Introduction
Pulmonary alveolar proteinosis (PAP) is a rare
disorder first described in 1958 by Rosen et al (1). Alveolar spaces are progressively
filled with a phospholipoproteinaceous material presumably caused
by malfunction of the balance between surfactant production by type
II pneumocytes and surfactant removal. The latter is affected
primarily by alveolar macrophages. A diagnosis of PAP can be
confirmed by typical histopathological findings of lung biopsy
specimens or the appearance of bronchoalveolar lavage. Whole-lung
lavage (WLL) introduced by Ramirez in the late 1960s, is still the
gold standard therapy (2). Therapy
with granulocyte macrophage colony-stimulating factor (GM-CSF) is
another option, but its long-term safety has not yet been confirmed
(3). The severity and natural
history of alveolar proteinosis is variable, and severe hypoxemia
may occur during the course of the disease. In patients with poor
clinical condition and hypoxemia, WLL is difficult to perform due
to possible complications involving general anesthesia. WLL often
requires more than 4 hours per lung to perform (4). For surgery, the time is longer, and
complication rates can be high. In these patients, multiple
segmental lavage (MSL) with flexible bronchoscopy (FB) can be
initially carried out to prepare the patient for the long-lasting
general anesthesia required for WLL.
Materials and methods
Multiple segmental lavage
In our technique, FB is wedged as accurately as
possible in all of the segments without error during the procedure.
Before and during MSL, 2% xylocaine and low-dose parenteral
sedation with midazolam and phentanyl are administered. In general,
the lavage is preferably carried out on the lung part or lobe noted
to have the most extensive accumulation as determined by radiology.
While the patient breaths oxygen through a nasal cannula, a FB is
passed through the mouth and placed in each segmental bronchus.
Warm saline solution is instilled via a syringe in 50-ml aliquots
and is removed by suction. The returning lavage fluid is initially
milky and gradually becomes clear. The tip of the FB is then
switched to another segment of the orifice. Segments or subsegments
of all of the lobes in the right or left lung are cleaned one by
one using this method. As it is successful and minimally stressful
for the physician and patient, MSL can be referred to as
‘prewash’.
Whole-lung lavage
In our technique, WLL of the preferred lung is
performed under general anesthesia via isolation of the two lungs
with a double-lumen endotracheal tube. Performance of single-lung
ventilation lasts for a duration of 5 to 10 hours. The correct
positioning of the tube is confirmed by fluoroscopy and fiberoptic
bronchoscopy. Invasive arterial access for frequent blood gas
analysis and continuous blood pressure monitoring should be carried
out in addition to routine anesthesia monitoring which consists of
O2 saturation, CO2 level and gas analysis and
ECG. Two large bore (18-G) IV access sites are initiated
immediately after induction of anesthesia for rapid infusion of
fluids. Central venous access is not preferred due to the risk of
pneumo-hemothorax during the insertion of the catheter. Anesthesia
induction can be performed with propofol 2 mg/kg, remiphentanyl 1
μg/kg, and rocuronium 0.6 mg/kg, and management is recommended to
be continued with sevoflurane 2% in 50% oxygen/50% air, remi
phentanyl 0.01 μg/kg IV bolus, when needed. Hemodynamic and
respiratory parameters of the patient should be monitored
throughout the procedure. Both lungs should be ventilated with 100%
oxygen for 10 min for the denitrogenation process. The isolation of
each lung is confirmed by water seal testing. Since the residual
nitrogen bubbles can diminish the access of lavage fluid to the
alveolar space and consequently the efficacy of WLL, after
separation of the lungs, the lumen of the endotracheal tube leading
to the lung to be lavaged is clamped proximally at the end of
expiration (at functional residual capacity) for 5 min to achieve
adequate degassing. Warmed isotonic saline solution at 37˚C is then
instilled by gravity 50 cm above the carina. After filling the lung
with approximately 800 ml of saline, the lavage process consists of
slowly instilling 500-ml aliquots in each cycle via a catheter.
Manual chest percussion should be performed throughout the
procedure as it has been noted that chest wall percussion enhances
the removal of proteinaceous material. During the procedure, the
position of the patient can be changed from supine to left or right
lateral position intermittently. The average procedure lasts
approximately 5–10 hours. An average range of 12 to 20 liters of
warmed saline should be used, and lavage should be discontinued
when the returning fluid becomes clear. The patient can be
repositioned several times to a lateral decubitis position in
relation to the non-lavaged lung. The major concerns which should
be considered during repositioning of the patient include the
possible malpositioning of the double-lumen tube and ischemic
complications of the extremities. Extra precautions should be taken
to prevent these complications by checking the positioning of the
tube, monitoring the airway pressure changes, air leakage and
placing supporting pillows under the thighs, head and axilla.
Case reports
Case 1
A female patient, 31 years of age, was admitted to
the hospital with exertional dyspnea experienced for two years. She
had a three pack-year history of smoking. Her dyspnea had
progressively increased, and hemoptysis and cough had developed one
month earlier. She had never been exposed to occupational dust, and
her past medical history was unremarkable. On physical examination,
her pulse was 122/min and respiratory rate, 28/min. Bilateral
crackles were audible at both lung bases. On laboratory analyses,
the white cell count was 9100/mm3, the erythrocyte
sedimentation rate (ESR) 8 mm/h and lactate dehydrogenase was 523
mg/dl (normal range (N), 240–480). Cholesterol and triglyceride
levels were 219 mg/dl (N, <200 mg/dl) and 164 mg/dl (N, <150
mg/dl), respectively. Blood gas analysis showed moderate hypoxemia
(PaO2, 49.2 mmHg). Pulmonary function tests revealed a
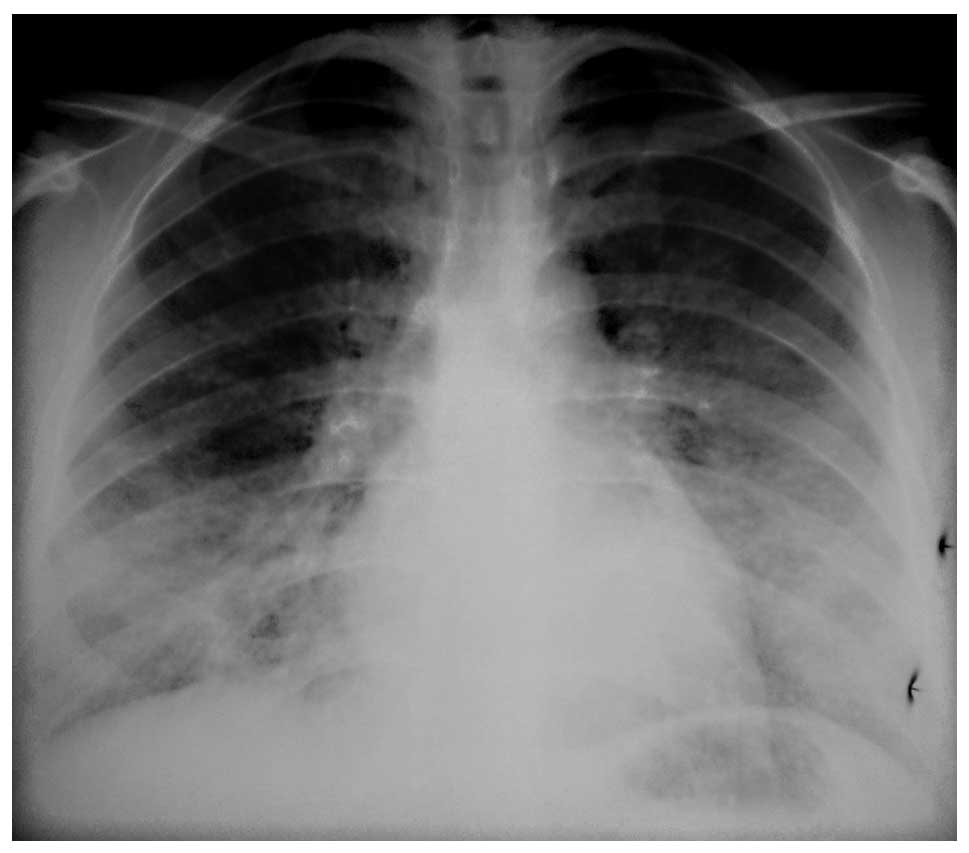
restrictive pattern with a reduced DLCO (55%) value. A chest X-ray
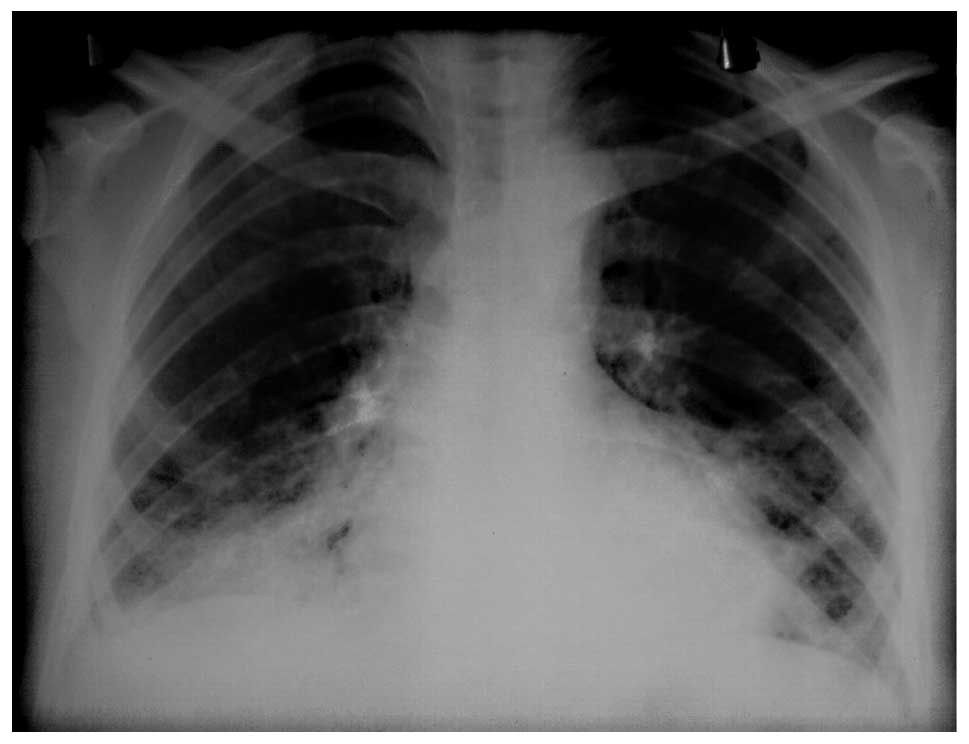
detected bilateral opacities at the mid-lower zones (Fig. 1). A thoracic high resolution
computerized tomography (HRCT) revealed bilateral diffuse ground
glass opacities and interlobular septal thickening. Bronchoalveolar
lavage (BAL) fluid revealed a milky turbid appearance. A
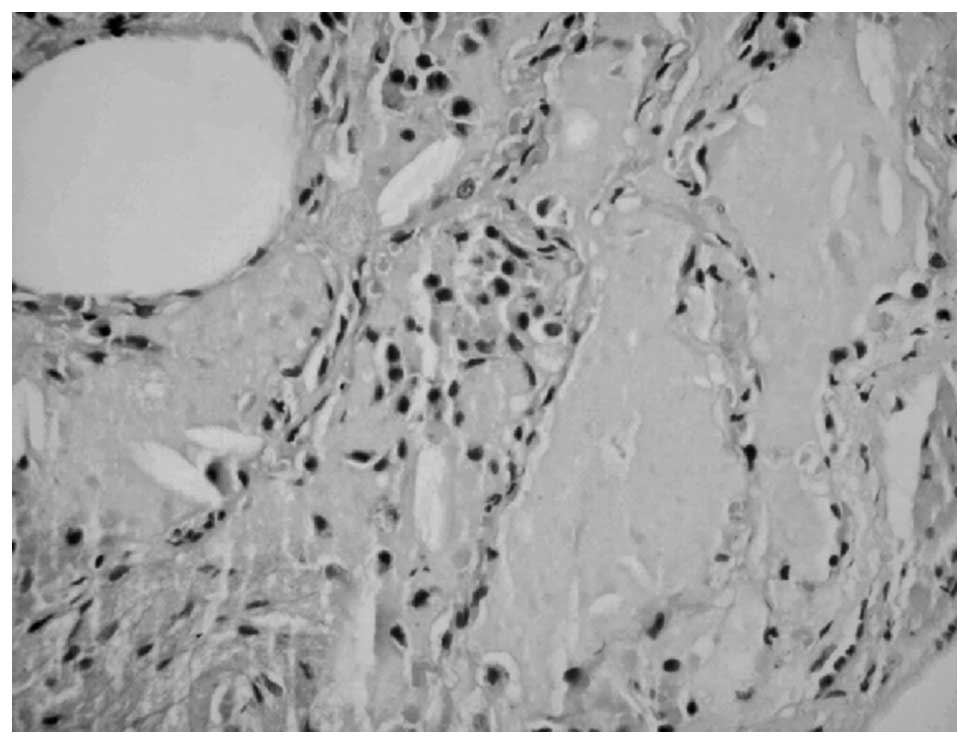
transbronchial lung biopsy (TBLB) was taken, and histopathologic
examination revealed PAS (+) lipoproteinaceous material deposition
(Fig. 2). Accordingly, a diagnosis
of PAP was made. WLL was planned; however, due to the poor clinical
condition of the patient with hypoxemia and her relatively
high-risk profile, she was not considered as a suitable candidate
for general anesthesia. Therefore, MSL under local anesthesia was
performed. A total of 2000 ml saline was instilled, and
approximately 1600 ml was aspirated from the right lung. MSL lasted
approximately 1 hour without any complication. Following MSL, the
PaO2 level increased to 60.3 mmHg on breathing ambient
air and remained stable until the WLL procedure scheduled a few
days later. The patient was re-evaluated and consequently scheduled
for general anesthesia.
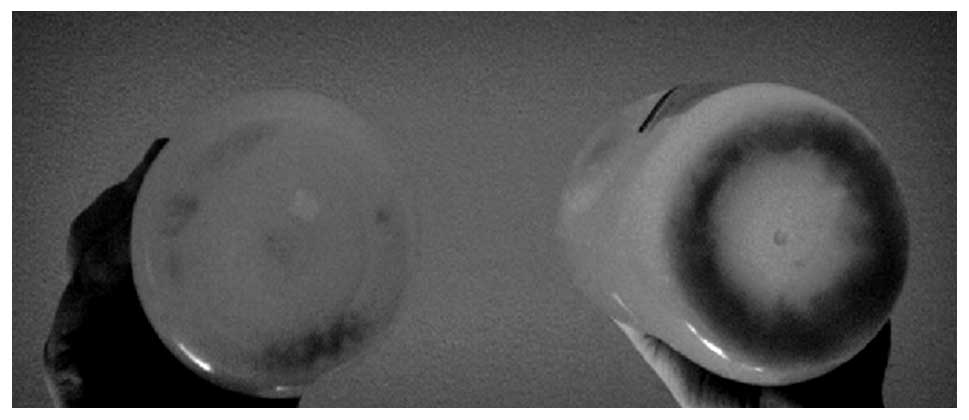
In total, 13 liters of warmed saline solution were
instilled, and 12 liters were obtained (Figs. 3 and 4). Immediately after the procedure, the
double-lumen tube was replaced with a single-lumen tube by an
anesthesiologist. The patient was transferred to the ICU, and
mechanical ventilation was continued for 12 hours. There was no
complication except mild hypokalemia and metabolic acidosis.
After one month, WLL of the right lung was performed
using the same technique mentioned above. During WLL, severe
hypoxia (SpO2 <70%) and an increase in airway
pressure (40 cmH20) developed 2 hours after the
initiation of the single-lung ventilation due to malpositioning of
the double-lumen tube. The patient was immediately extubated and
reintubated with the double-lumen tube, and correct positioning of
the tube was confirmed by fluoroscopy and fiberoptic bronchoscopy.
The rest of the procedure was uneventful. In this second procedure,
a total of 12 liters of saline was instilled, and 11 liters was
drained without any complications. After these combined lavage
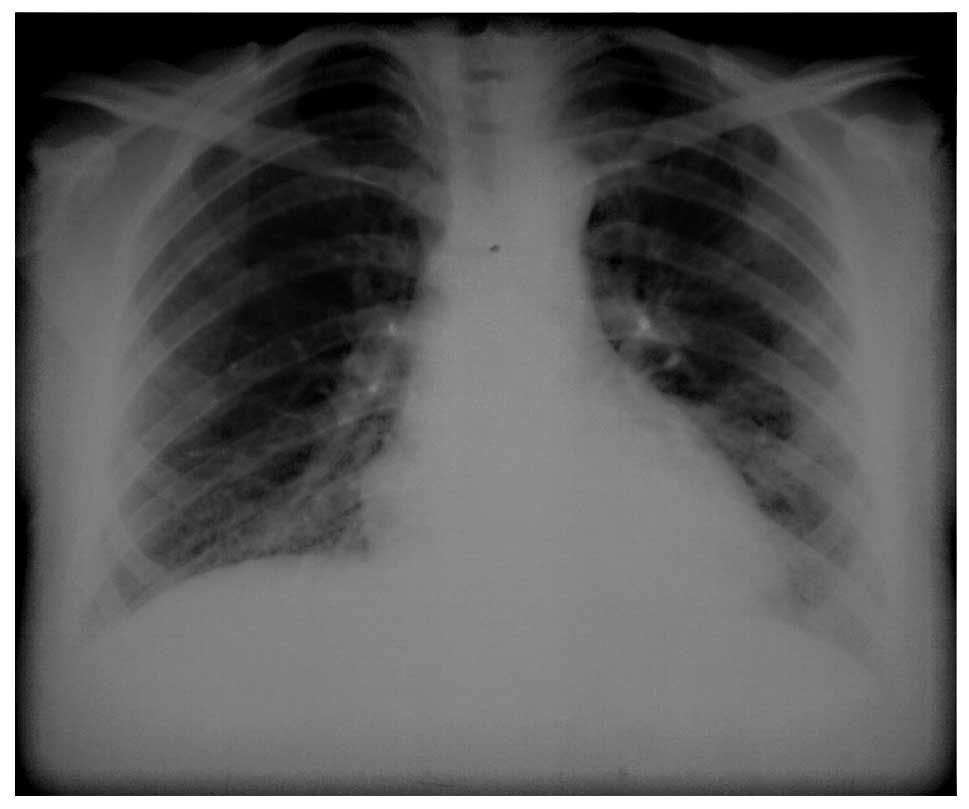
procedures, marked clinical, physiological and radiological
improvements (Fig. 5) were
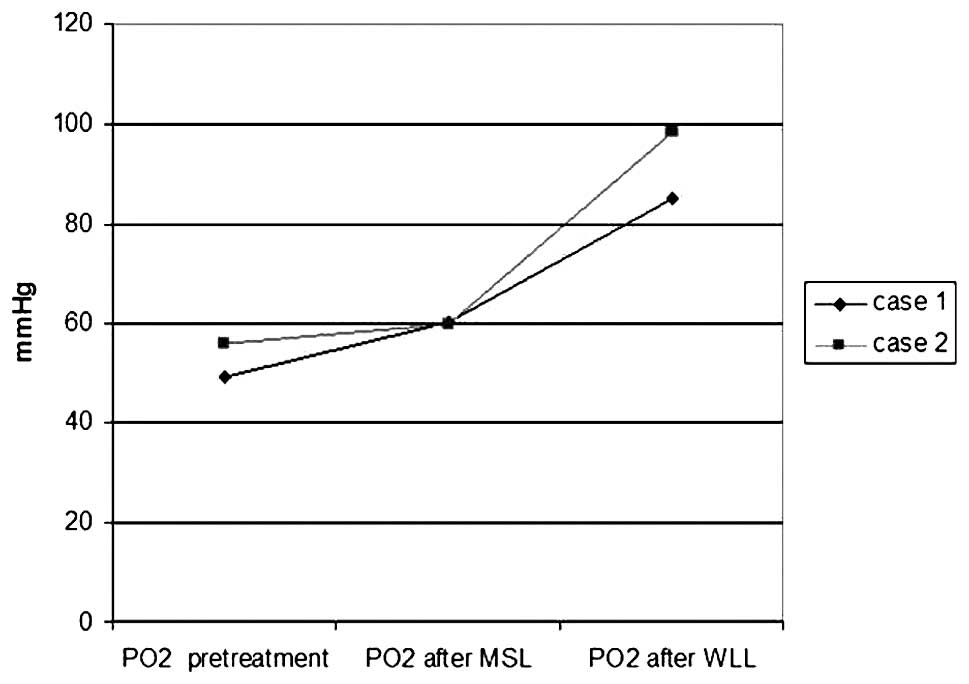
observed. PaO2 and diffusion capacity increased to 85
mmHg and 75%, respectively (Fig.
6).
Case 2
A female, 44 years of age, was admitted to the
hospital with exertional dyspnea for a one-year period. She had a
five pack-year history of smoking and had worked in a cloth factory
for five years. There was no significant exposure to organic or
inorganic material. On physical examination, her pulse and
respiratory rate were 76/min and 26/min, respectively. Bilateral
crackles were audible at both lung bases. On laboratory analyses,
the white cell count and ESR were normal. The blood gas analysis
showed hypoxemia (PaO2, 56 mmHg), and pulmonary function
tests revealed a restrictive pattern with a decreased value of DLCO
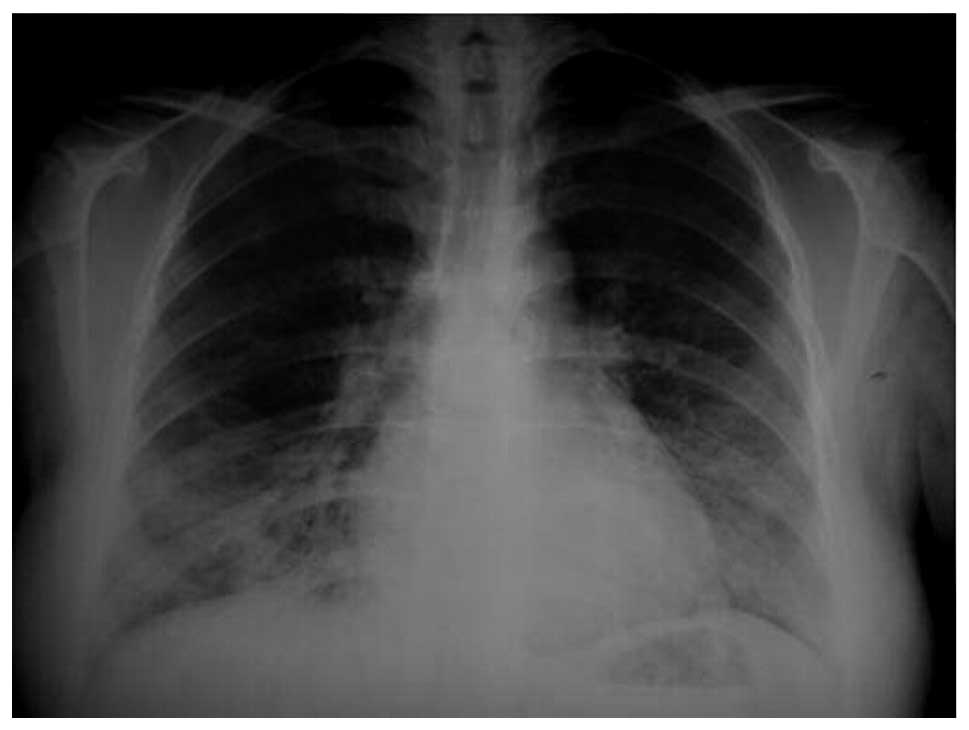
(58%). Chest radiograph revealed bilateral infiltrations at the
mid-lower zones (Fig. 7) and
thoracic HRCT showed bilateral diffuse ground glass opacities and
interlobular septal thickening showing a crazy paving pattern.
Bronchoalveolar lavage fluid revealed a milky turbid appearance.
The diagnosis of PAP was established by open lung biopsy.
A combined lavage procedure was planned as in case
1. MSL was performed using a total of 2600 ml warmed saline for the
right lung and 2300 ml for the left lung until blood gases became
high enough (above 60 mmHg) for general anesthesia. Two WLL
procedures were performed separated by an interval of 1 month. The
first WLL was carried out using 17 liters of saline for the right
lung (Fig. 8) and the second using
15 liters for the left lung. No complications were noted for either
MSL or the WLLs. After the procedures, clinical and radiological
improvements (Fig. 9) were noted,
and the PaO2 level rose to 98.5 mmH along with increased
diffusion capacity (82%) (Fig.
6).
Discussion
Pulmonary alveolar proteinosis is a rare disorder
characterized by the accumulation of lipoproteinaceous material
within the alveolar compartment (1). PAP is classified as congenital,
secondary and idiopathic. The pathophysiology of PAP is
characterized by several mechanisms; surfactant protein B mutations
and granulocyte monocyte colony-stimulating factor receptor
defects, inability of the macrophage to catabolize surfactant, and
the presence of certain systemic disorders and exposure to various
materials. Idiopathic PAP is rare, with a prevalence of
0.37/100,000 individuals, yet it constitutes 90% of all cases
(5,6). Surgical lung biopsy is the gold
standard for the diagnosis of PAP, but in an appropriate clinical
setting, a diagnosis can be confirmed by BAL and/or TBB (7). In this report, the diagnosis of PAP
was made by TBLB in case 1 and surgical lung biopsy in case 2.
According to the aforementioned descriptions, these two cases were
determined to be idiopathic PAP lacking a secondary reason.
According to literature studies, treatment of PAP
includes corticosteroids (8),
potassium iodide (9),
streptokinase (10) and
aerosolized trypsin. However, none of these agents has adequate
efficacy (11). More recently,
therapy with GM-CSF has been attempted. Although a positive effect
of GM-CSF has been shown in PAP, its long-term safety has not been
determined, and the optimal dose, optimal duration of treatment and
the optimal route of GM-CSF remain unclear (12–15).
Whole-lung lavage is now considered to be the most
effective treatment for PAP (4,15–16).
There are no standard indications for WLL, although the following
criteria have been proposed: daily life activity-impairing dyspnea,
PaO2 <60 mmHg and shunt fraction >10–12% (6). The major adverse effect of WLL is
hypoxemia that can be improved with a high inspired oxygen
concentration. Hemodynamic changes can also occur with single-lung
ventilation which may necessitate invasive monitoring during the
procedure. The major risks of WLL concern the correct placement of
the double-lumen endotracheal tube. In the case of wrong
replacement, spilling of fluid from the lavaged lung to the
ventilated lung can occur. Other complications include pleural
collections, hydropneumothorax, barotrauma and hypothermia. Due to
these potential complications and since the patients are usually
hypoxemic and in poor clinical condition, WLL is frequently
impossible to be performed. In such cases, multiple segmental or
lobar lavage by FB has been reported as a possible alternative to
WLL. In several case reports, it has been reported that MSL is a
simple and safe procedure which has led to an improvement in PAP
(3,17). However, the fluid yielded by this
method is small, and the volume of lavage fluid is limited. Hence,
it is useful for patients with less advanced disease (3). In contrast, our cases had advanced
disease and poor clinical condition with hypoxemia, thus they were
not good candidates for therapy with MSL alone. MSL was carried out
initially in order to prepare the patients for WLL. Although there
have been many publications, there is no published report of the
use of MSL and WLL together in the same case.
Cheng et al reported three cases treated with
MSL under local anesthesia (3).
They instilled warm saline solution via a syringe in 50-ml aliquots
at the orifice of the lobar bronchus and all segments which was
removed by suction. They stopped the procedure when the returning
fluid became clear or the patient could no longer tolerate the
discomfort. They repeated the procedure two to five times, and in
each procedure, one lobe was lavaged. The volume of instilled fluid
for one lobe was changed from 1700 to 2050 ml. In contrast, since
our main aim was to prepare patients for WLL, we instilled the same
volume of solution, and we discontinued the procedure earlier as
the aspirated fluid became bright. After MSL or ‘prewash’, in both
cases, we observed clinical and physiological improvements with an
increase in PaO2 level. Thus, WLL was subsequently
performed.
There are several important concerns when performing
WLL. The first step should include appropriate degassing of the
lung to be lavaged. Preoxygenization prior to degassing is very
important to ensure replacement of alveolar nitrogen with oxygen,
as residual bubbles can diminish the access of lavage fluid to the
alveolar space and consequently the efficacy of WLL. In the
presented cases, adequate degassing was noted. Second, performing
chest percussion can enhance the removal of the accumulated
material (18). Hammon et
al reported that during WLL, manual chest percussion is
superior to mechanical percussion (19). Indeed, in case 1, the initial fluid
returns were typically milky, but after the fifth cycle, the fluid
became clearer (Figs. 3 and
4). Then, manual chest percussion
was performed, and we observed that the manual percussion enhanced
the removal of proteinaceous material making the receiving fluids
turbid again. Therefore, we strongly recommend manual chest
percussion throughout the procedure. We halted the procedure when
the returning fluid became clear. After WLL, in both patients,
marked clinical and physiological improvements were noted without
any serious complications (Fig.
6).
Anesthesia for WLL is a challenging procedure for
several reasons. As discussed above, these patients are commonly
associated with severe hypoxia increasing the anesthesiaassociated
risks. The risk of pneumothorax is also increased. Moreover,
single-lung ventilation is required for anesthesia for WLL.
Single-lung ventilation increases the risk of shunting, hypoxia and
carbon dioxide retention. Pre-existing respiratory failure
exacerbates the detrimental effects of single-lung ventilation. In
addition, alveolar lavage leads to more ventilation-perfusion
mismatch. An extensive evaluation is required during the recruiting
phase to evaluate whether the patient is able to undergo WLL under
general anesthesia. Possible complications should be discussed with
the patients and relatives. The anesthesiology team should be
familiar with lung separation and single-lung ventilation
techniques, possible complications and pathological changes during
long-lasting anesthesia procedures (20). In the present cases, patients were
strictly examined and evaluated for their ability to undergo
general anesthesia. Moreover, after the initiation of general
anesthesia, proper monitoring was started and close hemodynamic and
respiratory data were obtained. Positioning of the double-lumen
tube was confirmed several times during the procedure. These
measures were the determining factors for the successful outcome of
the patients during the immediate postoperative period. Without any
doubt, the successful outcome of WLL requires close collaboration
of the thoracic physicians and the anesthesiology team.
In this report, we described our experience using
combined lavage techniques for the management of PAP. MSL, or
‘prewash’, is recommended for hypoxemic patients prior to WLL to
improve oxygenation. These two techniques (MSL and WLL) can be used
consecutively in patients with PAP who are initially unable to
undergo general anesthesia. Several precautions should be taken in
the surgical room for the safety and the success of the procedure
such as close monitoring and repositioning of the patient,
maintenance and inspection of the correct tube position, and manual
chest wall percussion.
References
|
1.
|
Rosen SH, Castleman B and Liebow AA:
Pulmonary alveolar proteinosis. N Engl J Med. 258:1123–1142. 1958.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ramirez J, Nyka W and McLaughlin J:
Pulmonary alveolar proteinosis: diagnostic techniques and
observations. N Engl J Med. 268:165–171. 1963. View Article : Google Scholar
|
|
3.
|
Cheng SL, Chang HT, Lau HP, Lee LN and
Yang PC: Pulmonary alveolar proteinosis: treatment by
bronchofiberscopic lobar lavage. Chest. 122:1480–1485. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Beccaria M, Luisetti M, Rodi G, et al:
Long-term durable benefit after whole lung lavage in pulmonary
alveolar proteinosis. Eur Respir J. 23:526–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ben-Dov I, Kishinevski Y, Roznman J, et
al: Pulmonary alveolar proteinosis in Israel: ethnic clustering.
Isr Med Assoc J. 1:75–78. 1999.PubMed/NCBI
|
|
6.
|
Ioachimescu OC and Kavuru MS: Pulmonary
alveolar proteinosis. Chron Respir Dis. 3:149–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wang BM, Stern EJ, Schmidt RA and Pierson
DJ: Diagnosing pulmonary alveolar proteinosis: a review and an
update. Chest. 111:460–466. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Davidson JM and Macleod WM: Pulmonary
alveolar proteinosis. Br J Dis Chest. 463:13–28. 1969. View Article : Google Scholar
|
|
9.
|
Larson RK and Gordinier R: Pulmonary
alveolar proteinosis: report of six cases, review of the literature
and formulation of a new theory. Ann Intern Med. 62:292–312. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bala RM and Snidal DP: Pulmonary alveolar
proteinosis. A case report and review of the literature. Dis Chest.
49:643–651. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Pallav LS, Hansell D, Lawson PR, Reid KB
and Morgan C: Pulmonary alveolar proteinosis: clinical aspects and
current concepts on pathogenesis. Thorax. 55:67–77. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kavuru MS, Sullivan EJ, Piccin R,
Thomassen MJ and Stoller JK: Exogenous granulocyte-macrophage
colony-stimulating factor administration for pulmonary alveolar
proteinosis. Am J Respir Crit Care Med. 161:1143–1148. 2000.
View Article : Google Scholar
|
|
13.
|
Seymour JF, Dunn AR, Vincent JM, Persneill
JJ and Pain MC: Efficacy of granulocyte-macrophage
colony-stimulating factor in acquired alveolar proteinosis. N Engl
J Med. 335:1924–1925. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Seymour JF, Persneill JJ, Schoch OD, et
al: Therapeutic efficacy of granulocyte-macrophage
colony-stimulating factor in patients with idiopathic acquired
alveolar proteinosis. Am J Respir Crit Care Med. 163:524–531. 2001.
View Article : Google Scholar
|
|
15.
|
Trapnell BC, Nakata K and Kavuru MS:
Pulmonary alveolar proteinosis syndrome. Textbook of Respiratory
Medicine. 5th edition. Mason RJ, Broaddus VC, Martin TR, King TE,
Schraufnagel DE, Murray JF and Nadel JA: Saunders Elsevier;
Philadelphia: pp. 1516–1536. 2010
|
|
16.
|
Prakash UB, Barham SS, Carpenter HA, Dines
DE and Marsh HM: Pulmonary alveolar phospholipoproteinosis:
experience with 34 cases and a review. Mayo Clin Proc. 62:499–518.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Edis EC, Tabakoglu E, Caglar T, Hatipoglu
ON, Cevirme L and Alagol A: Treatment of a primary pulmonary
alveolar proteinosis case with severe hypoxaemia by using segmental
lavage technique. Ann Acad Med Singapore. 36:871–872.
2007.PubMed/NCBI
|
|
18.
|
Perez A and Rogers RM: Enhanced alveolar
clearance with chest percussion therapy and positional changes
during whole-lung lavage for alveolar proteinosis. Chest.
125:2351–2356. 2004. View Article : Google Scholar
|
|
19.
|
Hammon WE, McCaffree DR and Cucchiara AJ:
A comparison of manual to mechanical chest percussion for clearance
of alveolar material in patients with pulmonary alveolar
proteinosis (phospholipidosis). Chest. 103:1409–1412. 1993.
View Article : Google Scholar
|
|
20.
|
Webb ST, Evans AJ, Varley AJ and Klein AA:
Anaesthesia for serial whole-lung lavage in a patient with severe
pulmonary alveolar proteinosis: a case report. J Med Case Reports.
2:3602008. View Article : Google Scholar : PubMed/NCBI
|