Introduction
Traditionally, patients undergoing breast-conserving
surgery (BCS) receive standard whole breast irradiation (WBI)
(1–3). However, patients with early-stage
breast cancer are a heterogeneous group, with a variable risk and
pattern of recurrence. Accordingly, alternative treatment options
besides conventional WBI should be available. Based on findings
that most recurrences after BCS for early breast cancer usually
occur within or near the original tumor bed, partial breast
irradiation (PBI) has become the focus of many cancer centers.
Moreover, various centers have investigated the indications for BCS
without radiotherapy (BCS only) (4,5).
Thus, BCS and WBI, BCS and PBI or BCS only jointly constitute the
individualized treatment strategy for early-stage breast
cancer.
PBI is still in the clinical research stage. No
consensus has been reached on numerous issues, such as indications,
target area identification and fractionation methods. The aim of
this study was to examine the use of PBI with a conventional
fractionated three-dimensional conformal modality. Since the target
volume was the only factor of this PBI protocol that varied from
standard WBI, local tumor control was the focus and sole end point
of this trial. Only once PBI has been determined to confer the same
local tumor control benefits as WBI can this technique be examined
in phase II trials.
Materials and methods
Patient eligibility
All patients had AJCC clinical stage I breast
cancer, and the specific eligibility requirements were as follows:
patients were ≥40 years of age, had no history of prior malignancy
and had unifocal breast cancer (single focus that could be
encompassed by 1 tylectomy), T ≤2 cm (T1), pN0, and pathological
types that included invasive ductal carcinoma of grade I–II and
non-lobular invasive carcinoma with no extensive intraductal
components (EIC). In addition, patients were required to have
surgical margins negative (≥5 mm) for cancer.
Simulation, treatment planning and
treatment
Treatment planning and delivery were performed with
the patient in a supine position with a vacuum phantom fixed to
expose the breasts. All patients underwent a CT breast simulation.
The clinical breast borders and lumpectomy scar were marked with
radiopaque catheters. CT images were acquired at 5-mm intervals
starting at or above the mandible and extending 2 cm below the
inframammary fold to the costophrenic angle (including the entire
lung). The lumpectomy cavity, ipsilateral breast, ipsilateral and
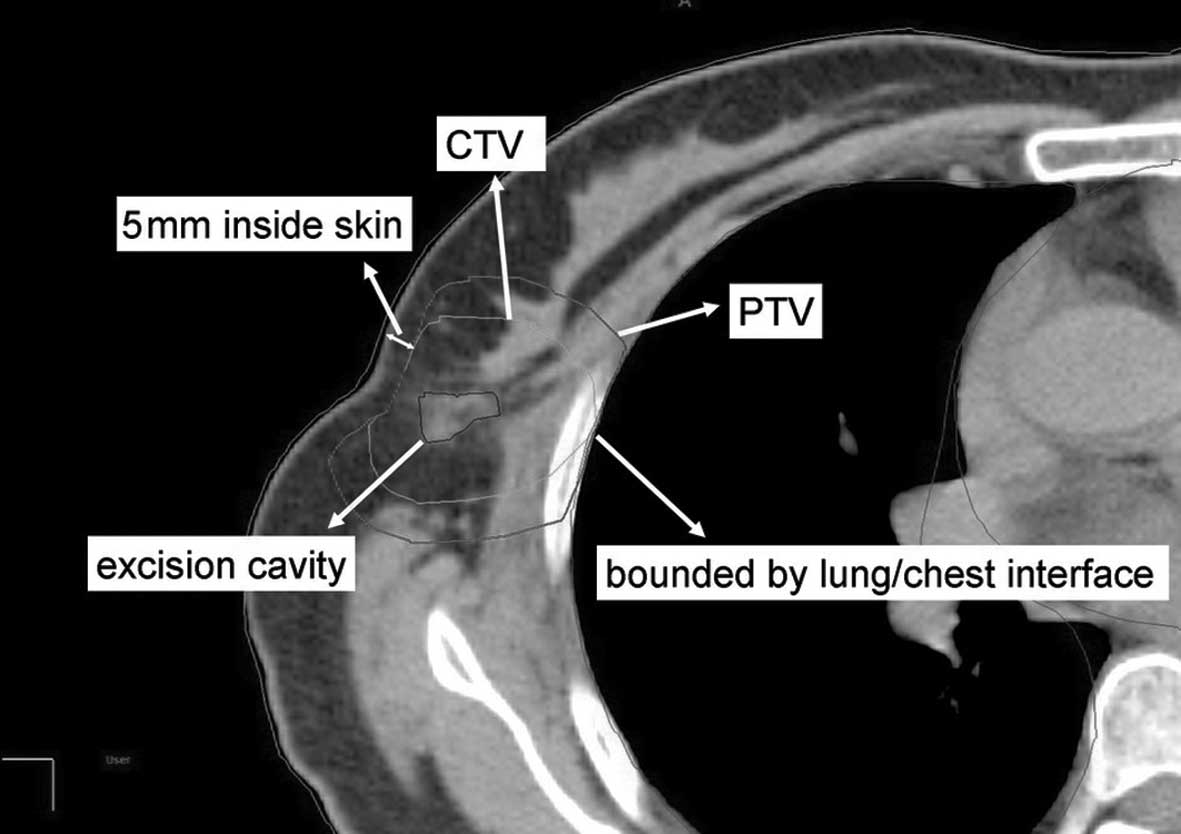
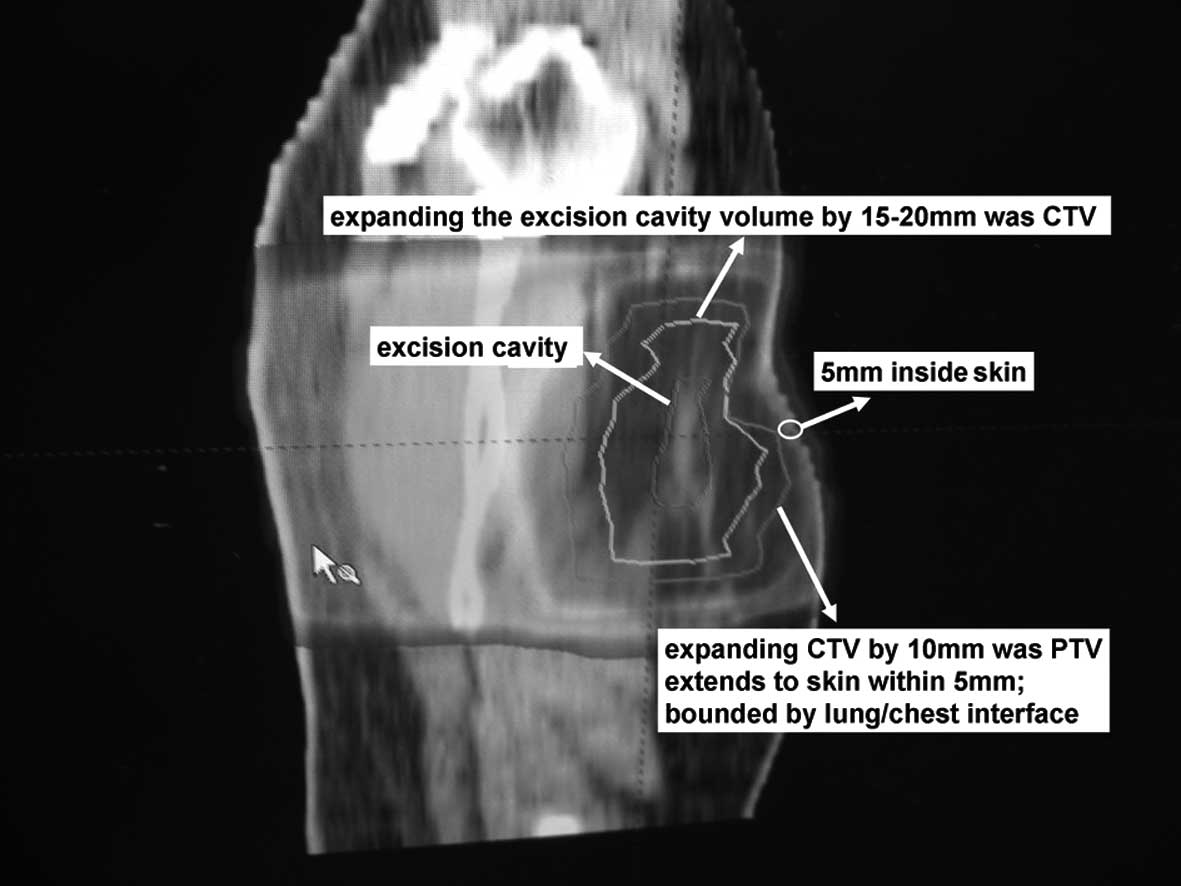
contralateral lungs, and heart were contoured. The clinical target
volume (CTV) was defined as uniform expansion of the excision
cavity volume by 15–20 mm (Figs. 1
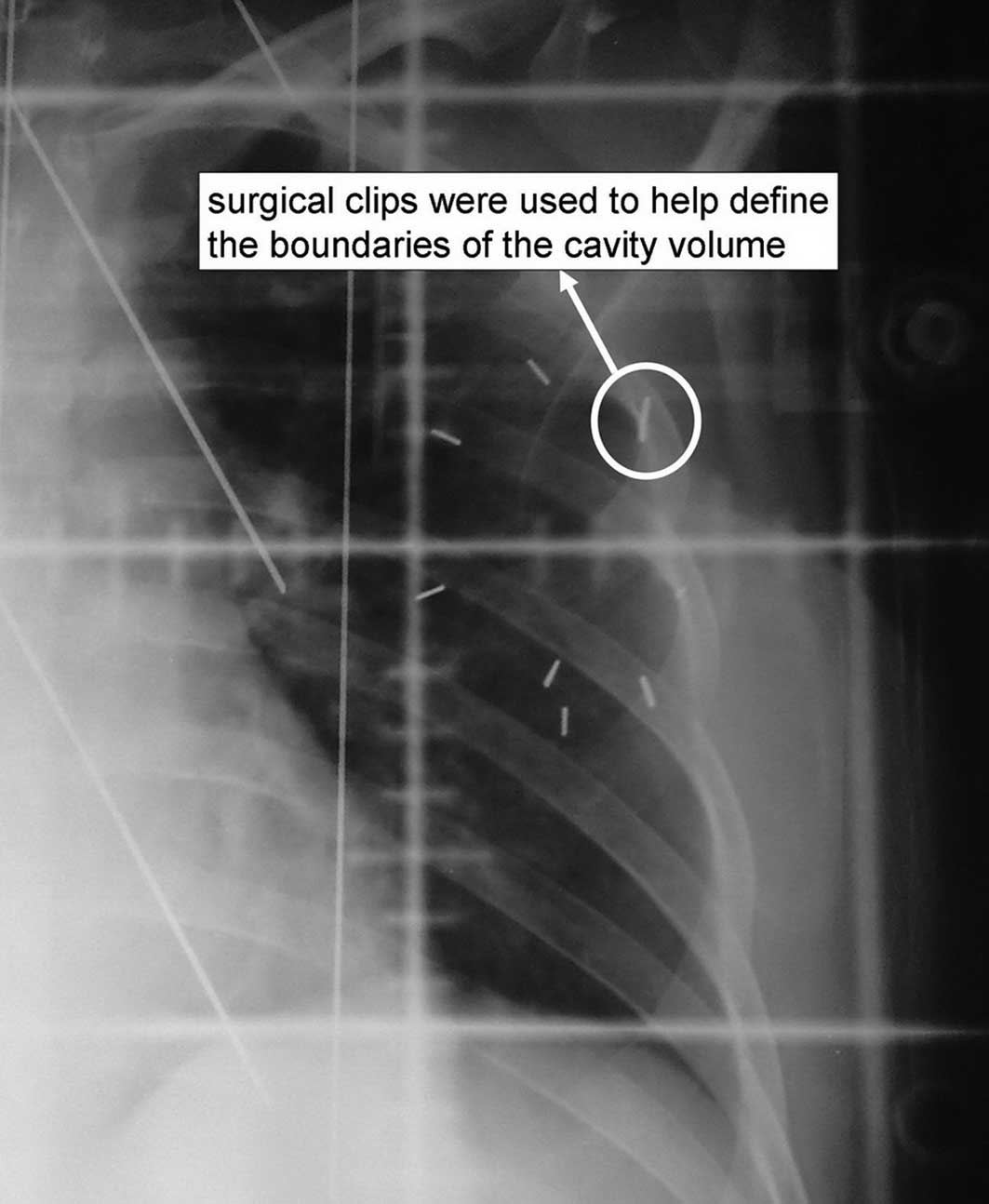
and 2). The post-operative scar,
post-operative B ultrasonic and six surgical clips (Fig. 3) were used to determine the
boundaries of the cavity volume. In order to ensure satisfactory
cosmetic results and to reduce adverse reactions, the CTV was
limited to an area 5 mm from the skin surface and lung-chest wall
interface. The planning target volume (PTV) was designed to provide
a margin around the CTV to compensate for the variability of
treatment set-up and motion of the breast with breathing. A minimum
10-mm region surrounding the CTV was required (superior, inferior,
medial and lateral dimension). Moreover, PTV was limited to exclude
the region outside the patient and the first 5 mm of tissue under
the skin and to also exclude (if applicable) the PTV expanded area
within the lung. Dose calculation was carried out using the
organization’s non-uniformity correction. Treatment planning was
made by the Varian Eclipse TPS system. Three-to-five fields were
arranged using 6-MV photons for lesions. Field arrangements were
made at the discretion of the physician and determined by 3D
treatment planning to produce the optimal conformal plan in
accordance with volume definitions (described below). The treatment
plan used for each patient was based on an analysis of the
volumetric dose, including DVH analyses of the PTV and critical
normal tissues.
When no chemotherapy was administered, it was
recommended that radiotherapy start within 8 weeks of surgery. When
chemotherapy was administered first, it was recommended that
radiotherapy to start a minimum of 2 weeks after the last cycle of
chemotherapy. A prescribed total dose of 60 Gy was administered, 2
Gy daily administered on 5 consecutive working days (from Monday to
Friday).
Normal tissue dose-limiting
requirement
Breast tissue dose limits
It was recommended that ≥50% of the ipsilateral
whole breast receive <60% of the prescribed dose, and that any
point in the contralateral breast receive <5% of the prescribed
dose.
Lung tissue dose limits. It was recommended
that <15% of the ipsilateral lung receive 30% of the prescribed
dose, and that <15% of the contralateral lung receive 5% of the
prescribed dose.
Heart tissue dose constraints. For
right-sided lesions, it was recommended that <5% of the heart
receive 5% of the prescribed dose, while for left-sided lesions it
was recommended that <5% of the heart receive 40% of the
prescribed dose.
Quality assurance evaluation
Ninety-five percent of the isodose surface covered
100% of the PTV. All critical normal tissue DVH limits were
met.
Statistical requirement
Reproducibility and safety were the early clinical
trial primary end points. The optimal two-stage design by Simon was
used (6). The p-value was taken as
the true indicator of whether the final result was acceptable or
marginally acceptable, and hence that the technique was
reproducible. The statistical test hypothesis was: H0, p≤p0 (=80%)
vs. H1, p≥p1 (=95%), two types of errors, α=5%, β=10%. Given these
parameters, 19 eligible patients were required for the first stage
of the two-stage design. However, in our experiments only 12
patients were eligible. When ≥2 treatments from these 12 were
scored as unacceptable, early discontinuation of the study was
recommended; otherwise accrual of data was continued.
Follow-up study
The final date for the follow-up study was June
2010. Overall survival time was calculated as time from disease
diagnosis to death or until the final date of the follow-up
interval.
Results
The result of the follow-up study
All patients were followed up for at least 41
months. No case was lost and the follow-up rate was 100%.
Patient characteristics
The pre-treatment characteristics for all eligible
patients including the first 12 evaluable patients are shown in
Table I.
 | Table I.Pre-treatment characteristics for all
eligible patients. |
Table I.
Pre-treatment characteristics for all
eligible patients.
| Patients (n=12) |
|---|
| Age, years | |
| Median | 53 |
| Range | 40–71 |
| Surgery, n (%) | |
| Lumpectomy +
axillary | |
| lymph node
dissection | 3 (25) |
| Quadrantectomy +
axillary | |
| lymph node
dissection | 9 (75) |
| Axillary lymph node
involvement | |
| Median no. | 12 |
| Range | 9–19 |
| Histology, n (%) | |
| Invasive
ductal | 11 (92) |
| Ductal carcinoma
in situ | 1 (8) |
| Menopausal status, n
(%) | |
| Premenopausal | 7 (58) |
| Postmenopausal | 5 (42) |
| Chemotherapy, n
(%) | |
| None | 5 (42) |
| Yes | 7 (58) |
| Hormonal therapy, n
(%) | |
| None | 9 (75) |
| Yes | 3 (25) |
| ER status, n (%) | |
| Positive | 7 (58) |
| Negative | 2 (17) |
| No test | 3 (25) |
| PR status, n (%) | |
| Positive | 5 (42) |
| Negative | 4 (33) |
| No test | 3 (25) |
| PS2 status, n
(%) | |
| Positive | 4 (33) |
| Negative | 5 (42) |
| No test | 3 (25) |
| HER-2 status, n
(%) | |
| Positive | 0 (0) |
| Negative | 11 (92) |
| No test | 1 (8) |
Treatment efficacy and adverse
reactions
All patients were followed up for at least 41
months, and presented a 0% rate of local recurrence and distant
metastasis. Data regarding adverse reactions are presented in
Table II.
 | Table II.Adverse reactions related to
radiotherapy in the studied patients (n=12). |
Table II.
Adverse reactions related to
radiotherapy in the studied patients (n=12).
| Adverse reactions, n
(%) | Grade 1 | Grade 2 | Grade 3 |
|---|
| Blood/bone
marrow | 2 (17) | 0 | 0 |
| Gastrointestinal | 0 | 0 | 0 |
| Lymphatic | 1 (8) | 0 | 0 |
| Breast atrophy/tissue
fibrosis | 0 | 0 | 0 |
| Pain | 1 (8) | 0 | 0 |
| Pigmentation | 10 (83) | 0 | 0 |
| Acute skin
reaction | 8 (67) | 2 (17) | 0 |
| Breast hematoma | 0 | 0 | 0 |
| Pulmonary/lung
fibrosis | 0 | 0 | 0 |
Discussion
Individualized treatment for patients with
early-stage breast cancer consists of BCS and irradiation (WBI or
PBI) or BCS only, based on the fact that two subgroups of patients
co-exist: those who are presumed to have occult disease and those
who are free of tumor residue. However, it is not currently
possible to identify these two patient subgroups.
To date, all trials regarding the omission of
irradiation therapy, even in highly selected low-risk early breast
cancer patients treated with BCS, have been unsuccessful (7–9). In
addition, it has been consistently demonstrated that the local
recurrence rate is significantly lower when adjuvant radiotherapy
is administered to the mammary gland (10,11).
As a result, combined with the concept of the multicentric origin
of breast cancer, WBI has become the standard procedure after BCS.
However, many authors have questioned the necessity of WBI in
early-stage breast cancer, since most of the local recurrences have
been observed to occur near or close to the tumor bed, justifying
the administration of PBI (12,13).
If PBI proves to be efficacious, it will offer the potential
advantage of reduced treatment-related toxicity and improvement in
the quality of life of patients by confining treatment to a limited
volume of breast tissue adjacent to the lumpectomy cavity and by
decreasing the volume of lung and heart that is irradiated as
compared to WBI (14).
A number of studies on PBI have been carried out in
North America and Europe, and most clinical results are promising
(15–17). Recently, a detailed systematic
review analyzed almost all aspects of accelerated PBI (APBI),
including the history of the patients and the rationale for the
selection of the APBI, treatment techniques used, as well as the
radiobiological aspects (18). In
this review, the ipsilateral breast recurrence rates for most APBI
cases were lower than 8% compared to patients receiving WBI. The
most comprehensive study was from the William Beaumont Hospital,
USA, where 199 patients were enrolled from 1993. The inclusion
criteria included patients ≥40 years of age with ductal carcinoma
only, T ≤3 cm, no EIC, pN0 (prior to 1997 also pN1) and negative
surgical margins ≥2 mm. The 5-year actuarial local failure rate was
only 1.2% after APBI with interstitial brachytherapy, as compared
to WBI. We based our inclusion criteria for APBI on those of the
William Beaumont Hospital (19),
with certain adjustments: negative surgical margins were increased
to ≥5 mm and only patients who placed extreme emphasis on the
protection of normal tissues were included.
The only non-invasive technique for APBI is
three-dimensional conformal radiotherapy (3D-CRT). The local
failure rate for APBI by 3D-CRT is comparable to that of
brachytherapy or other invasive techniques, such as mammosite or
intraoperative radiation (20).
3D-CRT is more acceptable as it eliminates the need for an
additional surgical procedure, has an improved dose homogeneity,
and reduces the risk of fat necrosis. Furthermore, 3D-CRT has
become common practice at most cancer centers, including those in
developing countries, and does not require additional medical staff
training, unlike brachytherapy. In the present study, 12 patients
were treated with 3D-CRT; 95% of the isodose surface covered 100%
of the PTV, the average area of the breast that received 115% of
the prescribed dose was zero. The most common adverse reactions
were grade 2 radioactive erythema in 2 (17%) cases and pigment
deposition in 10 (83%). All patients had satisfactory cosmetic
results.
In China, BCS is not a commonly opted for procedure
compared to Western countries. In the West, younger women with a
higher education level and a good socioeconomic status generally
tend to undergo BCS. Some patients have no alternative besides BCS
due to combined heart and/or lung morbidity. Almost all patients
treated with BCS fulfill 5–6 weeks of conventional fractionated
irradiation. In mainland China, patients who decide to undergo BCS
are usually prepared for conventional fraction irradiation, but
consider tumor control and improvement in the quality of life.
Thus, our PBI trial design was focused on controlling the local
tumor recurrence rate. In our opinion, the goal of any PBI trial is
the comparability of the local tumor control of PBI to that of WBI.
Although each of the APBI dose-fractionation schedules is
determined by referring to the equivalent biological dose (BED)
(21), in comparison with conventional fractionation, the BED is an
indeterminate factor that may impact the results of an APBI trial.
Therefore, a combination of conventional fractionation with 3D-CRT
was used to ensure a logistically simpler and more practical method
for PBI testing.
In our study, 12 patients complied with the
conditions of the trial. The patients were followed up for more
than 41 months, and no cases of recurrence or distant metastasis
were noted. PBI and WBI appeared to demonstrate an equivalent local
tumor control rate. In addition, the present study demonstrated the
superior efficacy and cosmetic results of BPI, and the patients
experienced few adverse reaction.
Due to the slow nature of local recurrences in
breast cancer, a very long follow-up is required. This research is
therefore still in its initial stages. Moreover, the number of
cases was limited. Thus, further studies are necessary in order to
reach an objective conclusion.
Abbreviations:
|
PBI,
|
partial breast irradiation;
|
|
APBI,
|
accelerated partial breast
irradiation;
|
|
BCS,
|
breast-conserving surgery;
|
|
CTV,
|
clinical target volume;
|
|
PTV,
|
planning target volume;
|
|
WBI,
|
whole breast irradiation;
|
|
EIC,
|
extensive intraductal components;
|
|
3D-CRT,
|
three-dimensional conformal
radiotherapy;
|
|
BED,
|
equivalent biological dose
|
Acknowledgements
The authors wish to thank Ms. Xiaohong
Wang, Ms. Jingya Wang and Mr. Fei Peng for their excellent
technical assistance.
References
|
1.
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241.
2002.PubMed/NCBI
|
|
2.
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty year
follow-up of a randomized study comparing breast conserving surgery
with radical (Halstead) mastectomy for early breast cancer. N Engl
J Med. 347:1227–1232. 2002.PubMed/NCBI
|
|
3.
|
Nold RJ, Beamer RL, Helmer SD and McBoyle
MF: Factors influencing a women’s choice to breast-conserving
surgery versus modified radical mastectomy. Am J Surg. 180:413–418.
2000.
|
|
4.
|
Fyles AW, McCready DR, Manchul LA, Trudeau
ME, Merante P, Pintilie M, Weir LM and Olivotto IA: Tamoxifen with
or without breast irradiation in women 50 years of age or older
with early breast cancer. N Engl J Med. 351:963–970.
2004.PubMed/NCBI
|
|
5.
|
Malmström P, Holmberg L, Anderson H,
Mattsson J, Jönsson PE, Tennvall-Nittby L, Balldin G, Lovén L,
Svensson JH, Ingvar C, Möller T, Holmberg E and Wallgren A; Swedish
Breast Cancer Group: Breast conservation surgery, with and without
radiotherapy, in women with lymph node-negative breast cancer: a
randomised clinical trial in a population with access to public
mammography screening. Eur J Cancer. 39:1690–1697. 2003.
|
|
6.
|
Simon R: Optimal two-stage designs for
phase II clinical trials. Control Clin Trials. 10:1–10. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schnitt SJ, Hayman J, Gelman R, Eberlein
TJ, Love SM, Mayzel K, Osteen RT, Nixon AJ, Pierce S, Connolly JL,
Cohen P, Schneider L, Silver B, Recht A and Harris JR: A
prospective study of conservative surgery alone in the treatment of
selected patients with stage I breast cancer. Cancer. 77:1094–1100.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Liljegren G, Holmberg L, Adami HO, Westman
G, Graffman S and Bergh J: Sector resection with or without
postoperative radiotherapy for stage I breast cancer: five-year
results of a randomized trial. J Natl Cancer Inst. 86:717–722.
1994.PubMed/NCBI
|
|
9.
|
Mannino M and Yarnold J: Local relapse
rates are falling after breast conserving surgery and systemic
therapy for early breast cancer: can radiotherapy ever be safely
withheld. Radiother Oncol. 90:14–22. 2008. View Article : Google Scholar
|
|
10.
|
Clarke M: Meta-analyses of adjuvant
therapies for women with early breast cancer: the Early Breast
Cancer Trialists’ Collaborative Group overview. Ann Oncol.
17(Suppl. 10): S59–S62. 2006.
|
|
11.
|
Winzer KJ, Sauer R, Sauerbrei W, Schneller
E, Jaeger W, Braun M, Dunst J, Liersch T, Zedelius M, Brunnert K,
Guski H, Schmoor C and Schumacher M; German Breast Cancer Study
Group: Radiation therapy after breast-conserving surgery; first
result of a randomized clinical trial in patients with low risk of
recurrence. Eur J Cancer. 40:998–1005. 2004.PubMed/NCBI
|
|
12.
|
Rosen PP, Fracchia AA, Urban JA,
Schottenfeld D and Robbins GF: ‘Residual’ mammary carcinoma
following simulated partial mastectomy. Cancer. 35:739–747.
1975.
|
|
13.
|
Touboul E, Buffat L, Belkacémi Y, Lefranc
JP, Uzan S, Lhuillier P, Faivre C, Huart J, Lotz JP, Antoine M,
Pène F, Blondon J, Izrael V, Laugier A, Schlienger M and Housset M:
Local recurrences and distant metastases after breast-conserving
surgery and radiation therapy for early breast cancer. Int J Radiat
Oncol Biol Phys. 43:25–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Park SS, Grills IS, Chen PY, Kestin LL,
Ghilezan MI, Wallace M, Martinez AM and Vicini FA: Accelerated
partial breast irradiation for pure ductal carcinoma in
situ. Int J Radiat Oncol Biol Phys. Aug. 26–2010.(Epub ahead of
print).
|
|
15.
|
Baglan KL, Sharpe MB, Jaffray D, Frazier
RC, Fayad J, Kestin LL, Remouchamps V, Martinez AA, Wong J and
Vicini FA: Accelerated partial breast irradiation using 3D
conformal radiation therapy. Int J Radiat Oncol Biol Phys.
55:302–311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Vicini F, Winter K, Straube W, Wong J,
Pass H, Rabinovitch R, Chafe S, Arthur D, Petersen I and McCormick
B: A phase I/II trial to evaluate three-dimensional conformal
radiation therapy confined to the region of the lumpectomy cavity
for stage I/II breast carcinoma: initial report of feasibility and
reproducibility of Radiation Therapy Oncology Group (RTOG) study
0319. Int J Radiat Oncol Biol Phys. 63:1531–1537. 2005.
|
|
17.
|
Vicini FA, Kestin L, Chen P, Benitez P,
Goldstein NS and Martinez A: Limited field radiation therapy in the
management of early-stage breast cancer. J Natl Cancer Inst.
95:1205–1210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Offersen BV, Overgaard M, Kroman N and
Overgaard J: Accelerated partial breast irradiation as part of
breast conserving therapy of early breast carcinoma: a systematic
review. Radiother Oncol. 90:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Azria D and Hennequin C: Impact of
radiotherapy modalities on local control and survival in adjuvant
breast cancer treatment. Cancer Radiother. 13:434–445.
2009.PubMed/NCBI
|
|
20.
|
Bovi J, Qi XS, White J and Li XA:
Comparison of three accelerated partial breast irradiation
techniques: treatment effectiveness based upon biological models.
Radiother Oncol. 84:226–232. 2007. View Article : Google Scholar
|