4 weeks (ultrashort-term survivors).
Of the 80 patients in this group, 51% underwent
macroscopically total resection, 38% partial resection and 11%
biopsy only.
EPOR score
The median score for EPOR in 80 probes was 31
(19–40). The patients were
divided into groups based on scores lower (n=30) or higher and
equal to (n=50) the median score.
Low EPOR scores were observed in 36% of the female
and in 38% of the male patients, and high scores in 64% of the
females and in 62% of the males.
The median age in the low-EPOR group was 67 years
and in the high-EPOR group 62.5 years.
A total of 47% of patients in the low-EPOR and 54%
in the high-EPOR group underwent gross total resection, while 53%
in the low-score and 46% in the high-score group received partial
resection or biopsy only. A total of 53% of the patients in the
low-score and 32% in the high-score EPOR group underwent surgery
only, while 47% patients in the low-score and 68% in the high-score
group received chemotherapy in addition to radiotherapy.
EPO score
The overall positivity of EPO protein was higher
than its receptor.
Patients were divided according to scores lower
(n=40) or higher and equal (n=40) to the median score of 35.
A total of 52% of the female and 49% of the male
patients were assigned to the low-score group. The median age in
the low-score group was 65 years, and the median age in the
high-score group was 60.5 years.
Gross total resection was possible in 45% of the
patients in the low-score group and in 57.5% of patients in the
high-score group. Surgery only was performed in 47.5% of the
patients in the low- and 32.5% in the high-score EPO group, while
adjuvant chemotherapy was administered to 52.5% in the low- and to
67.5% in the high-score group.
Correlation analyses
No correlation was found between EPO or EPOR and
gender or age. A highly significant correlation, however, was found
between EPO protein and its receptor EPOR (p<0.000001).
EPO, EPOR and overall survival
The median survival for patients with high
expression of EPOR was 12.5 months; significantly longer than that
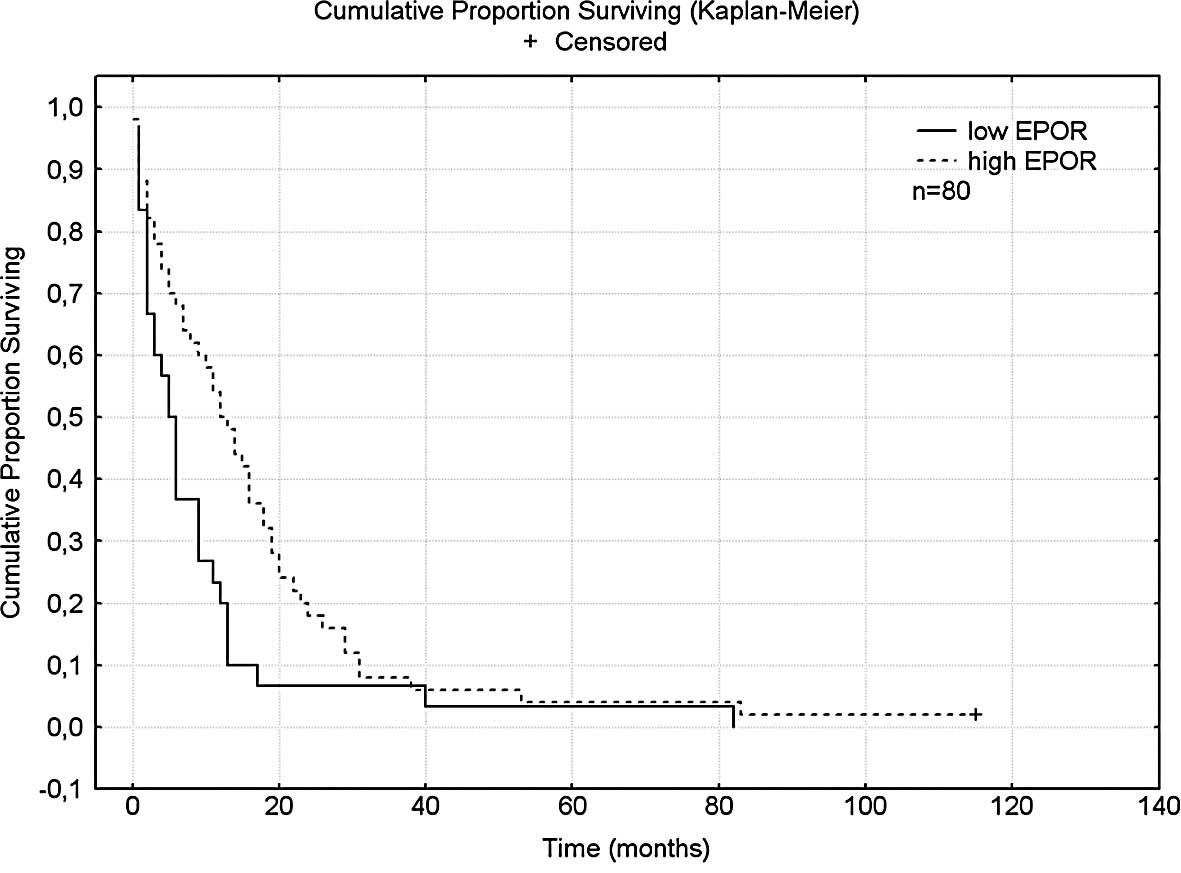
for patients with low expression (5.5 months, p<0.01) (Fig. 1). The median survival for patients
with high expression of EPO protein was 12 months and that for
patients with low expression of EPO protein was 6 months. The
advantage of overall survival, however, was not significant
(p=0.08).
The median survival of patients treated with
radiochemotherapy (n=48) with high and low expression of EPOR was
18 and 11.5 months, respectively (p<0.04).
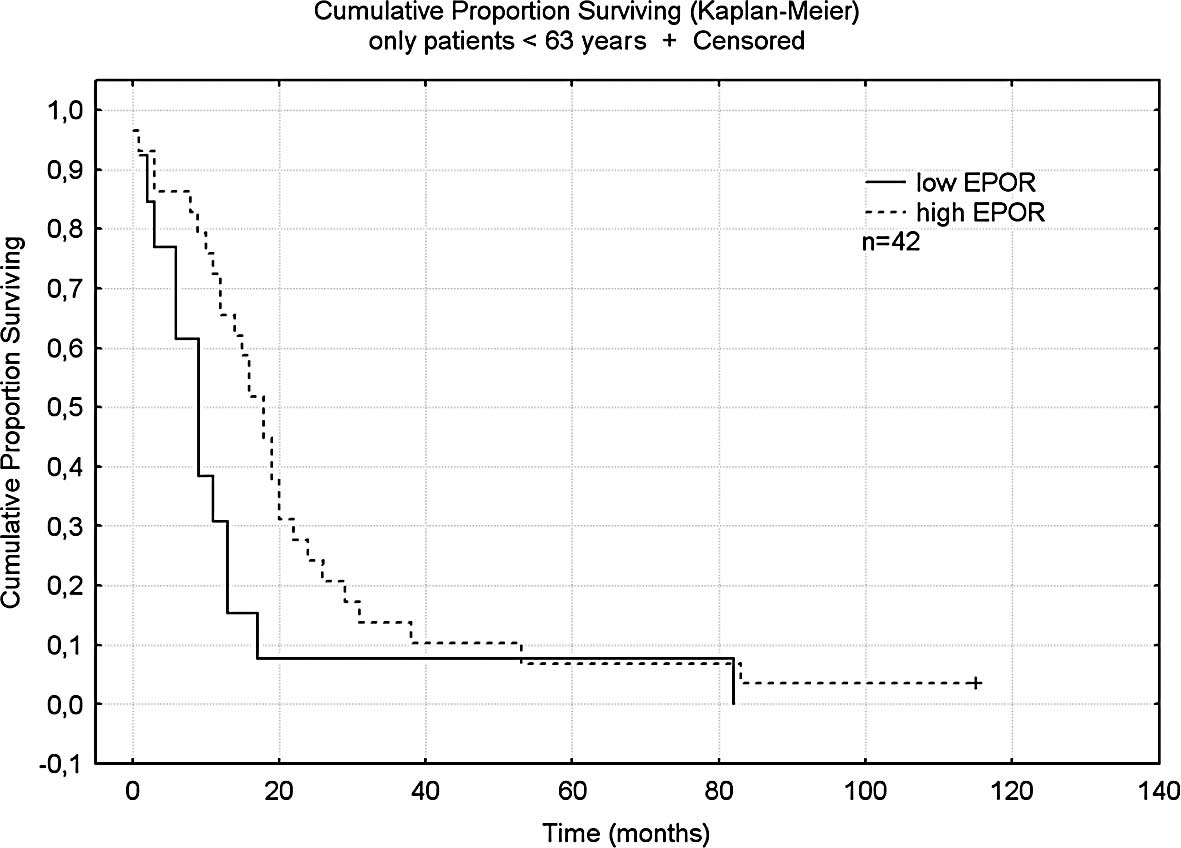
Patients younger than the median age of 63 years
(n=42) with high expression had a mean survival of 18 months, which
was significantly longer than patients in the same age group with
lower expression of EPOR who had a median survival of 9 months
(p<0.02) (Fig. 2). No
difference in survival was noted in patients with different EPO
expression (p=0.10). In separately assessed older patients, EPO or
EPOR had no influence on survival.
Multivariate regression analysis showed the
significant influence of extent of resection (p=0.005), age
(p=0.03) and type of chemotherapy (p=0.0002). The influence of EPOR
on survival failed to remain significant by multivariate analysis
(p=0.096). Likewise, in the EPO group, the extent of resection
(p=0.02), age (p=0.04) and type of chemotherapy (p=0.00005) had a
significant impact on the overall survival, while the expression of
EPO protein was not significant (p=0.133).
None of the analyses using the Cox proportional
hazard model and stratifying by age, extent of resection or
adjuvant chemotherapy revealed a significant influence of EPO or
EPOR expression on survival. This may indicate an intercorrelation
effect between the scores of expression and the extent of
resection, age or type of chemotherapy, probably due to an
imbalanced distribution of high and low expression of EPO and EPOR
in the subgroups of resection, age and chemotherapy.
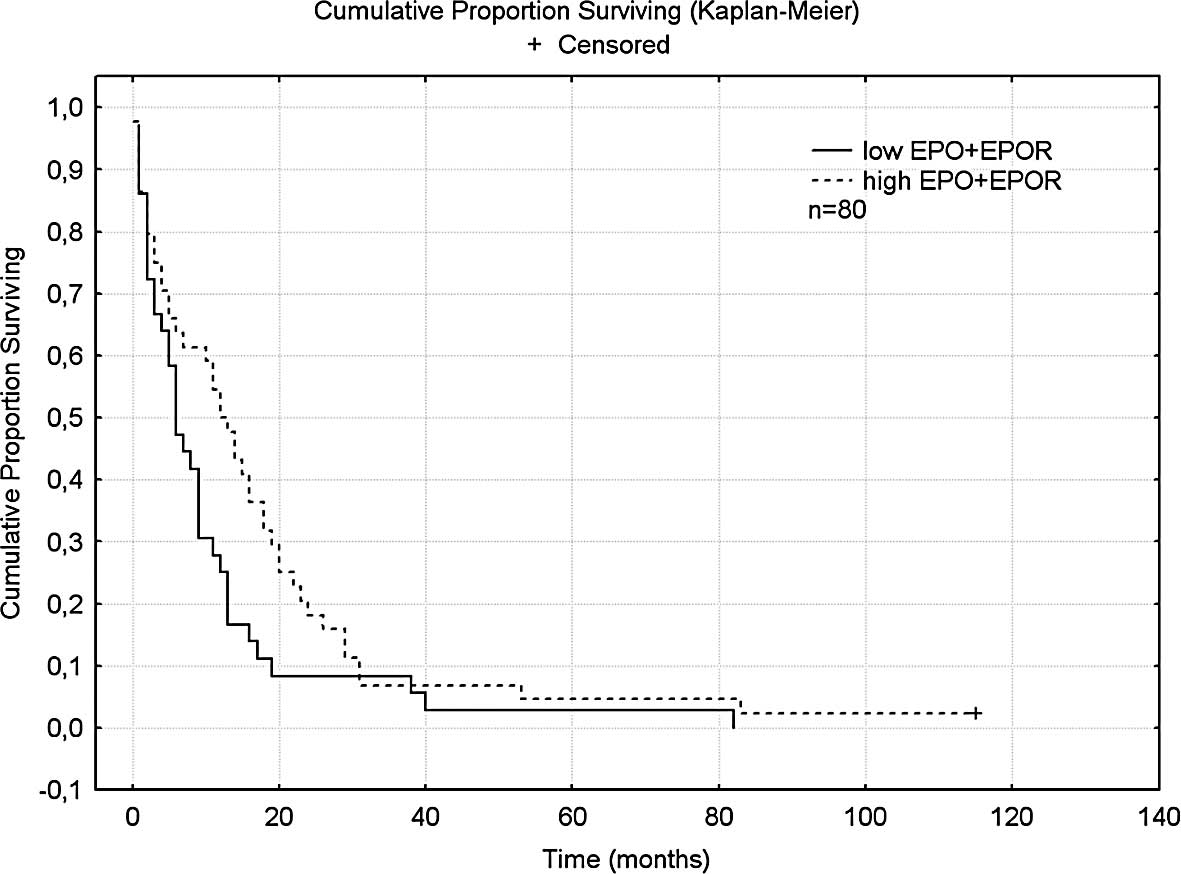
When EPO and EPOR were added for a cumulative score,
a longer survival of 12.5 months was found for patients with high
cumulative scores as compared to 6 months for patients with low
scores; this result just failed to reach significance (p=0.058)
(Fig. 3). This result also failed
to be significant in the multivariate analysis, including age,
extent of resection and adjuvant therapy (p=0.07).
The analysis of EPOR in 13 paired tumors showed a
mean score of 32.7 in primary and 29.6 in recurrent tumors, a
difference which was not significant.
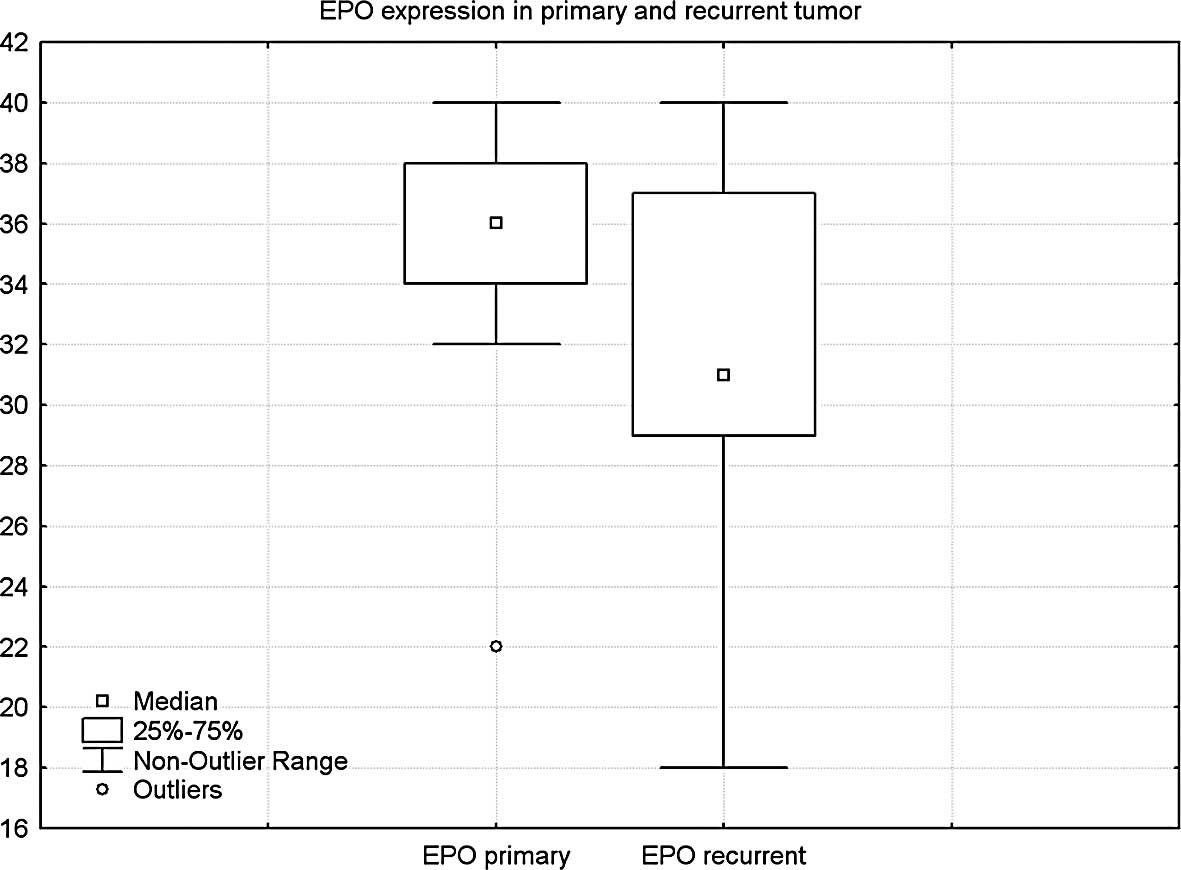
In contrast, in 13 pairs tested for EPO protein, a
significant difference was found between the mean score of 35.1 in
primary and 31.5 in recurrent tumors (p=0.05) (Fig. 4).
Discussion
The present study assessed the impact of EPO and
EPOR expression on the prognosis of patients with glioblastoma. No
negative association between EPO or EPOR and survival was found,
but rather a trend towards prolonged survival in certain subgroups.
These findings indicate that the therapeutic application of EPO may
not have a negative effect on glioma growth in vivo.
EPO is used for various indications in neoplastic
disease. Anemia, caused by systemic cancer or chemotherapy, may
require the use of hematopoietic growth factors as a symptomatic
treatment (8). The tumor itself
may be resistant to apoptosis-inducing stimuli such as ionizing
irradiation or chemotherapy through intratumoral hypoxia, which is
a frequent condition of solid tumors (12). An increased hemoglobin level
increases intratumoral oxygenation (13), which may lead to improved response
to radiation and chemotherapy (14,15).
Therefore, EPO has been used in various studies to increase tumor
oxygenation in order to augment the effect of antineoplastic
therapy (16–18). A specific indication during brain
tumor treatment may protect against the cognitive impairment caused
by radiochemotherapy. Neuroprotective effects of EPO have been
shown to be exerted via EPOR expressed in cerebral neurons during
radiotherapy (3,9).
Initial concerns concerning the use of EPO have been
raised by the detection of EPOR in glioma cells (10,19).
In functional studies, EPO protected cultured glioblastoma cells
from cisplatin cytotoxicity and promoted their invasiveness
(11). Hassouna et al
observed no growth-stimulating effect of EPO in cell culture and no
growth promotion in experimental mouse glioma (20). Resistance to induction of apoptosis
by ionizing irradiation and temozolomide, however, was found to
increase in 3 of 4 cell lines when EPO was applied concomitantly.
An initial immunohistochemical study revealed no negative impact of
EPOR expression on glioma cells. In the subgroup of younger
patients, a more favorable survival was found to be associated with
higher levels of EPOR (21). In
the present study, EPO protein was analyzed in addition to its
receptor. Moreover, we assessed the postoperative treatment and
thus were able to include relevant clinical prognostic factors into
our analysis. In line with the results of Mittelbronn et al,
a positive correlation was found between EPOR and survival
particularly in younger patients. Moreover, higher levels of EPO
were also associated with better survival. In light of the in
vitro results of Hassouna et al who found an increased
resistance to radiochemotherapy, we assessed the impact of EPO and
EPOR in patients receiving radiochemotherapy and found that higher
levels of EPOR tended to be associated with longer survival
(p<0.04). Notably, we observed a significant trend towards
reduced levels of EPO in recurrent as opposed to primary tumors.
Although this trend was not significant for EPOR in this small
group, the progression to more resistant recurrent tumors was
rather associated with a reduction than with increased activity of
the EPO-EPOR system.
These immunohistochemical results do not indicate a
negative effect of EPO on glioma growth in vivo. This
finding, however, is not sufficient to propagate the broad
prophylactic use of EPO, i.e., for neuroprotection during glioma
treatment. In various systemic tumors, EPO was applied to sensitize
tumors for radiation or chemotherapy. While certain studies
observed beneficial effects (16–18),
a lower survival rate was found in head-and-neck and breast cancer
(22,23). Apart from unclear effects on the
tumor growth itself, systemic side effects including deep venous
thrombosis or arterial hypertension may have worsened the patient
outcome (24).
Based on the data presented here, symptomatic
treatment of anemia in glioma patients with EPO appears to be safe
and without the risk of accelerated tumor growth. A prophylactic
use of EPO aimed at neuroprotection or at enhancing the effect of
adjuvant therapy is not recommended with respect to the results of
functional studies. Further experiments are required to address
this issue.
References
|
1.
|
Davis FG, McCarthy BJ, Freels S, Kupelian
V and Bondy ML: The conditional probability of survival of patients
with primary malignant brain tumors: surveillance, epidemiology,
and end results (SEER) data. Cancer. 85:485–491. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Erbayraktar S, de Lanerolle N, de
Lotbiniere A, et al: Carbamylated erythropoietin reduces
radiosurgically-induced brain injury. Mol Med. 12:74–80. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mulcahy L: The erythropoietin receptor.
Semin Oncol. 28:19–23. 2001. View Article : Google Scholar
|
|
5.
|
Sasaki R, Masuda S and Nagao M:
Erythropoietin: multiple physiological functions and regulation of
biosynthesis. Biosci Biotechnol Biochem. 64:1775–1793. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Leyland-Jones B: Evidence for
erythropoietin as a molecular targeting agent. Semin Oncol.
29:145–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nakamura Y, Komatsu N and Nakauchi H: A
truncated erythropoietin receptor that fails to prevent programmed
cell death of erythroid cells. Science. 257:1138–1141. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Khan FA, Shukla AN and Joshi SC: Anaemia
and cancer treatment: a conceptual change. Singapore Med J.
49:759–764. 2008.PubMed/NCBI
|
|
9.
|
Smith RE Jr: Erythropoietic agents in the
management of cancer patients. Part 2: studies on their role in
neuroprotection and neurotherapy. J Support Oncol. 2:39–49.
2004.PubMed/NCBI
|
|
10.
|
Berdel WE, Oberberg D, Reufi B and Thiel
E: Studies on the role of recombinant human erythropoietin in the
growth regulation of human nonhematopoietic tumor cells in vitro.
Ann Hematol. 63:5–8. 1991. View Article : Google Scholar
|
|
11.
|
Mohyeldin A, Dalgard CL, Lu H, et al:
Survival and invasiveness of astrocytomas promoted by
erythropoietin. J Neurosurg. 106:338–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Molls M, Stadler P, Becker A, Feldmann HJ
and Dunst J: Relevance of oxygen in radiation oncology. Mechanisms
of action, correlation to low hemoglobin levels. Strahlenther
Onkol. 174:13–16. 1998.PubMed/NCBI
|
|
13.
|
Kelleher DK, Mattheinsen U, Thews O and
Vaupel P: Blood flow, oxygenation, and bioenergetic status of
tumors after erythropoietin treatment in normal and anemic rats.
Cancer Res. 56:4728–4734. 1996.PubMed/NCBI
|
|
14.
|
Brizel DM, Sibley GS, Prosnitz LR, Scher
RL and Dewhirst MW: Tumor hypoxia adversely affects the prognosis
of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys.
38:285–289. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Höckel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
16.
|
Ning S, Hartley C, Molineux G and Knox SJ:
Darbepoietin alpha potentiates the efficacy of radiation therapy in
mice with corrected or uncorrected anemia. Cancer Res. 65:284–290.
2005.PubMed/NCBI
|
|
17.
|
Pinel S, Barberi-Heyob M, Cohen-Jonathan
E, Merlin JL, Delmas C, Plenat F and Chastagner P:
Erythropoietin-induced reduction of hypoxia before and during
fractionated irradiation contributes to improvement of
radioresponse in human glioma xenografts. Int J Radiat Oncol Biol
Phys. 59:250–259. 2004. View Article : Google Scholar
|
|
18.
|
Thews O, Koenig R, Kelleher DK, Kutzner J
and Vaupel P: Enhanced radiosensitivity in experimental tumours
following erythropoietin treatment of chemotherapy-induced anaemia.
Br J Cancer. 78:752–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Westphal G, Niederberger E, Blum C, et al:
Erythropoietin and G-CSF receptors in human tumor cells: expression
and aspects regarding functionality. Tumori. 88:150–159.
2002.PubMed/NCBI
|
|
20.
|
Hassouna I, Sperling S, Kim E, et al:
Erythropoietin augments survival of glioma cells after radiation
and temozolomide. Int J Radiat Oncol Biol Phys. 72:927–934. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mittelbronn M, Capper D, Bunz B, et al: De
novo erythropoietin receptor (EPO-R) expression in human neoplastic
glial cells decreases with grade of malignancy but is favourably
associated with patient survival. Neuropathol Appl Neurobiol.
33:299–307. 2007. View Article : Google Scholar
|
|
22.
|
Henke M, Laszig R, Rube C, et al:
Erythropoietin to treat head and neck cancer patients with anaemia
undergoing radiotherapy: randomised, double-blind,
placebo-controlled trial. Lancet. 362:1255–1260. 2003. View Article : Google Scholar
|
|
23.
|
Leyland-Jones B, Semiglazov V, Pawlicki M,
et al: Maintaining normal hemoglobin levels with epoetin alfa in
mainly nonanemic patients with metastatic breast cancer receiving
first-line chemotherapy: a survival study. J Clin Oncol.
23:5960–5972. 2005. View Article : Google Scholar
|
|
24.
|
Bohlius J, Wilson J, Seidenfeld J, et al:
Recombinant human erythropoietins and cancer patients: updated
meta-analysis of 57 studies including 9353 patients. J Natl Cancer
Inst. 98:708–714. 2006. View Article : Google Scholar : PubMed/NCBI
|