Introduction
N-myc downstream regulated gene 1
(NDRG1)/Ca2+-associated protein 43 (Cap43) has been
identified as a nickel- and calcium-induced gene, identical to the
homocysteine-inducible gene, reduced in tumor (RTP/rit42) and to
the differentiation-related gene-1 (Drg-1) (1–6). In
cancer progression, overexpression of NDRG1/Cap43 was found to
reduce cell proliferation and anchorage-independent growth in
vitro and tumor growth in vivo (2). However, NDRG1/Cap43 has no effect on
the primary tumor growth of colon and prostate cancer in
vivo (7,8). NDRG1/Cap43 expression has been found
to be increased in numerous types of human cancer in comparison to
normal tissues (8). However, other
studies have reported that the expression of NDRG1/Cap43 is
increased in normal cells and in well-differentiated cancer cells,
but decreased in poorly differentiated cancer cells and in cancer
of the colon, prostate, breast and pancreas (7–11).
This suggests a close association of NDRG1/Cap43 with the cancer
differentiation status.
NDRG1/Cap43 is a predictive marker of good prognosis
in patients with cancer of the prostate, esophagus, breast, colon
and pancreas, and with neuroblastoma (2,8,12–15).
However, the expression of NDRG1/Cap43 is a predictive marker of
poor prognosis in patients with liver, colon, esophagal and
cervical cancer (16–19). Taken together, the effectiveness of
NDRG1/Cap43 as a predictive marker of good or poor prognosis in
cancer patients may depend on the type of human malignancy and the
histological type or differentiation status of the tumor (20).
We previously reported that NDRG1/Cap43 expression
suppressed tumor growth and angiogenesis in a human pancreatic
cancer xenograft model (11).
Overexpression of NDRG1/Cap43 resulted in a marked inhibition of
the production of the potent angiogenesis factors VEGF-A and IL-8
(CXCL8) and of matrix metalloproteinase-9 (MMP-9) by pancreatic
cancer cells, suggesting a possible role of NDRG1/Cap43 in
angiogenesis and extracellular remodeling in the tumor stroma
(11). Our recent study further
demonstrated that NDRG1/Cap43 decreased the expression of various
chemo attractants, including CXC chemokines (CXCL5, CXCL1 and
CXCL8) for inflammatory cells, and the recruitment of macrophages
and neutrophils with the suppression of angiogenesis and growth in
pancreatic cancer (21). The
underlying mechanism whereby NDRG1/Cap43 suppressed tumor growth
and angiogenesis as well as the production of CXC chemokines in
pancreatic cancer appeared to be due to the attenuation of NF-κB
signaling through marked decreases in IKKβ expression and IKBα
phosphorylation (21,22). In patients with pancreatic cancer,
the NDRG1/Cap43 expression levels were inversely correlated with
the number of infiltrating macrophages and tumor angiogenesis in
the tumor stroma.
The key role infiltrating macrophages play in the
tumor stroma, promoting the malignant progression of cancer through
interaction with cancer cells, has recently been highlighted
(23–25). Tumor-supportive macrophages play an
active role in extracellular matrix remodeling, tissue repair and
angiogenesis, whereas tumor-suppressive macrophages exert
antimicrobial and antitumor activities through immunostimulatory
functions (26,27). Tumor-supporting macrophages, also
known as tumor-associated macrophages (TAMs), promote invasion,
metastasis and angiogenesis through the production of inflammatory
cytokines, chemokines, proteases, prostanoids, growth factors and
angiogenic factors. Clinical studies have demonstrated a close
association between the abundance of TAMs and poor prognosis or
tumor angiogenesis in various types of solid tumors (28). A number of chemotactic cytokines
are expected to play important roles in the recruitment and
accumulation of macrophages in the tumor stroma, and TAMs that are
recruited to the tumor play a key role in the angiogenic switch and
malignant transition of cancer. We previously reported that
depletion of these TAMs, as well as macrophages, by
macrophage-targeting bisphosphonate encapsulated in liposomes
markedly inhibits tumor growth, angiogenesis and bone metastasis
(24–31), suggesting the involvement of
macrophages in tumor growth, metastasis and angiogenesis (25).
In the present study, we investigated whether the
expression of NDRG1/Cap43 is a biomarker for the favorable or poor
prognosis of gastric cancer, as no previous study has focused on
the role of NDRG1/Cap43 in the differentiation status of gastric
cancer. We also examined whether NDRG1/Cap43 modulates the
infiltration of macrophages and angiogenesis in the tumor stroma of
gastric cancer, in association with histological type and
progression. We also discuss whether NDRG1/Cap43 plays a role in
tumor stromal responses, thus affecting tumor progression in
gastric cancer.
Materials and methods
Patients and tumor samples
The study comprised 129 patients with advanced
gastric cancer whose tumors had been surgically removed at the
Department of Surgery, Kurume University, between 2001 and 2004.
The age of the gastric cancer patients ranged from 33 to 86 years
(median 69); 91 were male and 38 were female. Histological types
were classified according to the criteria of the Lauren
classification (32), and tumor
stage was classified according to the TNM classification. Patient
characteristics are summarized in Table I. Cancer stages included 30 (23.2%)
cases of stage I (IA + IB), 18 (14.0%) stage II, 27 (20.9%) stage
III (IIIA + IIIB) and 54 (41.9%) stage IV. At the time of surgery,
79 (61.2%), 17 (13.2%) and 29 (22.5%) patients had lymph node
metastasis, liver metastasis or peritoneal dissemination,
respectively. No patients had been administered drugs before
surgery, including neo-adjuvant chemotherapy, and the standard
chemotherapy was performed after surgery: stage II or III patients
were administered TS-1 and stage IV patients were administered a
combination of TS-1 and cisplatin.
 | Table I.NDRG1/Cap43 expression and
clinicopathological characteristics in the gastric cancer
patients. |
Table I.
NDRG1/Cap43 expression and
clinicopathological characteristics in the gastric cancer
patients.
| Gastric cancer
patients
|
|---|
| Total (n=129) | Intestinal type
(n=65) | Diffuse type
(n=64) |
|---|
| Age (years) | | | |
| <66 | 52 | 21 | 31 |
| ≥66 | 77 | 44 | 33 |
| Gender | | | |
| Male | 91 | 56 | 35 |
| Female | 38 | 9 | 29 |
| Pathological
stage | | | |
| I | 30 | 17 | 13 |
| II | 18 | 9 | 9 |
| III | 27 | 14 | 13 |
| IV | 54 | 25 | 29 |
| Membrane
NDRG1/Cap43 | | | |
| Positive | 76 | 50 | 26 |
| Negative | 53 | 15 | 38 |
| Nuclear
NDRG1/Cap43 | | | |
| Positive | 29 | 20 | 9 |
| Negative | 100 | 45 | 55 |
Immunohistochemistry (IHC)
Paraffin-embedded tissue samples were cut into 4-μm
sections, examined on a coated glass slide and labeled with the
following antibodies using the BenchMark XT (Ventana Automated
Systems, Inc., Tucson, AZ, USA) and ChemMate Envision
(DakoCytomation, Glostrup, Denmark) methods: NDRG1/Cap43 (x200,
produced in our laboratory) (11),
CD68 (x1,200; KP-1; DakoCytomation) and CD34 (x200; Novo Castra,
Newcastle, UK). For NDRG1/Cap43, the BenchMark XT was used. Each
slide was heat-treated using Ventana's CC1 retrieval solution for
30 min and incubated with the NDRG1/Cap43 antibody for 30 min. This
automated system used the streptavidin biotin complex method with
3,3′ diaminobenzidine as the chromogen (Ventana iVIEW DAB detection
kit). The ChemMate Envision method was used for CD68 and CD34.
Endogenous peroxidase activity was inhibited by incubating the
slides in 3% H2O2 for 5 min. CD68 and CD34
antigen retrieval was performed by treatment with proteinase K for
5 min. Each slide was incubated for 30 min with the antibody at
room temperature. For staining detection, the ChemMate Envision
method was used with DAB as the chromogen. Healthy non-cancerous
mucosal lesions were used as controls.
Evaluation of NDRG1/Cap43 expression in
the membrane and nucleus of gastric cancer cells
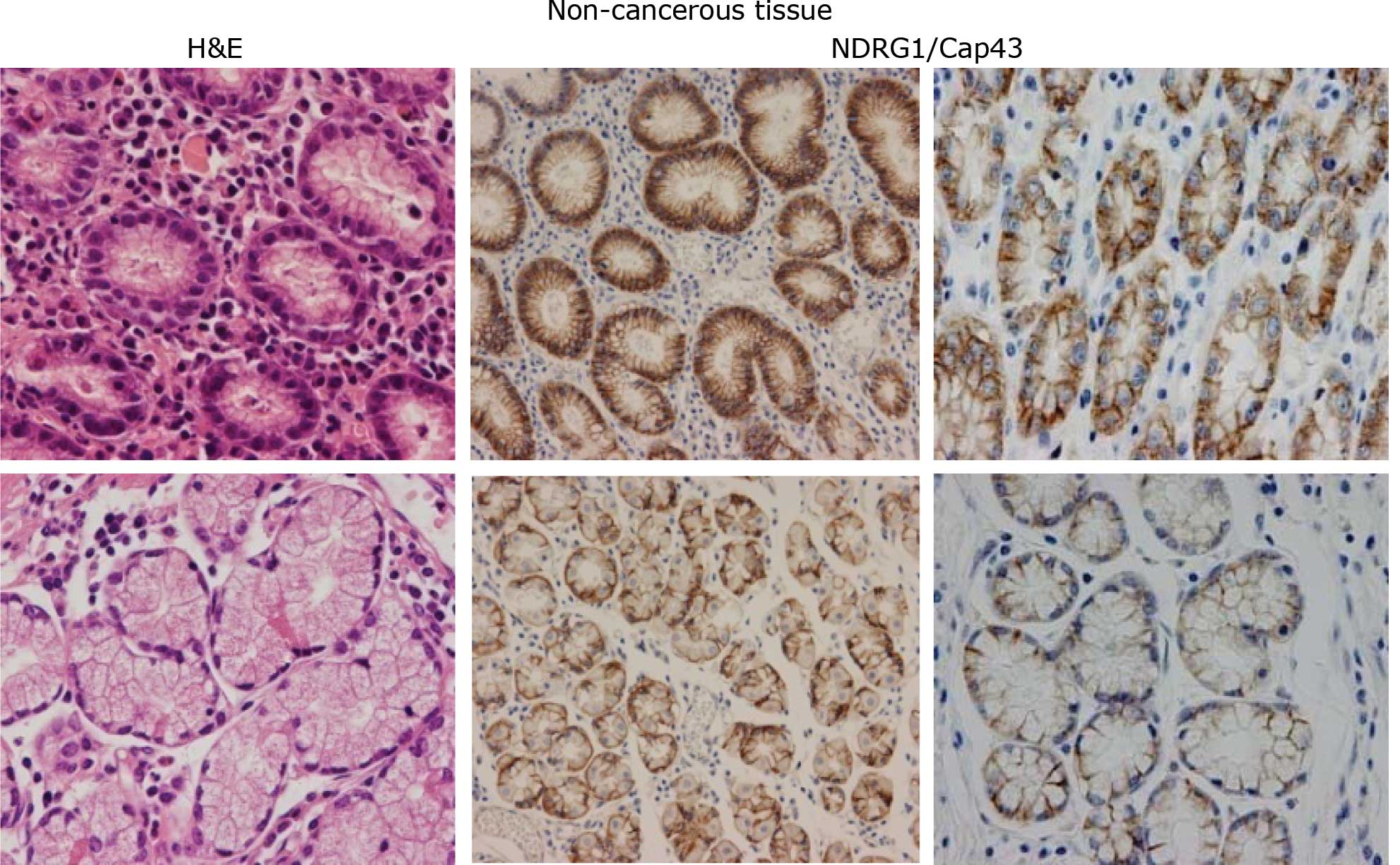
With regard to the expression of NDRG1/Cap43,
membranous and/or cytoplasmic and nuclear staining was observed in
gastric cancer tissues by IHC. Expression of NDRG1/Cap43 was
predominantly found in the membrane and/or cytoplasm of gastric
mucosal cells (Fig. 1A), and in
the cancerous cells in both the intestinal and diffuse types of
gastric cancer (Fig. 1B). By
contrast, the expression of NDRG1/Cap43 in the nucleus was
consistently observed only in the gastric cancer cells (Fig. 1B). Based on the IHC profiles of
membranous and nuclear staining, the presence and absence of
NDRG1/Cap43 expression was evaluated. The intensity of membranous
NDRG1/Cap43 expression was scored using the following scale: no
staining, 0; weak staining, 1+; moderate staining, 2+; and strong
staining, 3+ in >10% of cancer cells. Scores of 0 and +1 were
classified as negative, and scores of 2+ and 3+ as positive. The
expression of nuclear NDRG1/Cap43 was classified based on the
percentage of cancer cells with strongly stained nuclei: ≥5%
indicated that the cancer tissue was positive and ≤4% indicated
that it was negative. NDRG1/Cap43 expression was evaluated by two
experienced observers (A.K. and M.K.) blinded to the condition of
the patients.
Determination of the number of
CD68+ macrophages and CD34+ microvessel
density (MVD)
Digital expression data were extracted using the
following image analysis systems: CD68- and CD34-stained specimens
were examined to identify the areas of expression with high
density. Images of the areas of expression were selected for
clarity from at least 6 fields at x200 for each IHC specimen using
a CCD digital camera (Nikon, DXM1200). Expression analysis was
performed to measure the areas of expression of the number of
macrophages and MVD in all cases using ‘Win ROOF’ software (version
5.7; Mitani Corporation, Osaka, Japan) (33). The digitized data of the expression
areas were measured and averaged.
Statistical analysis
The distribution of CD68+ and
CD34+ was compared between NDRG1/Cap43-negative and
-positive patients with the Wilcoxon rank-sum test and was
displayed with box plots. Associations between CD68+,
CD34+ and stage were examined by comparing the
distribution of CD68+ and CD34+ among
patients of each stage with the Kruskal-Wallis test and displayed
with box plots. Associations between NDRG1/Cap43 and stage were
examined by the Mantel-Haenszel linear trend test. Overall survival
was defined as days from surgery until death due to any cause. The
log-rank test and the Kaplan-Meier method were applied to examine
the effect of NDRG1/Cap43 on overall survival. The hazard ratio of
NDRG1/Cap43-positive patients relative to NDRG1/Cap43-negative
patients was estimated by applying the Cox regression model. When
adjusting for possible confounding factors in the Cox regression
model, stage was not adjusted, since it may be an intermediate
variable in evaluating the effects of NDRG1/Cap43 on patient
prognosis (34). Statistical
analysis was performed by SAS version 9.2 (SAS Institute Inc.,
Cary, NC, USA), StatXact (Cytel Inc., Cambridge, MA, USA) and R
version 2.8.1.
Results
Clinicopathological features and
expression of NDRG1/Cap43 in non-cancerous gastric mucosal cells
and gastric cancer cells
In non-cancerous gastric mucosal cells, the
expression of NDRG1/Cap43 was observed in the membrane and/or
cytoplasm of almost all the cells, and no nuclear expression was
evident (n=77) (Fig. 1A). Among
the 129 gastric cancer specimens analyzed, 65 were classified as
the intestinal type and 64 as the diffuse type.
NDRG1/Cap43-positive expression in the membrane was observed in 50
(76.9%) patients with the intestinal type and in 26 (40.6%)
patients with the diffuse type. Nuclear expression of NDRG1/Cap43
in gastric cancer cells was evident in 29/129 (22.5%) patients
(Fig. 1B).
Increasing nuclear NDRG1/Cap43 expression
during progression of pathological stage in gastric cancer
patients
Nuclear NDRG1/Cap43 expression was not increased at
stage I in the intestinal type and at stages I and II in the
diffuse type, but nuclear NDRG1/Cap43 expression was significantly
increased at later pathological stages in both the intestinal and
diffuse types (Table II). Among
the 20 patients with positive nuclear expression of NDRG1/Cap43 in
the intestinal type, 17 (85%) were classified as stages III and IV,
compared to only 22 (48.9%) of the 45 patients who exhibited
negative nuclear NDRG1/Cap43 expression. All 9 patients with
nuclear NDRG1/Cap43-positive expression in the diffuse type were at
stages III and IV, compared to 32/55 (58.2%) patients with nuclear
NDRG1/Cap43-negative expression. The P-value of the linear trend
was statistically significant for both the intestinal (P=0.002) and
the diffuse type (P=0.039). The linear trend test for membranous
NDRG1/Cap43 expression was statistically significant for the
intestinal type (P=0.002), but not for the diffused type (P=0.926)
(Table II).
 | Table II.Association of nuclear and membranous
NDRG1/Cap43 expression with pathological stage. |
Table II.
Association of nuclear and membranous
NDRG1/Cap43 expression with pathological stage.
| Histological
type | Pathological stage
| Total | P-value |
|---|
| I | II | III | IV |
|---|
| NDRG1/Cap43
(nucleus) | | | | | | |
| Intestinal type,
n (%) | | | | | | |
| Negative | 17 (37.8) | 6 (13.3) | 10 (22.2) | 12 (26.7) | 45 | 0.002 |
| Positive | 0 (0.0) | 3 (15.0) | 5 (25.0) | 12 (60.0) | 20 | |
| Diffuse type, n
(%) | | | | | | |
| Negative | 13 (23.6) | 10 (18.2) | 9 (16.4) | 23 (41.8) | 55 | 0.039 |
| Positive | 0 (0.0) | 0 (0.0) | 3 (33.3) | 6 (66.7) | 9 | |
| NDRG1/Cap43
(membrane) | | | | | | |
| Intestinal type,
n (%) | | | | | | |
| Negative | 10 (66.7) | 1 (6.7) | 0 (0.0) | 4 (26.7) | 15 | 0.002 |
| Positive | 7 (14.0) | 8 (16.0) | 15 (30.0) | 20 (40.0) | 50 | |
| Diffuse type, n
(%) | | | | | | |
| Negative | 8 (21.0) | 5 (13.1) | 9 (23.7) | 16 (42.1) | 38 | 0.926 |
| Positive | 5 (19.2) | 5 (19.2) | 3 (11.5) | 13 (50.0) | 26 | |
Association of NDRG1/Cap43 expression
with infiltrating macrophages and tumor angiogenesis in the
intestinal, but not in the diffuse type
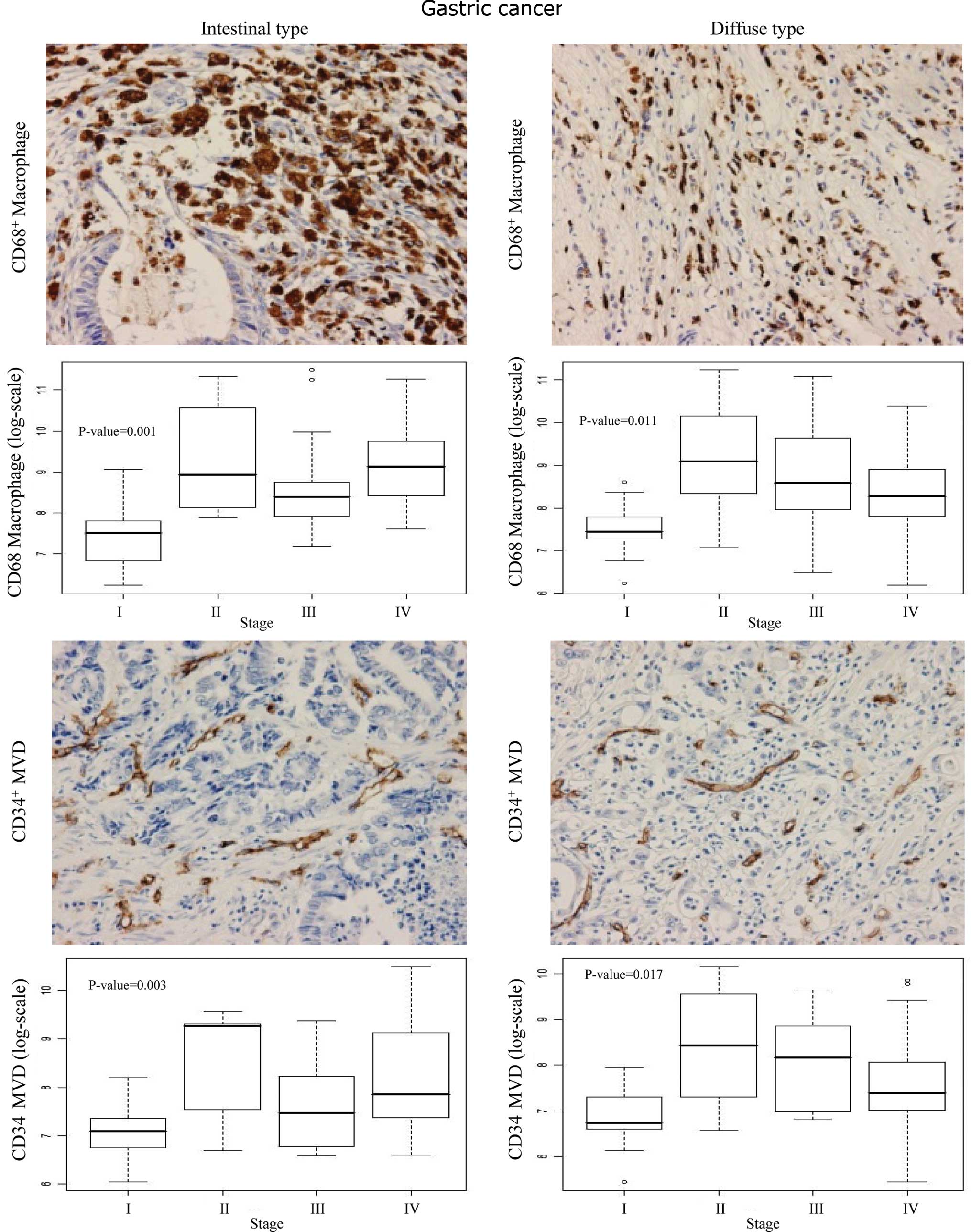
As shown in Fig. 2,
the CD68+ macrophage count and CD34+ MVD were
high in the cancerous region in both the intestinal and diffuse
types, compared to the non-cancerous gastric mucosal cells. Box
blots showed significantly higher numbers of infiltrating
CD68+ macrophages (P<0.001) and higher MVD (P=0.003)
at stages II, III and IV compared to stage I in the intestinal type
(Fig. 2). A stage-dependent
increase in both macrophage count (P=0.011) and MVD (P=0.017) was
also observed in the diffuse type of gastric cancer.
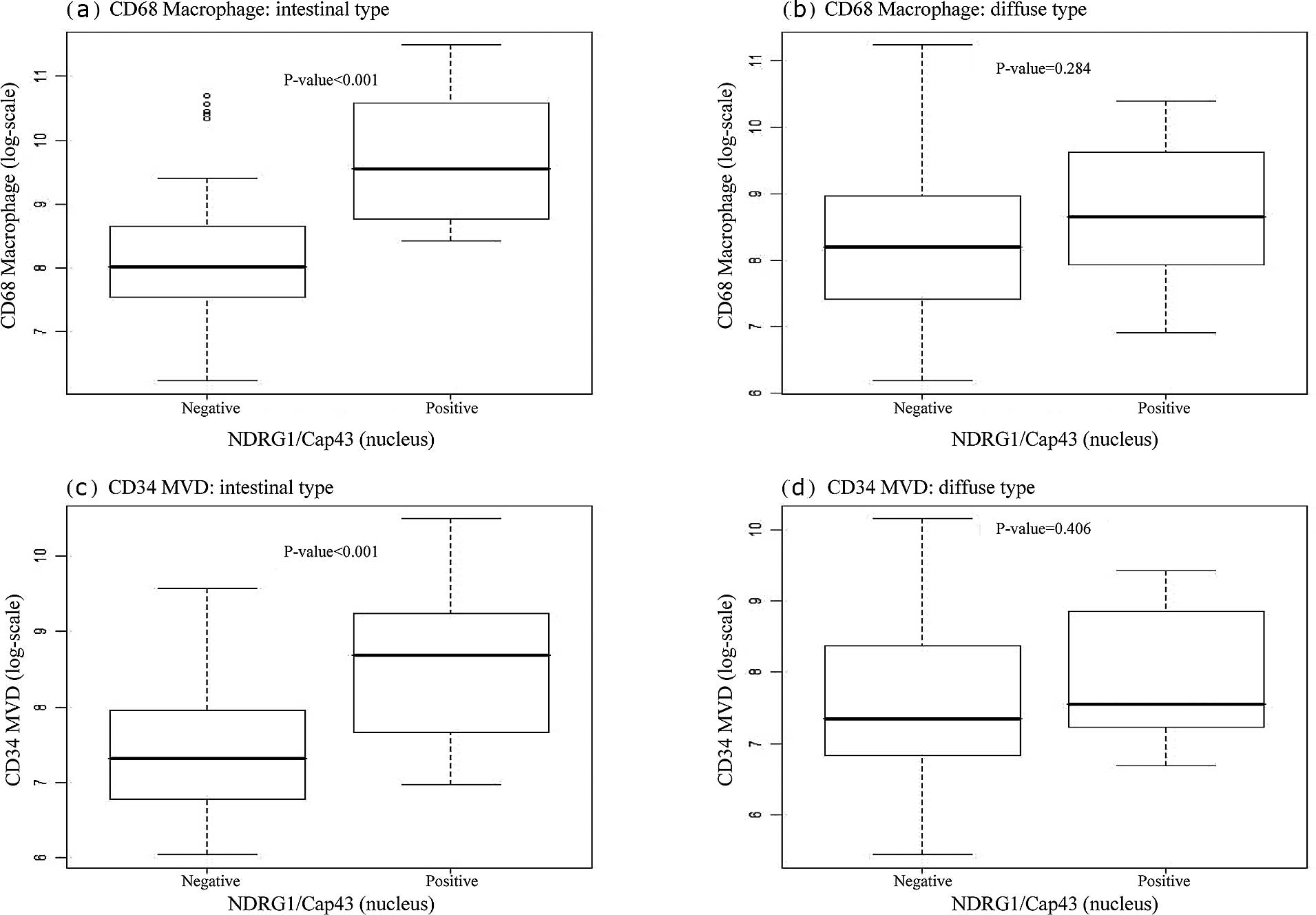
We next examined whether nuclear or membranous
NDRG1/Cap43 expression was correlated with the infiltrating
CD68+ macrophage count and MVD. Box plots indicated that
the number of infiltrating CD68+ macrophages was
significantly correlated with nuclear NDRG1/Cap43 expression in the
intestinal type (P<0.001) (Fig.
3A-a), but not in the diffuse type (Fig. 3A-b). There was also a significant
association of nuclear NDRG1/Cap43 expression with MVD, in the
intestinal type (P=0.001) (Fig.
3A-c), but not in the diffuse type (Fig. 3A-d). Furthermore, membranous
NDRG1/Cap43 expression was found to be significantly correlated
with the number of infiltrating CD68+ macrophages
(P=0.001), but not with MVD in the intestinal type (Fig. 3B-a and -c). There was no
significant association between membranous NDRG1/Cap43 expression
and the number of infiltrating CD68+ macrophages or MVD
in the diffuse type of gastric cancer (Fig. 3B-b and -d).
Association of NDRG1/Cap43 expression
with the survival of patients with intestinal type gastric
cancer
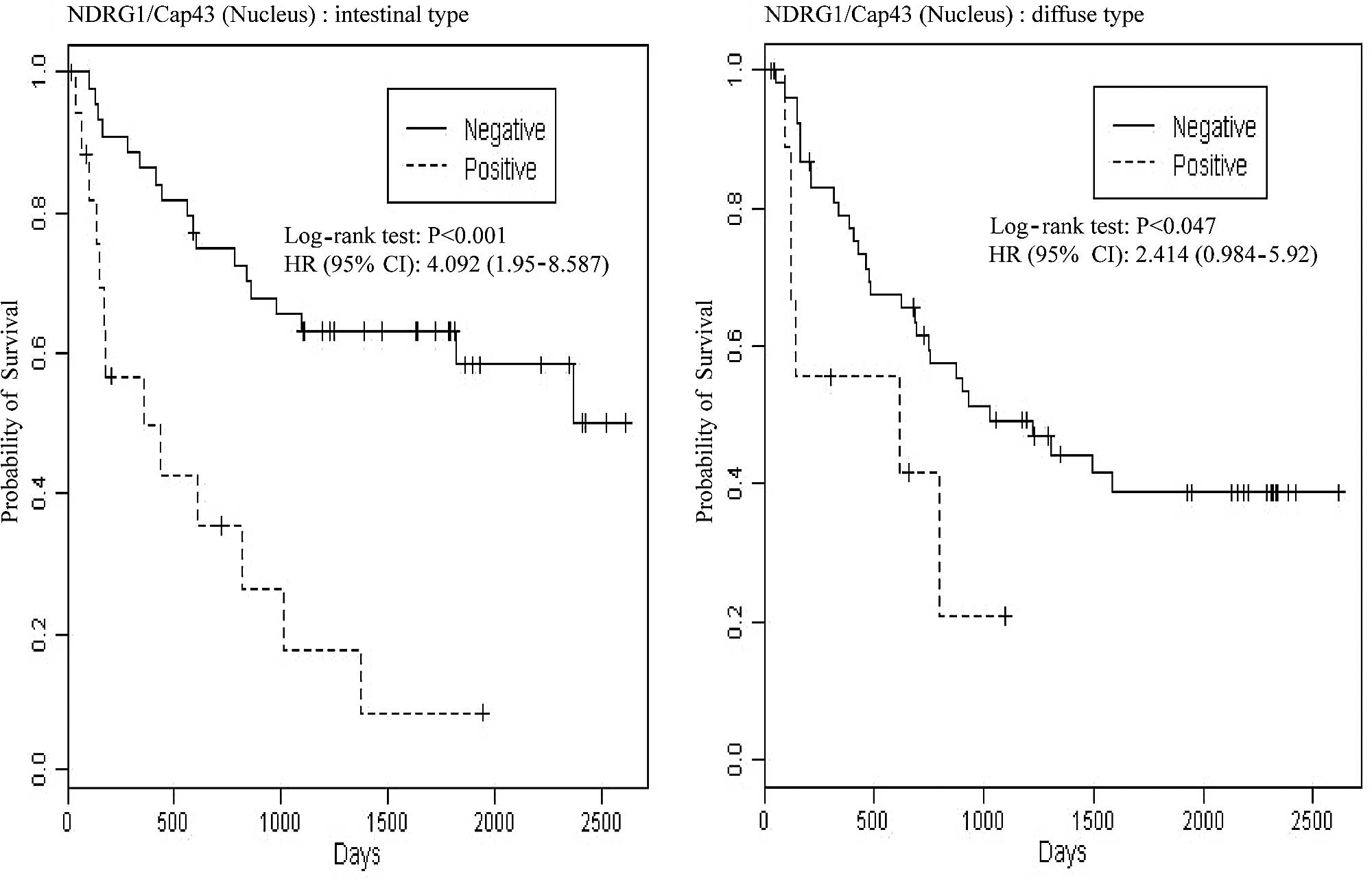
We further examined whether NDRG1/Cap43 expression
in the membrane and nucleus was associated with the overall
survival of gastric cancer patients. Log-rank P-values for nuclear
expression of NDRG1/Cap43 and Kaplan-Meier plots are shown in
Fig. 4A. Among patients with the
intestinal type, those who were nuclear NDRG1/Cap43-positive had
significantly shorter survival than those who were nuclear
NDRG1/Cap43-negative [P<0.001, hazard ratio (HR)=4.092,
confidence interval (CI) 1.950–8.547]. Among patients with the
diffuse type, a similar tendency was observed, with statistical
significance (P=0.047, HR=2.414, CI 0.984–5.920). The results
remained similar even after adjustment for possible confounding
factors, such as age and gender, by Cox regression (P=0.002,
HR=4.163, CI 1.970–8.797 for the intestinal type and P=0.111,
HR=2.086, CI 0.845–5.145 for the diffuse type). By contrast,
membranous NDRG1/Cap43 expression was not associated with overall
survival in either the intestinal or the diffuse type of gastric
cancer (Fig. 4B).
Discussion
In the present study, we observed that the
expression of NDRG1/Cap43 in the nucleus was specifically increased
during stage progression of both the intestinal and diffuse types
of gastric cancer. We evaluated whether nuclear or membranous
NDRG1/Cap43 expression affected tumor angiogenesis, the
infiltration of macrophages and patient survival, and demonstrated
that nuclear NDRG1/Cap43 expression, rather than its membranous
expression, was significantly correlated with the number of
infiltrating macrophages and tumor angiogenesis in the intestinal,
but not in the diffuse, type of gastric cancer. Furthermore,
nuclear NDRG1/Cap43 expression was associated with a poor prognosis
in both the intestinal and diffuse types of gastric cancer.
During normal postnatal development, NDRG1/Cap43 is
expressed in the membrane and/or cytoplasm of cells in the rat
kidney and brain (35).
NDRG1/Cap43 is also a membrane and/or cytoplasm protein in human
tissues, but its cellular localization is dependent on cell type
(35). For instance, epithelial
cells of the prostate predominantly show membranous expression of
NDRG1/Cap43. Analysis of human NDRG1/Cap43 localization has
demonstrated that the probability of NDRG1/Cap43 expression in the
membrane and/or cytoplasm, nucleus and mitochondria is 47.8, 26.1
and 8.7%, respectively (36).
Overexpression of NDRG1/Cap43 is an indicator of poor prognosis in
hepatocellular carcinoma (17) and
cervical adenocarcinoma (18), and
NDRG1/Cap43 is specifically expressed in the membrane and/or
cytoplasm. In hepatic cancer cells, NDRG1/Cap43 is localized in the
membrane and/or cytoplasm both in vivo and in vitro
(37). In the present study,
NDRG1/Cap43 was localized in the membrane and/or cytoplasm of
normal gastric mucosal cells and in early-stage gastric cancer. By
contrast, NDRG1/Cap43 was not expressed in the nucleus of normal
gastric mucosal cells or in cancer cells at earlier stages, but its
nuclear expression was markedly increased in cancer cells at later
stages of progression. Thus, in gastric cancer, nuclear NDRG1/Cap43
expression may play a role in tumor progression.
NDRG1/Cap43 is a specific differentiation-related
gene first identified by van Belzen et al (1). NDRG1/Cap43 expression suppresses the
expression of angiogenic factors and MMP-9 in pancreatic cancer
cells, and also suppresses tumor growth and angiogenesis in human
pancreatic cancer (11,21). Furthermore, macrophage infiltration
and tumor angiogenesis have been shown to be significantly
correlated with the expression level of NDRG1/Cap43 in patients
with pancreatic cancer (20).
Contrary to the inverse association of NDRG1/Cap43 expression with
tumor angiogenesis and prognosis in pancreatic cancer, our present
study demonstrated that NDRG1/Cap43 expression was positively
correlated with tumor angiogenesis in the intestinal type of
gastric cancer. Thus, depending on tumor type, NDRG1/Cap43 may be a
positive or negative biomarker of malignant progression, including
tumor angiogenesis, infiltration of TAMs and the prognosis of
cancer patients. Nuclear NDRG1/Cap43 may positively regulate the
infiltration of macrophages, including TAMs in tumors, as well as
tumor angiogenesis, in the intestinal type of gastric cancer. We
favor the idea that NDRG1/Cap43 induces the accumulation and
activation of macrophages/monocytes, resulting in an angiogenic
switch in the tumor stroma (25),
probably in close connection with the differentiation status.
Further study is required to ascertain how NDRG1/Cap43 functions in
association with histological type in gastric cancer.
In gastric cancer, tumor angiogenesis and
lymphangiogenesis are known to be closely associated with malignant
progression and poor prognosis (38). Macrophage infiltration and tumor
angiogenesis, which are stimulated by nuclear NDRG1/Cap43, may play
roles in the promotion of metastasis to the lymph nodes and liver
in intestinal type gastric cancer cells. NDRG1/Cap43 may
specifically modulate tumor angiogenesis and metastasis in close
correlation with the recruitment of macrophages and TAMs, depending
on the histological type of gastric cancer. In both the intestinal
and diffuse types of gastric cancer, infiltration of macrophages
and tumor angiogenesis were found to be increased during
progression of pathological stages (Fig. 2). However, there was no such
significant correlation between infiltrating macrophages or MVD and
NDRG1/Cap43 expression in the nucleus and membrane in the diffuse
type of gastric cancer (Fig. 3).
By contrast, our present study showed that the expression of
NDRG1/Cap43 in the nucleus significantly affected the survival of
patients with intestinal type gastric cancer and those with the
diffuse type after surgical treatment (Fig. 4). Although it remains unclear which
biological function of NDRG1/Cap43 is specifically responsible for
survival in gastric cancer, the localization of NDRG1/Cap43
expression to the nucleus rather than the membrane appears to be a
better indicator of poor prognosis in gastric cancer patients.
Inagaki et al (39) recently reported that nuclear
localization of NDRG1/Cap43 is significantly related to lymph node
metastasis as well as to the survival of patients with diffuse type
gastric cancer, and also that nuclear localization of NDRG1/Cap43
is significantly correlated with p53 expression in the nucleus. Of
the various suppressor genes linked to the expression of
NDRG1/Cap43 (40), p53 is known to
be closely associated with NDRG1/Cap43 in tumor growth and/or
cancer cell apoptosis (2,41,42),
but the molecular interaction between p53 and NDRG1/Cap43 remains
unclear. In the present study, we did not examine whether any
suppressor gene was linked to NDRG1/Cap43 expression in gastric
cancer cells. Consistent with the study by Inagaki et al
(39), nuclear NDRG1/Cap43
expression was found to be significantly correlated with lymph node
metastasis. Further study is required to understand how the
expression of NDRG1/Cap43 affects the peritoneal dissemination of
gastric cancer.
In conclusion, the present study demonstrated for
the first time a close association of nuclear NDRG1/Cap43
expression with the infiltration of macrophages and tumor
angiogenesis in the intestinal type of gastric cancer, whereas no
such association was evident in the diffuse type. The present
findings suggest that the nuclear expression of NDRG1/Cap43 may
serve as a novel biomarker for the molecular diagnosis of gastric
cancer, and also for the development of new therapeutic strategies.
Further study is required to ascertain how the nuclear localization
of NDRG1/Cap43 is controlled at the molecular level.
References
|
1.
|
Van Belzen N, Dinjens WN, Diesveld MP, et
al: A novel gene which is up-regulated during colon epithelial cell
differentiation and down-regulated in colorectal neoplasms. Lab
Invest. 77:85–92. 1997.PubMed/NCBI
|
|
2.
|
Kurdistani SK, Arizti P, Reimer CL, Sugrue
MM, Aaronson SA and Lee SW: Inhibition of tumor cell growth by
RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res.
58:4439–4444. 1998.PubMed/NCBI
|
|
3.
|
Zhou D, Salnikow K and Costa M: Cap43, a
novel gene specifically induced by Ni2+ compounds.
Cancer Res. 58:2182–2189. 1998.PubMed/NCBI
|
|
4.
|
Okuda T and Kondoh H: Identification of
new genes Ndr2 and Ndr3 which are related to Ndr1/RTP/Drg1 but show
distinct tissue specificity and response to N-myc. Biochem Biophys
Res Commun. 266:208–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Shimono A, Okuda T and Kondoh H:
N-myc-dependent repression of ndr1, a gene identified by direct
subtraction of whole mouse embryo cDNAs between wild type and N-myc
mutant. Mech Dev. 83:39–52. 1999. View Article : Google Scholar
|
|
6.
|
Qu X, Zhai Y, Wei H, et al:
Characterization and expression of three novel
differentiation-related genes belong to the human NDRG gene family.
Mol Cell Biochem. 229:35–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM and
Pardee AB: Drg-1 as a differentiation-related, putative metastatic
suppressor gene in human colon cancer. Cancer Res. 60:749–755.
2000.PubMed/NCBI
|
|
8.
|
Bandyopadhyay S, Pai SK, Gross SC, et al:
The Drg-1 gene suppresses tumor metastasis in prostate cancer.
Cancer Res. 63:1731–1736. 2003.PubMed/NCBI
|
|
9.
|
Angst E, Sibold S, Tiffon C, et al:
Cellular differentiation determines the expression of the
hypoxia-inducible protein NDRG1 in pancreatic cancer. Br J Cancer.
95:307–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fotovati A, Fujii T, Yamaguchi M, et al:
17Beta-estradiol induces down-regulation of Cap43/NDRG1/Drg-1, a
putative differentiation-related and metastasis suppressor gene, in
human breast cancer cells. Clin Cancer Res. 12:3010–3018. 2006.
View Article : Google Scholar
|
|
11.
|
Maruyama Y, Ono M, Kawahara A, et al:
Tumor growth suppression in pancreatic cancer by a putative
metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of
angiogenesis. Cancer Res. 66:6233–6242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ando T, Ishiguro H, Kimura M, et al:
Decreased expression of NDRG1 is correlated with tumor progression
and poor prognosis in patients with esophageal squamous cell
carcinoma. Dis Esophagus. 19:454–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Fukahori S, Yano H, Tsuneoka M, et al:
Immunohistochemical expressions of Cap43 and Mina53 proteins in
neuroblastoma. J Pediatr Surg. 42:1831–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Koshiji M, Kumamoto K, Morimura K, et al:
Correlation of N-myc downstream-regulated gene 1 expression with
clinical outcomes of colorectal cancer patients of different
race/ethnicity. World J Gastroenterol. 13:2803–2810.
2007.PubMed/NCBI
|
|
15.
|
Song YS, Oda Y, Hori M, et al: N-myc
downstream regulated gene1/Cap43 may play an important role in
malignant progression of prostate cancer, in its close association
with E-cadherin. Human Pathol. 41:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shah MA, Kemeny N, Hummer A, et al: Drg1
expression in 131 colorectal liver metastases: correlation with
clinical variables and patient outcomes. Clin Cancer Res.
11:3296–3302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chua MS, Sun H, Cheung ST, et al:
Overexpression of NDRG1 is an indicator of poor prognosis in
hepatocellular carcinoma. Mod Pathol. 20:76–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nishio S, Ushijima K, Tsuda N, et al:
Cap43/NDRG1/Drg-1 is a molecular target for angiogenesis and a
prognostic indicator in cervical adenocarcinoma. Cancer Lett.
264:36–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sohda M, Mochida Y, Kato H, et al:
Overexpression of Cap43 is associated with malignant status of
esophageal cancer. Anticancer Res. 29:965–970. 2009.PubMed/NCBI
|
|
20.
|
Kovacevic Z, Fu D and Richardson DR: The
iron-regulated metastasis suppressor, Ndrg-1: identification of
novel molecular targets. Biochim Biophys Acta. 1783:1981–1992.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hosoi F, Izumi H, Kawahara A, et al: N-myc
downstream regulated gene 1/Cap43 suppresses tumor growth and
angiogenesis of pancreatic cancer through attenuation of IKKbeta
expression. Cancer Res. 69:4983–4991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Murakami Y, Hosoi F, Izumi H, et al:
Identification of sites subjected to serine/threonine
phosphorylation by SGK1 affecting N-myc downstream-regulated gene 1
(NDRG1)/Cap43-dependent suppression of angiogenic CXC chemokine
expression in human pancreatic cancer cells. Biochem Biophysical
Res Commun. 396:376–381. 2010. View Article : Google Scholar
|
|
23.
|
Mantovani A, Allavena P and Sica A:
Tumour-associated macrophages as a prototypic type II polarised
phagocyte population: role in tumour progression. Eur J Cancer.
40:1660–1667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ono M: Molecular links between tumor
angiogenesis and inflammation: inflammatory stimuli of macrophages
and cancer cells as targets for therapeutic strategy. Cancer Sci.
99:1501–1506. 2008. View Article : Google Scholar
|
|
26.
|
Lin EY and Pollard JW: Tumor-associated
macrophages press the angiogenic switch in breast cancer. Cancer
Res. 67:5064–5066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Nakao S, Kuwano T, Tsutsumi-Miyahara C, et
al: Infiltration of COX-2-expressing macrophages is a prerequisite
for IL-1 beta-induced neovascularization and tumor growth. J Clin
Invest. 115:2979–2991. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kimura YN, Watari K, Fotovati A, et al:
Inflammatory stimuli from macrophages and cancer cells
synergistically promote tumor growth and angiogenesis. Cancer Sci.
98:2009–2018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hiraoka K, Zenmyo M, Watari K, et al:
Inhibition of bone and muscle metastases of lung cancer cells by a
decrease in the number of monocytes/macrophages. Cancer Sci.
99:1595–1602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Lauren P: The two histological main types
of gastric carcinoma: diffuse and so-called intestinal-type
carcinoma: an attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.
|
|
33.
|
Kawahara A, Hattori S, Akiba J, et al:
Infiltration of thymidine phosphorylase-positive macrophages is
closely associated with tumor angiogenesis and survival in
intestinal type gastric cancer. Oncol Rep. 24:405–415. 2010.
View Article : Google Scholar
|
|
34.
|
Rothman KJ and Greenland S: Modern
Epidemiology. 3rd edition. Lippincott Williams & Wilkins;
Philadelphia: 1998
|
|
35.
|
Wakisaka Y, Furuta A, Masuda K, Morikawa
W, Kuwano M and Iwaki T: Cellular distribution of NDRG1 protein in
the rat kidney and brain during normal postnatal development. J
Histochem Cytochem. 51:1515–1525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lachat P, Shaw P, Gebhard S, van Belzen N,
Chaubert P and Bosman FT: Expression of NDRG1, a
differentiation-related gene, in human tissues. Histochem Cell
Biol. 118:399–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sibold S, Roh V, Keogh A, et al: Hypoxia
increases cytoplasmic expression of NDRG1, but is insufficient for
its membrane localization in human hepatocellular carcinoma. FEBS
Lett. 581:989–994. 2007. View Article : Google Scholar
|
|
38.
|
Maehara Y, Kabashima A, Koga T, et al:
Vascular invasion and potential for tumor angiogenesis and
metastasis in gastric carcinoma. Surgery. 128:408–416. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Inagaki Y, Tang W, Xu HL, et al:
Localization of N-myc downstream-regulated gene 1 in gastric cancer
tissue. Dig Liver Dis. 41:96–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Kovacevic Z and Richardson DR: The
metastasis suppressor, Ndrg-1: a new ally in the fight against
cancer. Carcinogenesis. 27:2355–2366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Rutherford MN, Bayly GR, Matthews BP, et
al: The leukemogenic transcription factor E2a-Pbx1 induces
expression of the putative N-myc and p53 target gene NDRG1 in Ba/F3
cells. Leukemia. 15:362–370. 2001. View Article : Google Scholar
|
|
42.
|
Stein S, Thomas EK, Herzog B, et al: NDRG1
is necessary for p53-dependent apoptosis. J Biol Chem.
279:48930–48940. 2004. View Article : Google Scholar : PubMed/NCBI
|