Introduction
Neuroblastoma is the most common extracranial solid
tumor in childhood and has been associated with marked clinical,
histological and genetic heterogeneity. Most often, the progression
of the clinical course results in a poor response to conventional
treatment. The prognosis of neuroblastoma depends on the genetic
alterations of the cancer cells, which may include non-random
deletions, rearrangements or amplification within the chromosome.
Among these alterations, the most influential appears to be an MYCN
amplification. Since more than 60% of patients die from aggressive
disease progression, it is critical to develop effective
therapeutic agents for the treatment of neuroblastoma, regardless
of the heterogeneous nature and genetic alterations involved.
Arsenic trioxide (As2O3) has
been shown to exert a cytotoxic effect on a variety of tumors,
including acute promyelocytic leukemia, multiple myeloma,
esophageal carcinoma and neuroblastoma, regardless of whether MYCN
is amplified (1). Arsenic trioxide
induces apoptosis via the generation of reactive oxygen species,
the disruption of mitochondrial transmembrane potential (ΔΨm), the
down-regulation of Bcl-2 and the activation of caspases. Arsenic
trioxide-treated cells were shown to manifest DNA damage and cell
cycle arrest at the G0 or G2/M phases.
Imatinib mesylate (Gleevec®, STI-571) is
a selective inhibitor of Bcr-Abl tyrosine kinase, as well as other
tyrosine kinases, such as the PDGF receptor (PDGFR) and c-Kit
(2). STI-571 is now employed for
the treatment of chronic myeloid leukemia and gastrointestinal
stromal tumors, and its potential use in the treatment of other
c-Kit-positive malignancies, including seminoma and acute myeloid
leukemia, is currently being investigated (3). Many neuroblastoma cell lines and
neuroblastoma tissue samples have been found to express PDGFR and
c-Kit. Furthermore, STI-571 has been reported to inhibit the growth
of neuroblastoma cells both in vitro and in vivo, via
the suppression of PDGFR and c-Kit phosphorylation (4).
Since arsenic trioxide and STI-571 have been
identified as good candidates for the treatment of neuroblastoma,
we aimed to determine the effects of STI-571 with arsenic treatment
on the growth of neuroblastoma cells in vitro, in relation
to the state of MYCN amplification and expression, using SH-SY5Y,
SK-N-SH and SK-N-BE(2) cells.
Materials and methods
Cell culture and chemicals
Imatinib mesylate (Gleevec, STI-571), which was
generously provided by Novartis (Basel, Switzerland), was prepared
as a 10-mM stock solution in a sterile solution in DMSO.
Neuroblastoma cell lines, SH-SY5Y (CRL-2266), SK-N-SH (HTB-11) and
SK-N-BE(2) (CRL-2271) (all from
ATCC, Manassas, VA, USA), as well as K562 cells, were cultured in
RPMI-1640 media supplemented with heat-inactivated 10% FBS and
antibiotics.
Proliferation and apoptosis assays
The cells (5×105 cells/ well) were
treated for 72 h with arsenic trioxide, then detached with trypsin,
washed twice in cold PBS, and resuspended in 1X Binding Buffer (10
mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) at a
concentration of 1×106 cells/ml. The cells were then
stained with Annexin V and propidium iodide (PI). After incubation
for 15 min at room termperature in the dark, the cells were
analyzed using the FACSCalibur system (BD, San Jose, CA, USA) with
CellQuest software (BD).
For the 3-(4,5-dimethylthizol-2-yl)2,5-diphenyl
tetrazolium bromide (MTT, M5655; Sigma) assay, the cells were
plated into 96-well microtiter plates at a density of
5x103/150 μl in fresh medium and treated with arsenic
trioxide and/or STI-571. After 72 h, 20 μl of MTT (5 mg/ml in PBS)
was added to each well, and the plates were returned to the
incubator for an additional 4 h. At the end of the incubation
period, the supernatants were discarded by suction and 200 μl of
DMSO was added to all wells in order to dissolve the dark blue
formazan crystals. The plates were then covered with aluminum foil
and gently agitated, then the absorbance was read at a wavelength
of 570 nm.
Immunoblots
The cells were lysed in a lysis buffer containing
150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 50 mM Tris-Cl (pH 7.5),
2 mM sodium orthovanadate, 20 μg/ ml phenylmethylsulfonyl fluoride
(PMSF) and 2 μg/ml of aprotinin. The lysates were sonicated with an
ultrasonic homogenizer (Cole-Parmer, Vernon Hills, IL, USA) and
then centrifuged for 10 min at 11,000 rpm at 4˚C. The clear lysates
were collected, and the protein concentrations of the lysates were
determined using BCA kits (Pierce Chemical Co., Rockford, IL, USA).
The protein was then loaded at 5 μg per lane on a 10% SDS-PAGE gel.
The antibodies used were anti-MYCN Ab (#9405; Cell Signaling
Technology) and β-actin Ab (Sigma). Secondary antibodies were
purchased from BioRad (Hercules, CA, USA).
Small interfering RNA (siRNA)
transfection
siRNA against MYCN (siMYCN; siGENOME SMARTpool
M-003913-01-0005) was purchased from Dharmacon, Inc. (Lafayette,
CO, USA) (5), and the
non-targeting control was obtained from Bioneer (Daejon, Korea).
Transfection was conducted with Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) in accordance with the manufacturer’s
recommendations. The cells were seeded on 96-well plates for the
MTT assay and on 6-well plates for RNA preparation.
RT-PCR
Total RNA was extracted from siRNA-transfected cells
using TRIzol reagent (Invitrogen), and RT-PCR was conducted for the
confirmation of gene expression in the cell lines. The primer
sequences were as follows: MYCN forward, 5′-ACC ACA AGG CCC TCA GTA
CC-3′ and reverse, 5′-GTG CAT CCT CAC TCT CCA CG-3′; GAPDH forward
5′-GTC TTC TCC ACC ATG GAG AA-3′ and reverse 5′-CAT GCC AGT GAG CTT
CCC GTT CA-3′. The PCR conditions were as follows: denaturation for
5 min at 94˚C, amplification for 30 cycles with 1 min at 94˚C, 1
min at 55˚C and a final extension for 1 min at 72˚C.
Results
Cellular apoptosis induced in
neuroblastoma cells upon STI-571 treatment
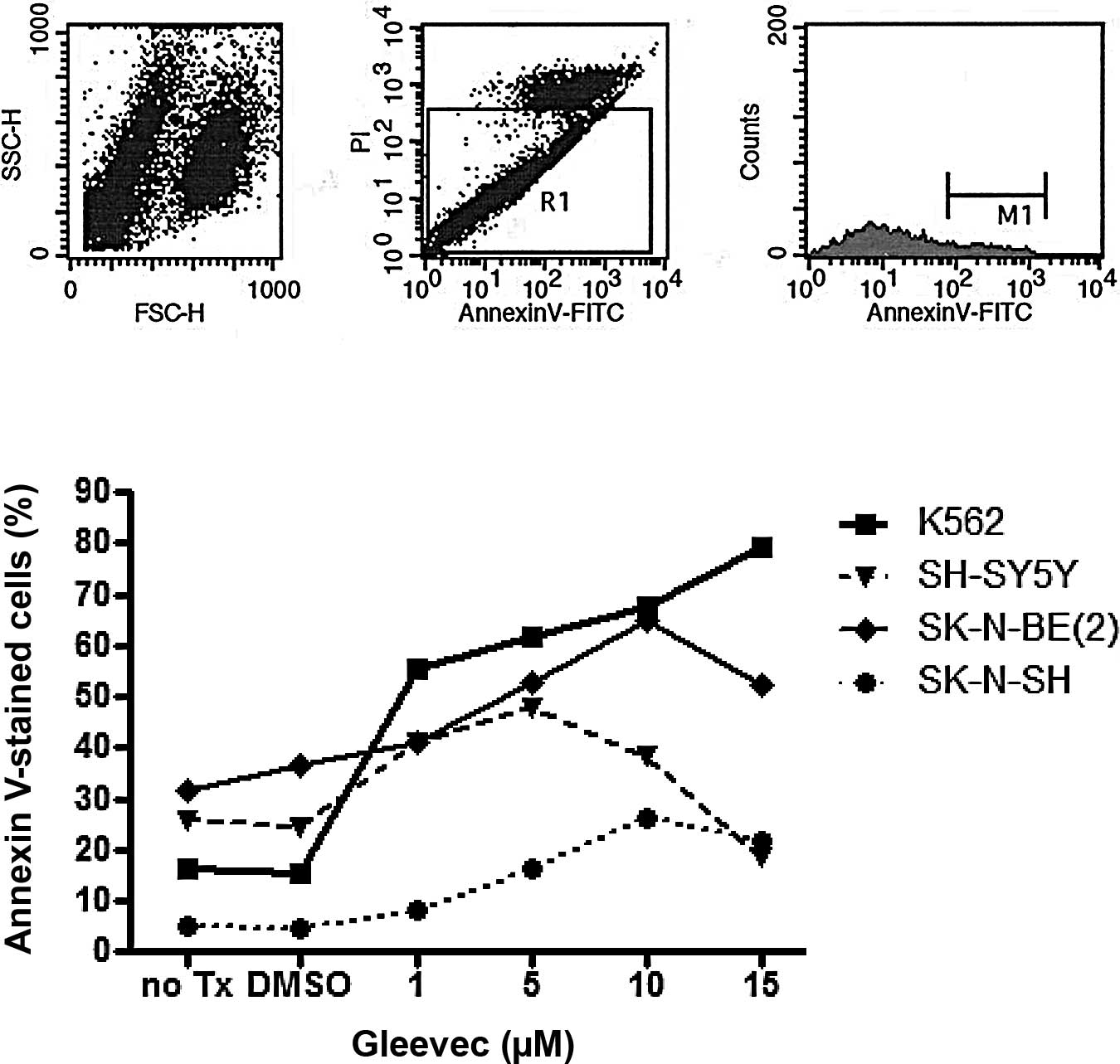
Initially, we assessed the effects of STI-571 on
three neuroblastoma cell lines: SK-N-BE(2), which has an MYCN amplication and
expresses the N-Myc protein; SH-SY5Y, which expresses the N-Myc
protein, but does not have an MYCN amplification; and SK-N-SH,
which does not express N-Myc protein or have an MYCN amplification
(6,7). K562 cells, which have a bcr-abl
translocation, were used as a positive control. The percentage of
Annexin V-positive neuroblastoma cells was increased in the cell
lines treated with concentrations >1 μM STI-571 (Fig. 1). Notably, the percentage of
Annexin V-positive cells was increased to a greater extent in the
SK-N-SH cells than in the SH-SY5Y or SK-N-BE(2) cells. Additionally, the percentage of
Annexin V-positive cells decreased at STI-571 concentrations of 10
and 15 μM in the neuroblastoma cell lines. This was attributed to
the removal of the dead cell fraction occurring during the washing
procedure.
Cytotoxic effects induced in
neuroblastoma cells after arsenic trioxide and STI-571
treatment
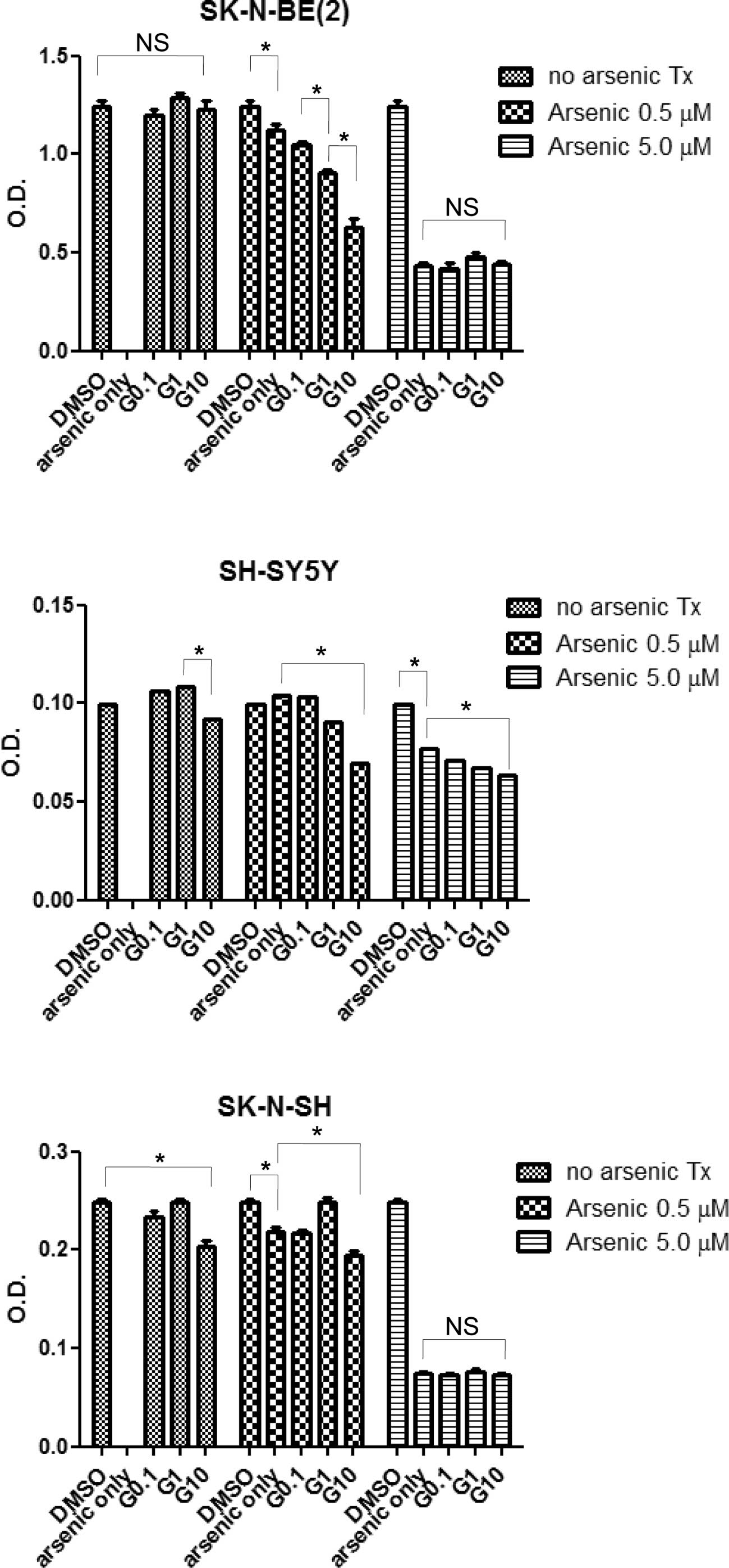
An MTT assay was conducted to measure the effects of
arsenic trioxide and/or STI-571 treatment on cell proliferation in
the neuroblastoma cell lines. As shown in Fig. 2, in the SK-N-SH and SH-SY5Y cell
lines, treatment with 10 μM of STI-571 suppressed proliferation,
while this effect was not noted in the SK-N-BE(2) cells. Arsenic trioxide also induced a
reduction in cell proliferation at 0.5 and 5 μM. In addition,
treatment with STI-571 and arsenic trioxide exerted an additive or
synergistic effect on the inhibition of cell proliferation. Upon
arsenic treatment at a concentration of 5 μM, proliferation was
inhibited independent of the STI-571 concentration in the
SK-N-BE(2) and SH-SY5Y cell lines.
In the SH-SY5Y cells, in the presence of arsenic trioxide (0.5 and
5 μM), STI-571 exerted marked effects on cellular proliferation in
a dose-dependent manner.
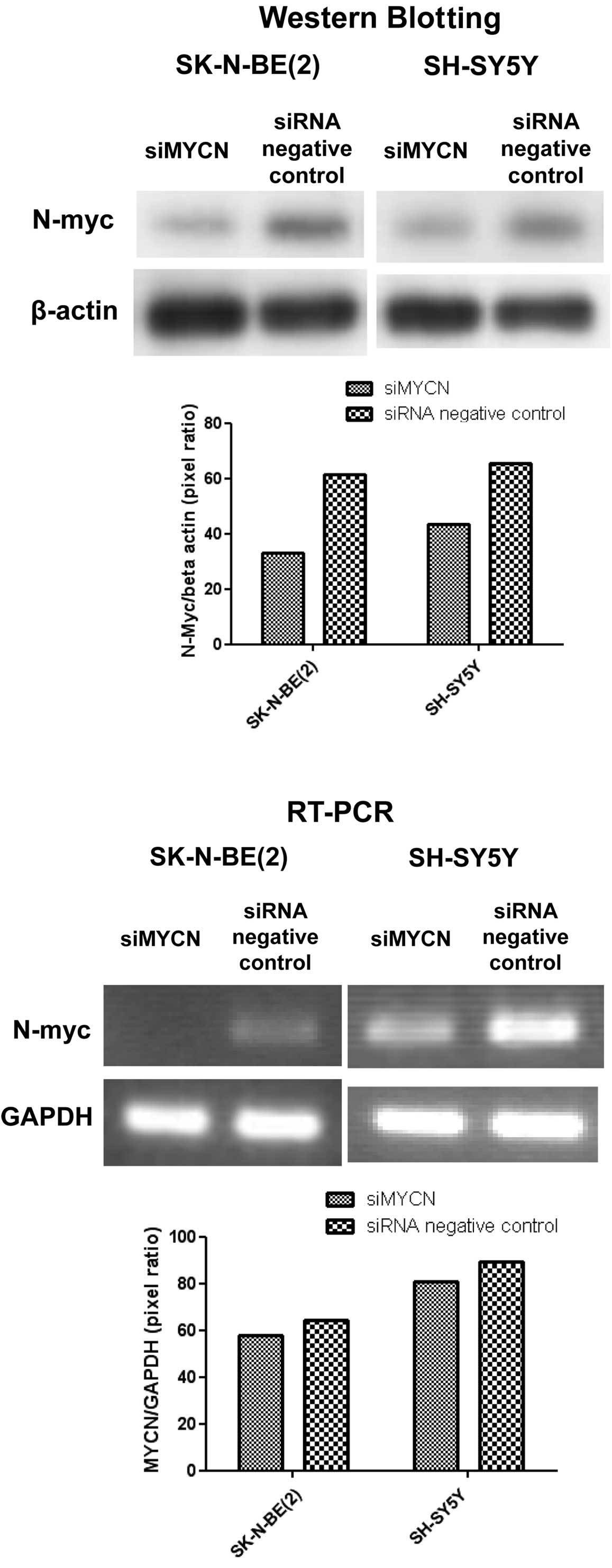
Expression of N-myc knocked down by
siRNA
Since the SK-NBE(2)
and SH-SY5Y cell lines have been reported to express N-myc, the
differences noted in sensitivity (Fig.
1) to STI-571 may be related to N-myc expression in
neuroblastoma cells. Thus, we aimed to characterize the effects of
N-myc knockdown on STI-571 and/or arsenic trioxide treatment. As
shown in Fig. 2, STI-571 treatment
applied to the SK-N-BE(2) cell
line manifesting an MYCN amplification had minimal influence on the
suppressive effect. However, in SH-SY5Y cells, a dose-dependent
reduction in cell proliferation was observed, even at a
concentration of 5 μM of arsenic trioxide. Using siRNA against MYCN
(siMYCN) as well as the control (negative), mRNA and protein
expression was inhibited after siRNA treatment (Fig. 3).
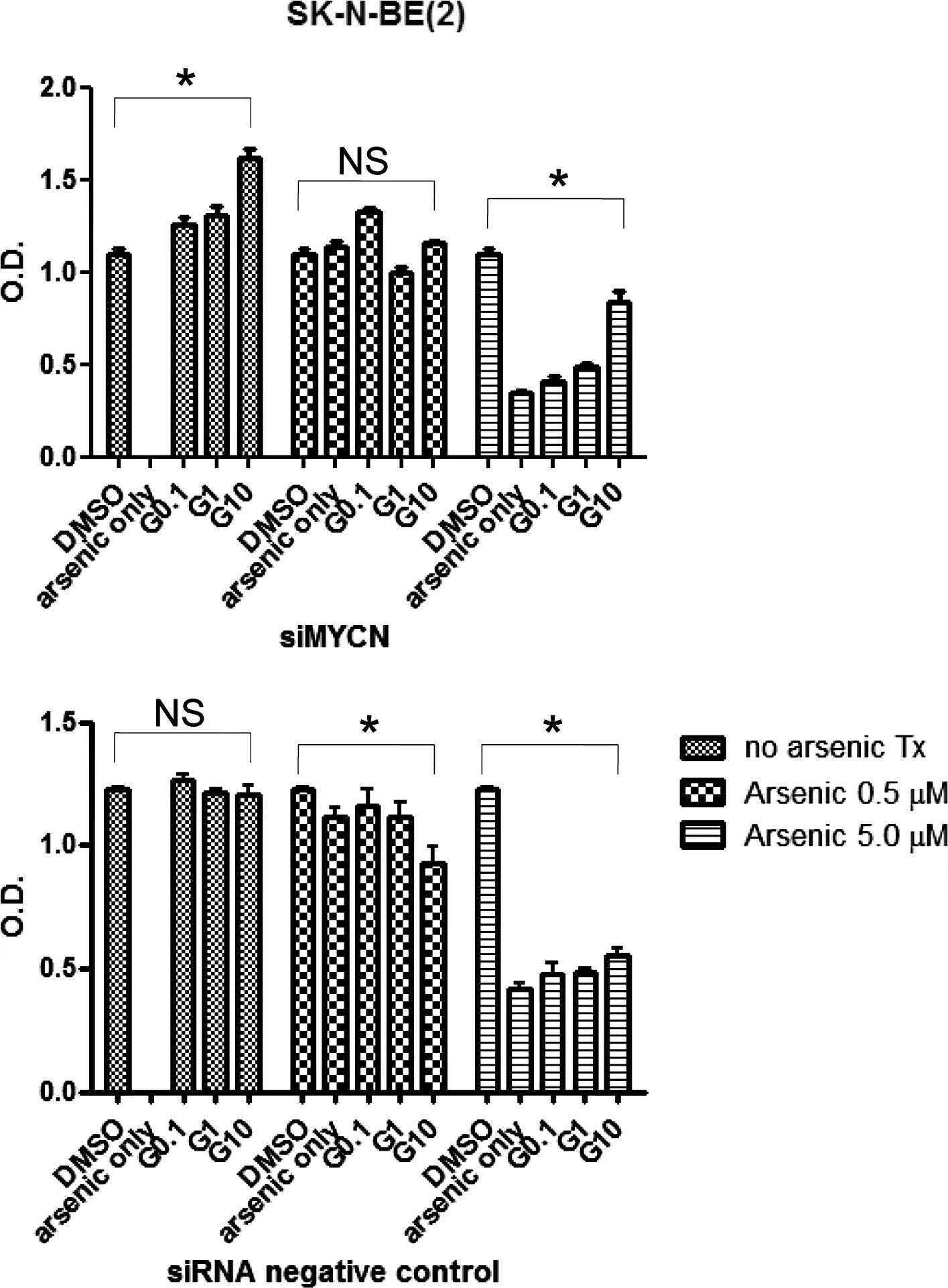
Cytotoxic effects reversed in
neuroblastoma cells after siMYCN treatment
In order to observe the effects of N-myc knockdown
on arsenic trioxide and STI-571 treatments, the cells were
transfected with siMYNC or siRNA negative controls and analyzed
using an MTT assay. The siRNA negative control-transfected cells
exhibited a higher sensitivity to STI-571 treatment than the
siMYCN-transfected cells (Fig. 4).
This suggests that N-myc knockdown does not render neuroblastoma
cells more vulnerable to STI-571 treatment.
Discussion
In the present study, we found that treatment with
STI-571 and arsenic trioxide exerted synergistic cytotoxic effects
on three neuroblastoma cell lines: SH-SY5Y, SK-N-SH and
SK-N-BE(2). In the
MYCN-expressing/amplified cell lines, gene knockdown of MYCN via
the siRNA method inhibited the therapeutic effects of STI-571
exposure.
MYCN amplification occurs in approximately 25% of
neuroblastoma cases and has been profoundly correlated with cancer
progression and treatment failure. Myc protein is a member of the
family of basic-helix-loop-helix-leucine zipper transcription
factors, and the increased expression of this protein results in
the entry of cells into the cell cycle. The amplification and
overexpression of MYCN in neuroblastoma have been correlated with
treatment failure and a poor prognosis. As MYCN-expressing cancer
cells often exhibit defects in the apoptotic pathway, the
inhibition of MYCN expression may prove to be a useful target for
the treatment of neuroblastoma. In pursuit of such targets, RNA
interference may be employed for the knockdown of specific proteins
in target cells. Therefore, the siRNA technique targeting MYCN was
recently highlighted as a potential new therapeutic modality for
the treatment of aggressive neuroblastoma (5).
However, it has also been reported that MYCN
expression may increase the antitumor effect of various drug
therapies, as MYCN expression was found to induce the transition
from the G1-S to G2/M phase of the cell cycle (8). In addition, in K562 cells, c-Myc
levels were not correlated with cell proliferation and c-Myc
down-regulation was correlated with STI-571 activity, but not with
STI-571-induced apoptosis (9).
Therefore, in the present study we evaluated the effects of N-myc
knockdown by siRNA in combination with STI-571 and arsenic trioxide
treatment, as both have been suggested as promising new therapeutic
agents for the treatment of aggressive neuroblastomas.
STI-571 treatment applied to neuroblastoma cells was
found to target c-Kit and PDGFR-α and -β (10). In particular, c-Kit and its ligand
stem cell factor were reported to be expressed in neuroblastoma
cells, as well as in primary tumors (3). Moreover, c-Kit has been reported to
be preferentially expressed in MYCN-amplified neuroblastoma and was
shown to inhibit STI-571-mediated proliferation (11).
In conclusion, STI-571 is a potential therapeutic
drug for the treatment of neuroblastoma, particularly in
MYCN-amplified/expressing cancers. However, for the development of
new therapeutic targets in cancer therapy, synergistic or possible
antagonistic effects should be considered when using a combined
therapeutic regimen.
Acknowledgements
This study was supported by the Korea
Research Foundation Grant funded by the Korean Government (MOEHRD,
Basic Research Promotion Fund) (KRF-2005-003-E00134).
References
|
1.
|
Woo SY, Lee MY, Jung YJ, Yoo ES, Seoh JY,
Shin HY, Ahn HS and Ryu KH: Arsenic trioxide inhibits cell growth
in SH-SY5Y and SK-N-AS neuroblastoma cell lines by a different
mechanism. Pediatr Hematol Oncol. 23:231–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Krause DS and van Etten RA: Tyrosine
kinases as targets for cancer therapy. N Engl J Med. 353:172–187.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mauro MJ, O’Dwyer M, Heinrich MC and
Druker BJ: STI571: a paradigm of new agents for cancer
therapeutics. J Clin Oncol. 20:325–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Beppu K, Jaboine J, Merchant MS, Mackall
CL and Thiele CJ: Effect of imatinib mesylate on neuroblastoma
tumorigenesis and vascular endothelial growth factor expression. J
Natl Cancer Inst. 96:46–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kang JH, Rychahou PG, Ishola TA, Qiao J,
Evers BM and Chung DH: MYCN silencing induces differentiation and
apoptosis in human neuroblastoma cells. Biochem Biophys Res Commun.
351:192–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Brodeur GM, Green AA, Hayes FA, Williams
KJ, Williams DL and Tsiatis AA: Cytogenetic features of human
neuroblastomas and cell lines. Cancer Res. 41:4678–4686.
1981.PubMed/NCBI
|
|
7.
|
Gilbert F, Feder M, Balaban G, Brangman D,
Lurie DK, Podolsky R, Rinaldt V, Vinikoor N and Weisband J: Human
neuroblastomas and abnormalities of chromosomes 1 and 17. Cancer
Res. 44:5444–5449. 1984.PubMed/NCBI
|
|
8.
|
Paffhausen T, Schwab M and Westermann F:
Targeted MYCN expression affects cytotoxic potential of
chemotherapeutic drugs in neuroblastoma cells. Cancer Lett.
250:17–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gomez-Casares MT, Vaque JP, Lemes A,
Molero T, Delgado MD and Leon J: C-myc expression in cell lines
derived from chronic myeloid leukemia. Haematologica. 89:241–243.
2004.PubMed/NCBI
|
|
10.
|
Buchdunger E, Cioffi CL, Law N, Stover D,
Ohno-Jones S, Druker BJ and Lydon NB: Abl protein-tyrosine kinase
inhibitor STI571 inhibits in vitro signal transduction mediated by
c-kit and platelet-derived growth factor receptors. J Pharmacol Exp
Ther. 295:139–145. 2000.
|
|
11.
|
Vitali R, Cesi V, Nicotra MR, McDowell HP,
Donfrancesco A, Mannarino O, Natali PG, Raschella G and Dominici C:
c-Kit is preferentially expressed in MYCN-amplified neuroblastoma
and its effect on cell proliferation is inhibited in vitro by
STI-571. Int J Cancer. 106:147–152. 2003. View Article : Google Scholar : PubMed/NCBI
|