Introduction
Benign prostatic hyperplasia (BPH) occurs frequently
in older men and is associated with lower urinary tract symptoms
causing obstruction of the proximal urethra and urinary flow
disturbances (1). In clinics,
medical treatments for BPH include widely used α-1 antagonists and
5-α-reductase inhibitors. However, the side effects, such as
postural hypotension, erectile dysfunction and ejaculatory
difficulty, disturb the quality of life of these patients (2,3).
Therefore, development of a more effective therapy for the
treatment of BPH is urgent and necessary.
Loperamide is widely used in the clinic for a
variety of diarrheal syndromes, including acute and nonspecific
(infectious) diarrhea (4,5). Recently, we identified opioid
μ-receptor expression in rat prostates, and prostatic
relaxation was induced by the activation of opioid
μ-receptors using loper-amide (6). Loperamide was introduced as a
peripheral agonist of opioid μ-receptors with poor ability
to penetrate the blood-brain barrier (7,8).
Some analgesic agents have also revealed relaxant effects in smooth
muscle (9,10). (+)-Tramadol was found to activate
peripheral opioid μ-receptors to exhibit a
concentration-dependent relaxation of aorta (11). Basically, the opioid
μ-receptors have been divided into 3 subtypes, including
μ-1, μ-2 and μ-3 opioid receptors (12). However, activation of opioid
μ-1 receptors was reported to be link mainly with the
PLC-PKC pathway (13). Since
PLC-PKC signals increase intracellular calcium to induce
vasoconstriction or bladder contraction (14,15),
prostatic relaxation ia not considered to be induced by the
activation of opioid μ-1 receptors.
ATP-sensitive K+ (KATP)
channels have been found to be involved in the relaxation of
urethral smooth muscle (16).
Actually, the opening of the KATP channel is introduced
to lower intracellular Ca+ concentrations (17,18).
Moreover, impairment of the KATP channel appears to be
associated with the dysfunction of the lower urinary tract
(19). However, the role of the
KATP channel in prostatic relaxation remains
obscure.
In an attempt to clarify the subtype of opioid
μ-receptor involved in the regulation of prostatic tone, we
used loperamide as an agonist in order to induce relaxation in
isolated prostates. Specific blockers or antagonists were then
applied to investigate the possible mechanism(s) of loperamide.
Materials and methods
Experimental animals
We obtained 12-week-old male Wistar rats from the
Animal Center of National Cheng Kung University Medical College.
Rats were maintained in a temperature-controlled room (25±1°C)
under a 12 h light-dark cycle (lights on at 06:00). All rats were
given water and fed standard chow (Purina Mills, LLC, St. Louis,
MO, USA) ad libitum. All animal-handling procedures were
performed according to the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health, as well as the
Guidelines of the Animal Welfare Act.
Preparation of isolated prostate
strips
In all prostatic experiments, the isolated prostates
from Wistar rats were used. Each rat was sacrificed by decapitation
under anesthesia with pento-barbital (50 mg/kg). Following our
previous study, the prostate strips were rapidly removed and placed
in oxygenated Krebs' buffer (95% O2, 5% CO2).
After the prostate strips were carefully freed from fat and
connective tissue, the strips were then mounted in organ baths
filled with 10 ml of oxygenated Krebs' buffer (95% O2,
5% CO2) at 37°C containing (in mmol/l) NaCl 135; KCl 5;
CaCl2 2.5; MgSO4 1.3;
KH2PO4 1.2; NaHCO3 20; and
D-glucose 10 (pH 7.4).
Each preparation was connected to strain gauges
(FT03; Grass Instrument, Quincy, MA, USA). Isometric tension was
recorded using Chart software (MLS023, Powerlab; ADInstruments,
Bella Vista, NSW, Australia). Strips were mounted and allowed to
stabilize for 2 h. Each preparation was then gradually stretched to
achieve an optimal resting tension of 0.5 g.
Prostatic relaxation caused by
loperamide
After the resting tension had stabilized, a solution
of phenylephrine (Sigma-Aldrich, St. Louis, MO, USA) or KCl
prepared in distilled water was added into bathing buffer to induce
a rapid increase in prostatic tone followed by stable constriction
(tonic contraction). The final concentration in the organ bath for
phenylephrine was 1 μmol/l and for KCl, 50 mmol/l,
respectively. Prostate strips in the treatment group were exposed
to loperamide (0.1–10 μmol/l) to observe the decrease in
tonic contraction (vasodilatation). Relaxation was expressed as the
percent decrease in maximal tonic contraction.
Concentration-relaxation curves were generated in cumulative
fashion.
Effects of blockers on loperamide-induced
prostatic relaxation
Prostate strips were exposed to glibenclamide
(Research Biochemical, Wayland, MA, USA) or opioid
μ-receptor antagonist, cyprodime or naloxonazine (Tocris
Cookson, Bristol, UK), for 15 min before the addition of loperamide
into the organ bath. In addition, the inhibitor of cyclic AMP
phosphodiesterase (IBMX) or protein kinase A (H-89) was
administered in the same manner. Changes in relaxation caused by
loperamide were compared with that in the vehicle (distilled
water)-treated controls.
Statistical analysis
All values are presented as the mean ± SEM for a
given number of animals or samples. Analysis of variance and
Dunnett's post hoc test were used to evaluate the significance
between groups. P<0.05 was considered to be a significant
difference.
Results
Effect of opioid receptor blockade on
loperamide-induced prostatic relaxation
Prostate strips were strongly contracted by the
application of phenylephrine (1 μmol/l) or KCl (50 mmol/l).
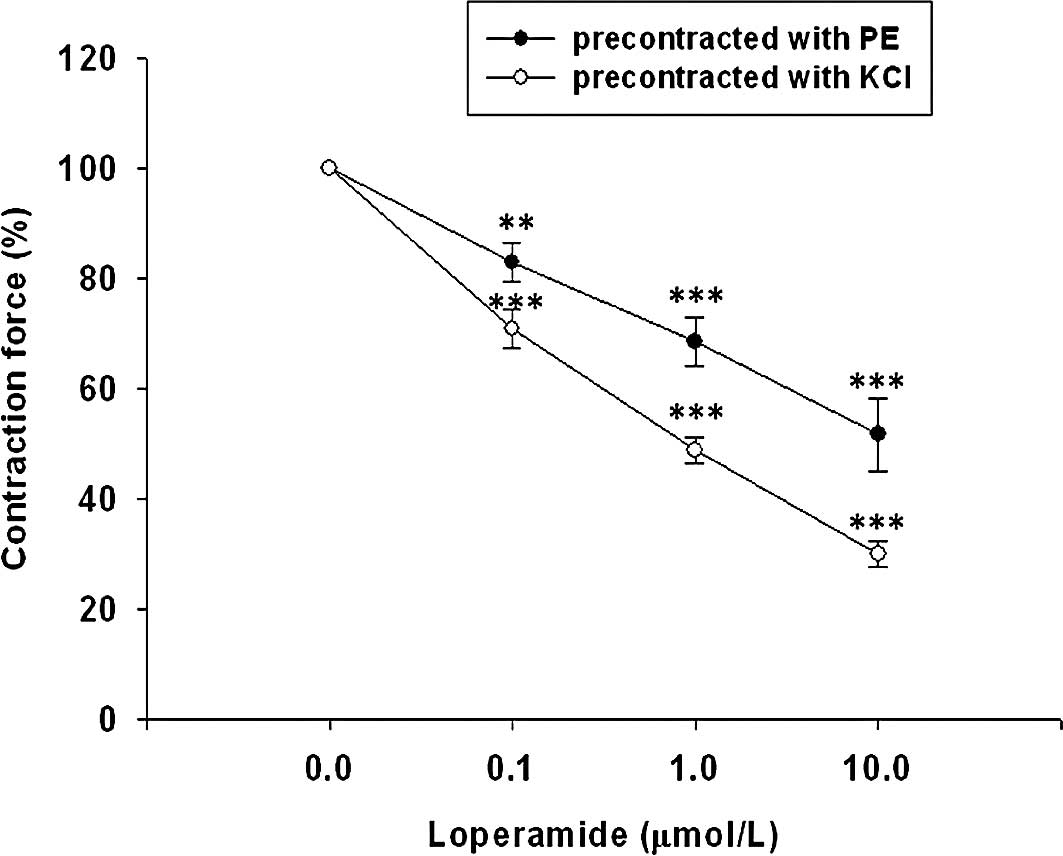
As shown in Fig. 1, loperamide
dilated both phenylephrine- and KCl-contracted prostate strips in a
concentration-dependent manner. At the maximal concentration tested
(10 μmol/l), loperamide significantly attenuated the tonic
contraction of prostate strips induced by phenylephrine to
52.79±6.10% of the maximal contraction. Similarly, 10 μmol/l
loperamide also lowered KCl-induced tonic constriction to
30.06±2.19% of the maximal contraction. Cyprodime (0.01–0.1
μmol/l) produced a significant and concentration-dependent
attenuation of the relaxant effect of loperamide on the tonic
contraction of phenylephrine-precontracted prostate strips. The
prostatic relaxation due to loperamide in KCl-pretreated prostate
strips was also abolished in a similar manner in the presence of
cyprodime (Table I). In addition,
naloxonazine failed to abolish the relaxant effect of loperamide on
tonic contraction in phenylephrine (1 μmol/l)-precontracted
prostate strips at a higher concentration (0.1 μmol/l). As
shown in Table I, the prostatic
relaxation by loperamide in KCl (50 mmol/l)-precontracted prostate
strips was also not reversed by naloxonazine even at a higher
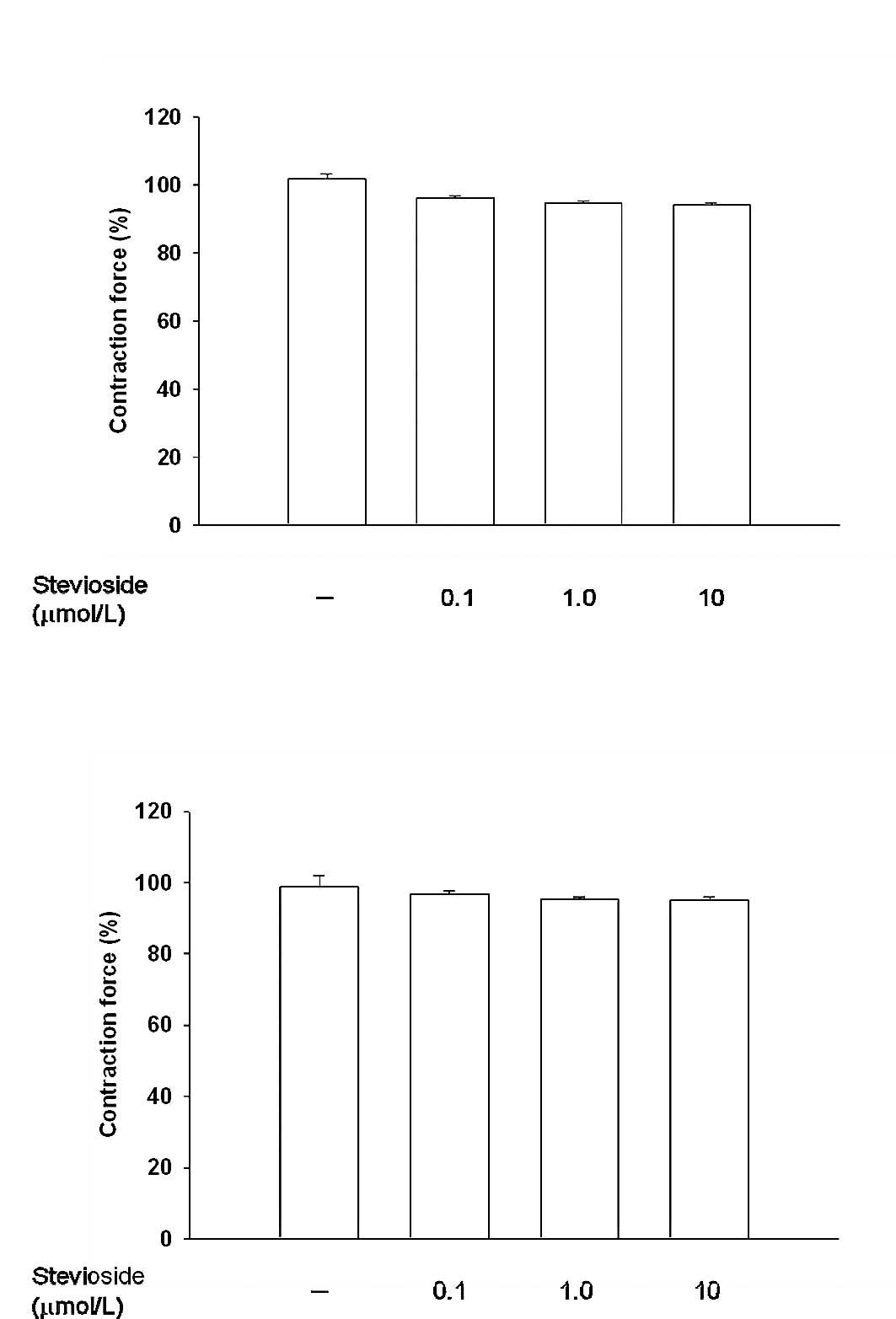
concentration. Also, treatment with stevioside at a dose sufficient
to activate the opioid μ-1 receptor as described previously
(20) failed to modify muscle tone
in either the phenylephrine- or KCl-contracted prostate strips
(Fig. 2).
 | Table I.Inhibitory effect of cyprodime or
naloxonazine on the relaxation of loperamide (10 μmol/l) in
isolated prostates precontracted with 1 μmol/l phenylephrine
(PE) or 50 mmol/l KCl. |
Table I.
Inhibitory effect of cyprodime or
naloxonazine on the relaxation of loperamide (10 μmol/l) in
isolated prostates precontracted with 1 μmol/l phenylephrine
(PE) or 50 mmol/l KCl.
| PE (%) | KCl (%) |
|---|
| Loperamide (10
μmol/l) | | |
| + Vehicle | 55.49±6.16 | 35.18±3.30 |
| + Cyprodime | | |
| 0.01
μmol/l | 76.48±1.75b | 72.58±1.47c |
| 0.10
μmol/l | 90.69±0.80c | 87.59±0.47c |
| +
Naloxonazine | | |
| 0.01
μmol/l | 57.90±2.73 | 38.58±3.38 |
| 0.10
μmol/l | 66.97±1.31 | 41.17±3.93 |
Role of ATP-sensitive K+
(KATP) channels in loperamide-induced prostatic
relaxation
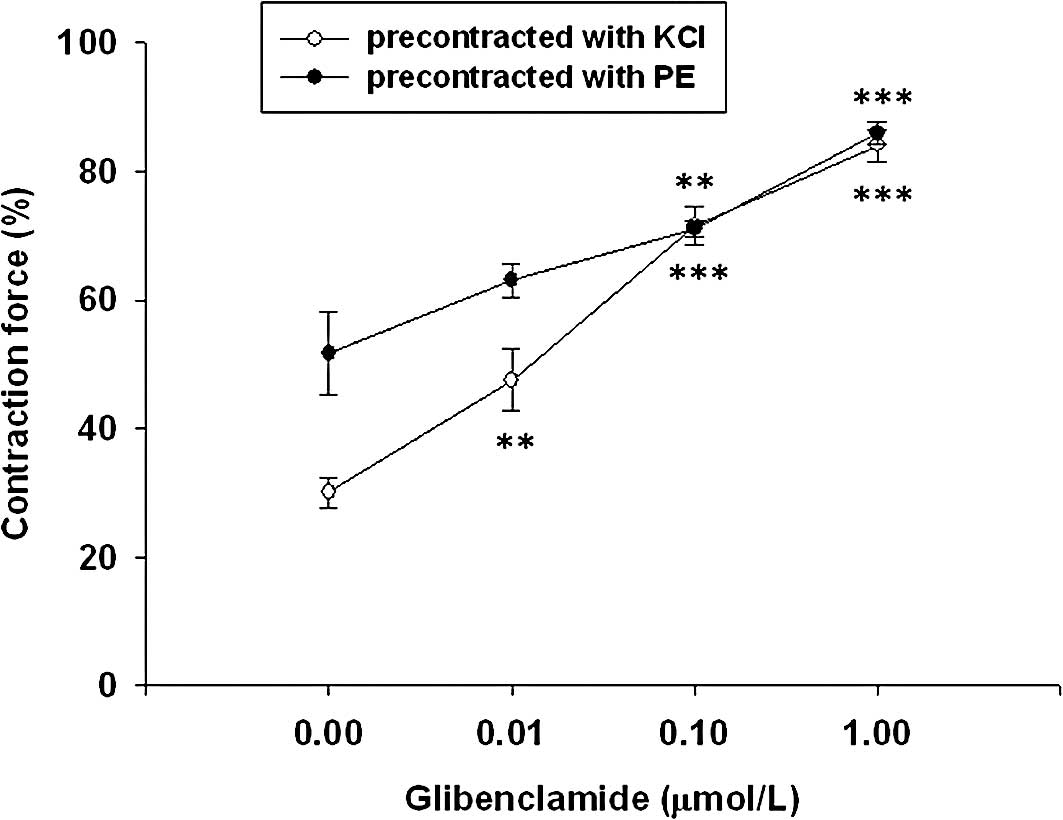
Glibenclamide produced a concentration-dependent
(0.01–1 μmol/l) attenuation of the relaxant effect of
loperamide on tonic contraction of phenylephrine (1
μmol/l)-precontracted prostate strips. The prostatic
relaxation by loperamide in KCl (50 mmol/l)-pre-treated prostate
strips was also abolished in a similar manner by the treatment of
glibenclamide (Fig. 3).
Role of cAMP and PKA in
loperamide-induced prostatic relaxation
In the present study, forskolin (10 μmol/l),
a direct activator of adenylate cyclase, was used as a positive
control to increase cyclic AMP (cAMP) as described previously
(21). In prostate strips
precontracted with phenylephrine (1 μmol/l) or KCl (50
mmol/l), forskolin-induced relaxation was also abolished by
pretreatment with glibenclamide (1 μmol/l). Moreover,
prostatic relaxation by forskolin was increased by
3-isobutyl-1-methylxanthine (IBMX) at a concentration (10
μmol/l) sufficient to inhibit cAMP-phosphodiesterase
(22), and was decreased by H-89
at a concentration (1 μmol/l) enough to abolish the protein
kinase A (PKA) (23). The
loperamide-induced prostatic relaxation was also modified by these
agents in a similar manner. Results showed that prostatic
relaxation induced by loperamide was increased by IBMX and
attenuated by H-89 (Table II).
 | Table II.Effects of the inhibitors for cAMP
phosphodiesterase or protein kinase A (PKA) on relaxation induced
by loperamide (10 μmol/l) or forskolin (10 μmol/l) in
isolated prostates precontracted with 1 μmol/l phenylephrine
(PE) or 50 mmol/l KCl. |
Table II.
Effects of the inhibitors for cAMP
phosphodiesterase or protein kinase A (PKA) on relaxation induced
by loperamide (10 μmol/l) or forskolin (10 μmol/l) in
isolated prostates precontracted with 1 μmol/l phenylephrine
(PE) or 50 mmol/l KCl.
| PE (%) | KCl (%) |
|---|
| Loperamide (10
μmol/l) | | |
| + Vehicle | 59.69±3.54 | 33.08±1.89 |
| + IBMX (10
μmol/l) | 46.58±1.87b | 25.12±0.86b |
| + H-89 (1
μmol/l) | 86.22±2.10c | 85.00±0.58c |
| Forskolin (10
μmol/l) | | |
| + Vehicle | 28.47±1.83 | 24.48±1.92 |
| + Glibenclamide
(1 μmol/l) | 83.17±2.17c | 80.21±1.77c |
| + IBMX (10
μmol/l) | 11.69±1.29c | 10.47±0.28c |
| + H-89 (1
μmol/l) | 86.08±1.69c | 83.03±1.17c |
| IBMX (10
μmol/l) | 94.64±0.88c | 90.26±1.01c |
| H-89 (1
μmol/l) | 95.78±1.07c | 91.25±1.18c |
| Glibenclamide (1
μmol/l) | 95.79±1.16c | 90.32±0.69c |
Discussion
In the present study, we found that loperamide
caused a dose-dependent relaxation in prostate strips of rats
precontracted with phenylephrine or KCl. The action of loperamide
appears to be related to the activation of opioid receptors in
peripheral tissue as loperamide does not cross the central nervous
system (8). Moreover,
loperamide-induced action is effectively abolished by cyprodime,
suggesting an activation of opioid μ-receptors by loperamide
in prostatic relaxation. However, the action of loperamide was not
reversed by naloxonazine even at the dose sufficient to block
opioid μ-1 receptors. In addition, relaxation was not
induced by agonist specific for opioid μ-1 receptors
(Fig. 2). Thus, mediation of
opioid μ-1 receptors was unlikely involved in the prostatic
relaxation of loperamide.
Thus, another opioid μ-receptor involved in
this action of loperamide was considered. There is no doubt that
loperamide is an agonist of peripheral opioid μ-receptors
(8,24). Recently, the opioid
μ-receptors have been divided into 3 subtypes, including
μ-1, μ-2 and μ-3 (25–27).
Analgesic action through the activation of opioid μ-1
receptors has been reported to exert spinal antinociception
(28,29). In addition, activation of the
opioid μ-1 receptors seems to be related to smooth muscle
contraction via the PLC-PKC pathway (14,15).
Activation of the opioid μ-2 receptors was found to
participate in the relaxation of guinea pig ileum and inhibition of
gastrointestinal transit (30,31).
Moreover, opioid μ-3 receptors are mostly present in
endothelial cells associated with the production of nitric oxide to
induce vasodilatation (32).
Therefore, mediation of opioid μ-1 or μ-3 receptors
in prostatic relaxation seems unlikely. Taken together, an
activation of opioid μ-2 receptors is more likely to
participate in the action of loperamide in the relaxation of
isolated prostate strips. Since there is no suitable tool or agent
which can aid us in identifying this receptor, we focused on the
subcellular signals of this receptor as an alternative method.
Potassium channels play an important role in the
regulation of prostatic contractility in guinea-pig prostate smooth
muscle cells (33). In a previous
study, potassium channel expression was reduced in human BPH and
prostate cancer (34). Moreover,
the ATP-sensitive K+ (KATP) channels are
composed of four inwardly rectifying K+ channel subunits
and four regulatory sulphonylurea receptors (35). The activation of KATP
channels causes hyperpolarization of the cell membrane and
consequently relaxes smooth muscle. In our previous study (36,37),
the relaxation of loperamide in rat prostate strips was abolished
by the pretreatment with glibenclamide at a concentration
sufficient to block KATP channels. Thus, we focused on
the involvement of KATP channels in the prostatic
relaxation induced by loperamide. We then applied forskolin as a
positive reference since forskolin was introduced as a direct
activator of adenylate cyclase which can increase the intracellular
cyclic AMP (cAMP) to activate cAMP-dependent protein kinase (PKA)
for opening KATP channels (23). As shown in Table II, we determined that
forskolin-induced prostatic relaxation was also blocked by
glibenclamide. The prostatic relaxation of forskolin was abolished
by H-89 at a concentration sufficient to block PKA (23) and was enhanced by IBMX at a
concentration sufficient to inhibit cAMP-phosphodiesterase
(22). Similar results were also
observed in prostate strips relaxed by loperamide (Table II). These data suggest that the
possible mechanism for loperamide-induced prostatic relaxation is
mediated through the cAMP-PKA pathway to open KATP
channels, which can explain previous phenomenon for
loperamide-induced prostatic relaxation (6). Therefore, the obtained results
provide novel insights into the action mechanisms of loperamide
particularly in the understanding of prostatic relaxation.
In conclusion, we suggest that activation of opioid
μ-2 receptors to open KATP channels is
responsible for loperamide-induced prostatic relaxation. Therefore,
activation of peripheral opioid μ-2 receptors may be a new
target in the development of agents for the management of benign
prostatic hyperplasia.
Acknowledgements
We thank Mr. K.F. Liu for the
technical assistance. The present study was supported in part by a
grant from Chi-Mei Medical Center (CLFHR9829).
References
|
1.
|
Tiwari A, Krishna NS, Nanda K and Chugh A:
Benign prostatic hyperplasia: an insight into current
investigational medical therapies. Expert Opin Investig Drugs.
14:1359–1372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hellstrom WJ and Sikka SC: Effects of
acute treatment with tamsulosin versus alfuzosin on ejaculatory
function in normal volunteers. J Urol. 176:1529–1533. 2006.
View Article : Google Scholar
|
|
3.
|
Roehrborn CG, Marks LS, Fenter T, et al:
Efficacy and safety of dutasteride in the four-year treatment of
men with benign prostatic hyperplasia. Urology. 63:709–715.
2004.PubMed/NCBI
|
|
4.
|
Hanauer SB: The role of loperamide in
gastrointestinal disorders. Rev Gastroenterol Disord. 8:15–20.
2008.PubMed/NCBI
|
|
5.
|
Corazziari E: Role of opioid ligands in
the irritable bowel syndrome. Can J Gastroenterol. 13:71A–75A.
1999.PubMed/NCBI
|
|
6.
|
Cheng JT and Tong YC: Loperamide induced
rat prostate relaxation through activation of peripheral opioid
μ-receptors. Neurourol Urodyn. (In press).
|
|
7.
|
Baker DE: Loperamide: a pharmacological
review. Rev Gastroenterol Disord. 7:S11–S18. 2007.
|
|
8.
|
Nozaki-Taguchi N and Yaksh TL:
Characterization of the anti-hyperalgesic action of a novel
peripheral mu-opioid receptor agonist–loperamide. Anesthesiology.
90:225–234. 1999.PubMed/NCBI
|
|
9.
|
Tagaya E, Tamaoki J, Chiyotani A and Konno
K: Stimulation of opioid mu-receptors potentiates beta
adrenoceptor-mediated relaxation of canine airway smooth muscle. J
Pharmacol Exp Ther. 275:1288–1292. 1995.PubMed/NCBI
|
|
10.
|
Ozdem SS, Batu O, Tayfun F, Yalcin O,
Meiselman HJ and Baskurt OK: The effect of morphine in rat small
mesenteric arteries. Vascul Pharmacol. 43:56–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Raimundo JM, Sudo RT, Pontes LB, Antunes
F, Trachez MM and Zapata-Sudo G: In vitro and in vivo
vasodilator activity of racemic tramadol and its enantiomers in
Wistar rats. Eur J Pharmacol. 530:117–123. 2006. View Article : Google Scholar
|
|
12.
|
Stefano GB: Endogenous morphine: a role in
wellness medicine. Med Sci Monit. 10:ED5:2004.PubMed/NCBI
|
|
13.
|
Liu IM, Liou SS, Chen WC, Chen PF and
Cheng JT: Signals in the activation of opioid mu-receptors by
loperamide to enhance glucose uptake into cultured C2C12 cells.
Horm Metab Res. 36:210–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bova S, Trevisi L, Cima L, Luciani S,
Golovina V and Cargnelli G: Signaling mechanisms for the selective
vasoconstrictor effect of norbormide on the rat small arteries. J
Pharmacol Exp Ther. 296:458–463. 2001.PubMed/NCBI
|
|
15.
|
Cheng TC, Lu CC, Chung HH, et al:
Activation of muscarinic M-1 cholinoceptors by curcumin to increase
contractility in urinary bladder isolated from Wistar rats.
Neurosci Lett. 473:107–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Teramoto N, Yunoki T, Ikawa S, et al: The
involvement of L-type Ca(2+) channels in the relaxant effects of
the ATP-sensitive K(+) channel opener ZD6169 on pig urethral smooth
muscle. Br J Pharmacol. 134:1505–1515. 2001.
|
|
17.
|
Mishra SK and Aaronson PI: A role for a
glibenclamide-sensitive, relatively ATP-insensitive K+
current in regulating membrane potential and current in rat aorta.
Cardiovasc Res. 44:429–435. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Quayle JM and Standen NB: KATP
channels in vascular smooth muscle. Cardiovasc Res. 28:797–804.
1994.
|
|
19.
|
Teramoto N: [Molecular and
electrophysiological investigation of ATP-sensitive K+
channels in lower urinary tract function: the aims for clinical
treatment of unstable detrusor]. Nippon Yakurigaku Zasshi.
121:317–324. 2003.
|
|
20.
|
Yang PS, Lee JJ, Tsao CW, Wu HT and Cheng
JT: Stimulatory effect of stevioside on peripheral mu opioid
receptors in animals. Neurosci Lett. 454:72–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhang L, Bonev AD, Mawe GM and Nelson MT:
Protein kinase A mediates activation of ATP-sensitive K+
currents by CGRP in gallbladder smooth muscle. Am J Physiol.
267:G494–G499. 1994.PubMed/NCBI
|
|
22.
|
Uder M, Heinrich M, Jansen A, et al: cAMP
and cGMP do not mediate the vasorelaxation induced by iodinated
radiographic contrast media in isolated swine renal arteries. Acta
Radiol. 43:104–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wellman GC, Quayle JM and Standen NB:
ATP-sensitive K+ channel activation by calcitonin gene-related
peptide and protein kinase A in pig coronary arterial smooth
muscle. J Physiol. 507:117–129. 1998.
|
|
24.
|
Liu IM, Chi TC, Chen YC, Lu FH and Cheng
JT: Activation of opioid mu-receptor by loperamide to lower plasma
glucose in streptozotocin-induced diabetic rats. Neurosci Lett.
265:183–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chen JC, Smith ER, Cahill M, Cohen R and
Fishman JB: The opioid receptor binding of dezocine, morphine,
fentanyl, butorphanol and nalbuphine. Life Sci. 52:389–396. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kristensen K, Christensen CB and Christrup
LL: The mu1, mu2, delta, kappa opioid receptor binding profiles of
methadone stereoisomers and morphine. Life Sci. 56:PL45–PL50. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Cadet P: Mu opiate receptor subtypes. Med
Sci Monit. 10:MS28–MS32. 2004.PubMed/NCBI
|
|
28.
|
Mizoguchi H, Watanabe H, Hayashi T, et al:
Possible involvement of dynorphin A-(1–17) release via mu1-opioid
receptors in spinal antinociception by endomorphin-2. J Pharmacol
Exp Ther. 317:362–368. 2006.
|
|
29.
|
Sakurada S, Sawai T, Mizoguchi H, et al:
Possible involvement of dynorphin A release via mu1-opioid receptor
on supraspinal antinociception of endomorphin-2. Peptides.
29:1554–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Gintzler AR and Pasternak GW: Multiple mu
receptors: evidence for mu2 sites in the guinea pig ileum. Neurosci
Lett. 39:51–56. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Matsumoto K, Hatori Y, Murayama T, et al:
Involvement of mu-opioid receptors in antinociception and
inhibition of gastrointestinal transit induced by
7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna
speciosa. Eur J Pharmacol. 549:63–70. 2006. View Article : Google Scholar
|
|
32.
|
Stefano GB, Hartman A, Bilfinger TV, et
al: Presence of the mu3 opiate receptor in endothelial cells.
Coupling to nitric oxide production and vasodilation. J Biol Chem.
270:30290–30293. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Oh SJ, Kim KM, Chung YS, Hong EK, Shin SY
and Kim SJ: Ion-channel currents of smooth muscle cells isolated
from the prostate of guinea-pig. BJU Int. 92:1022–1030. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Abdul M and Hoosein N: Reduced Kv1.3
potassium channel expression in human prostate cancer. J Membr
Biol. 214:99–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Mannhold R: KATP channel openers:
structure-activity relationships and therapeutic potential. Med Res
Rev. 24:213–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Tsai CC, Lai TY, Huang WC, Liu IM and
Cheng JT: Inhibitory effects of potassium channel blockers on
tetramethylpyrazine-induced relaxation of rat aortic strip in
vitro. Life Sci. 71:1321–1330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Wong KL, Chan P, Yang HY, et al:
Isosteviol acts on potassium channels to relax isolated aortic
strips of Wistar rat. Life Sci. 74:2379–2387. 2004. View Article : Google Scholar : PubMed/NCBI
|