Contents
Introduction
Malignant features of metastatic CTCs
Methods for separating CTCs
CTC enrichment
CTC identification
CTCs and cancer stem cells
Clinical relevance of CTCs
Advanced tools for tailored therapy?
Concluding remarks
Introduction
Metastasis to distant sites (e.g., lungs, liver,
bone and brain) via the bloodstream or lymph nodes is a major cause
of cancer-related mortality (1–3).
Circulating tumor cells (CTCs) play an important role in cancer
relapse and metastasis. CTCs identical to those in primary tumors
were first discovered by Ashworth as early as 1869 (4), and were later regarded as a hallmark
of the ‘leukemic phase’ of cancer (5). CTCs were proposed as a novel
minimally invasive prognostic and predictive marker that reflects
the biological characteristics of tumors, and have been the subject
of an increasing number of clinical studies. Identifying cancer
cells among the millions of normal blood cells during the early
stages of cancer, however, is challenging. In recent years, many
new methods have been developed to enrich and detect these rare
CTCs in peripheral blood (6–13).
The different technologies involved, coupled with the heterogeneity
of the screened populations, make the clinical significance of CTCs
difficult to interpret (7). Thus,
it is necessary to standardize the detection methods used to
identify CTCs in order to determine their biological and clinical
relevance. The detection of dynamic changes and malignant features
within these rare cells is closely associated with the efficacy of
therapy and with prognosis (6,14–24).
CTCs may play an important role in the detection of early relapse
and in the assessment of prognosis and the efficacy of the chosen
therapy for both established cancers and metastatic precursor
cells.
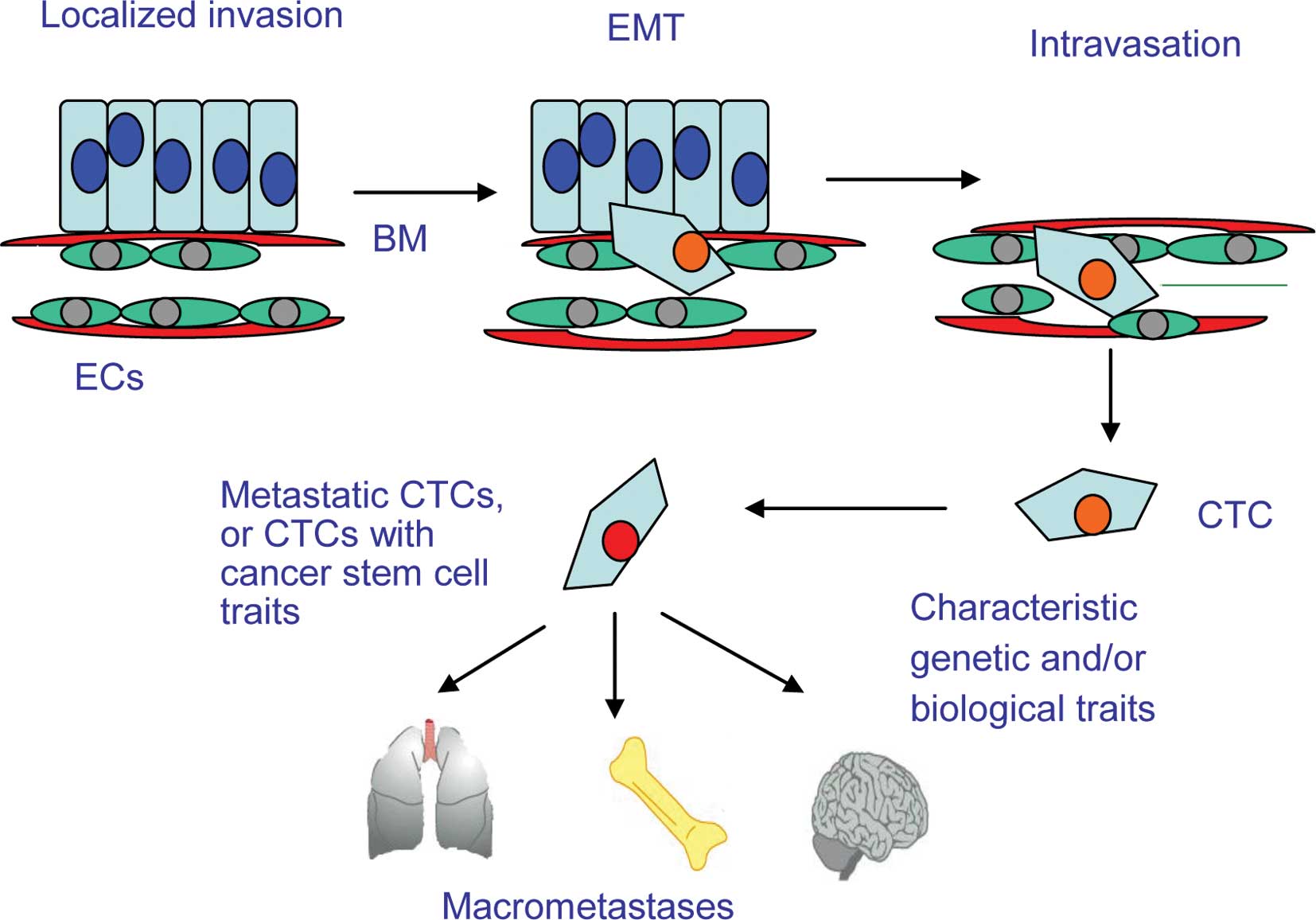
Malignant features of metastatic CTCs
Occult tumor cells may persist in a dormant or low
proliferative state after curative therapy. It is these cells that
are responsible for tumor relapse and metastasis. Such cells, which
are not detectable by current routine diagnostic methods, may play
an important role in recurrence as they may express different
biological characteristics and/or markers from those of the primary
tumor (25). Therefore, the
detection and characterization of CTCs is of the utmost clinical
relevance. The processes by which cancer cells proliferate
[angiogenesis, detachment from the primary tumor, epithelial to
mesenchymal transition (EMT) and intravasation into the vasculature
followed by extravasation into distal organs] are not yet fully
understood (Fig. 1). As cancer
cells invade through the basement membrane, they undergo EMT and
are shed into the circulation. This process is vital for
metastasis. Due to changes in the microenvironment and destruction
by shearing forces in the blood vessels and by immune cells, most
CTCs in the circulation undergo apoptosis. The result is that only
a very small proportion of CTCs survive (5). However, given ideal conditions, they
may extravasate and develop into micrometastases (26). A mouse model of tumor metastasis
showed that 106 cancer cells were shed into the blood
stream every day, and that most of these failed to form metastases
due to cellular apoptosis (27).
The malignant phenotype of CTCs is closely related
to their metastatic tendency. CTCs derived from breast cancer,
renal cell carcinoma, prostate cancer, colon cancer and melanoma
have been shown to be malignant by analyzing the aneusomy of
chromosomes 1, 3, 4, 7, 8, 11 or 17 using dual or tricolor
fluorescence in situ hybridization (FISH) (28,29).
During the early stages of tumor formation, hypoxia triggers
neovascularization within the lesions, which facilitates tumor
dissemination via the blood vessels. This process occurs prior to
proliferation and, of course, before the appearance of clinical
symptoms in the patients (30).
Therefore, early detection of CTCs may enable the early diagnosis
of cancer. As CTCs are shed into the blood of breast cancer
patients every few hours, apoptotic CTCs are replenished by cells
originating from the primary tumor, thus maintaining a balance
between apoptotic and proliferating CTCs. Several CTCs may remain
in the circulation for up to 22 years, which may explain tumor
dormancy (31).
Studies of the signaling pathways within CTCs have
found that breast cancer CTCs co-express p-FAK, p-PI3K and HER2,
suggesting that they possess activated protein kinases, which
regulate their metastatic ability (32,33).
Analysis of these proliferative or metastatic signaling pathways
may help to elucidate the mechanisms underlying the malignant
biology of CTCs.
Whether CTCs metastasize to other organs depends on
their genetic profile. The metastatic potential of CTCs is closely
related to their heterogeneity, the microenvironment and the
efficiency of the patient's immune system. The immune system sees
CTCs as ‘foreign’, but many CTCs escape immune surveillance and
manage to form micrometastases and macrometastases in distant
organs (34).
Methods for separating CTCs
Recent technological advances in the detection and
characterization of CTCs have proven helpful in understanding the
biology and clinical significance of these rare cells. Many methods
can be used to enrich and identify CTCs (35), such as immunomagnetic cell
enrichment (which includes magnetic activated cell separation;
MACS) (36), the CellSearch
system, isolation by the size of epithelial tumor cells (ISET)
(37,38), epithelial immunospot (EPISPOT),
immunological assays based on enzyme-linked immunosorbent assay
(ELISPOT) technology (7) and
microchips that enrich CTCs from millions of white blood cells
(12,13) (Table
I).
 | Table I.Detection of CTCs in solid tumors and
prognosis. |
Table I.
Detection of CTCs in solid tumors and
prognosis.
| Tumor type | TNM | Samples (n) | Methods | Markers | Positivity of CTC
(%) | Prognosis | Refs. |
|---|
| Breast cancer | IV | 80 | CellSearch |
Ck8/18/19+
CD45− cells | 61.0 | PFS | 16 |
| Breast cancer | IV | 177 | CellSearch |
CK8/18/19+
CD45− cells | 49.0 | PFS
OS | 19 |
| Breast cancer | I–II | 148 | RT-PCR | CK19 mRNA | 29.7 | DFS
OS | 66 |
| Breast cancer | I–II | 444 | RT-PCR | CK19 mRNA | 40.8 | DFS
OS | 67 |
| Hepatic cancer | I–IV | 101 | RT-PCR | Albumin mRNA | 45.0 | DFS no
OS no | 78 |
| Colorectal
cancer | I–IV | 196 | RT-PCR | CEA, CK19 mRNA | 85.0 | PFS | 68 |
| Colorectal
cancer | I–II | 66 | RT-PCR | CEA mRNA | 54.5 | OS no | 79 |
| Colorectal
cancer | IV | 413 | CellSearch |
CK8/18/19+
CD45− cells | 26 ≥3 CTCs | OS
PFS yes | 51,69 |
| Prostate
cancer | IV | 162 | RT-PCR | PSA mRNA | 44.0 | OS | 70 |
| Bladder cancer | T1G3 | 54 | RT-PCR | Survivin mRNA | 44.0 | PFS | 76 |
| Non-small cell lung
cancer | I–II | 61 | RT-PCR | TIF-1
CK19 mRNA | 36.1
42.6 | PFS | 77 |
CTC enrichment
CTCs in the peripheral blood of patients with solid
tumors are rare, and so the sensitivity and specificity of
detection is dependent upon the particular technological approach
used. In the past, immunomagnetic methods of enrichment have been
widely used (9,11). Basically, these techniques involve
either positive or negative selection of the chosen cell type. For
positive selection, beads are linked to the epithelial antibody,
EpCAM, to enrich the rare cells. For negative selection, beads are
linked the common leukocyte antigen, CD45, in order to deplete the
hematopoietic cells (11).
Positive enrichment systems include the CellSearch system (approved
by the FDA in 2004), CTC-chips, MACS (when used for positive
selection) and the OncoQuick system. The major negative enrichment
system is MACS. Although many methods enable the isolation of CTCs
with an epithelial phenotype, such as EpCAM and CK antigen
expression, the disadvantage is that epithelial proteins are
down-regulated during EMT (39),
which may affect the efficiency of CTC detection (Fig. 2). Therefore, positive enrichment
strategies may not detect EpCAM-negative CTCs (40). A comparison of two different
methods for enumerating CTCs in carcinoma patients showed that the
CellSearch system (mean detection rate, 20 CTCs/7.5 ml of blood) is
a more accurate and sensitive method for enumerating CTCs than the
OncoQuick system (mean detection rate 3, CTCs/7.5 ml; P<0.0001)
(41,42).
The newly developed microchip technology provides
the highest detection rate for CTCs. The chip separates CTCs from
whole blood using EpCAM-coated micro-posts under controlled laminar
flow conditions to ensure optimal interaction between the cells
(12,35,43).
The purity of the CTCs was found to be >100 times that obtained
using other methods (12).
However, this enrichment system requires further clinical
validation of its accuracy. In cancer cell spiking experiments
using the CellSearch system, recovery rates were 85 and 92%,
respectively, from two different patient groups (44,45).
New CTC-chip micro-fluidic technology showed a 10- to 100-fold
improvement in CTC yield from patient samples over the CellSearch
system (44). However, this method
needs further validation using large numbers of clinical samples.
Since not all CTCs express EpCAM, the use of magnetic beads labeled
with both CD146 and EpCAM antibodies increases the rate of
detection (46).
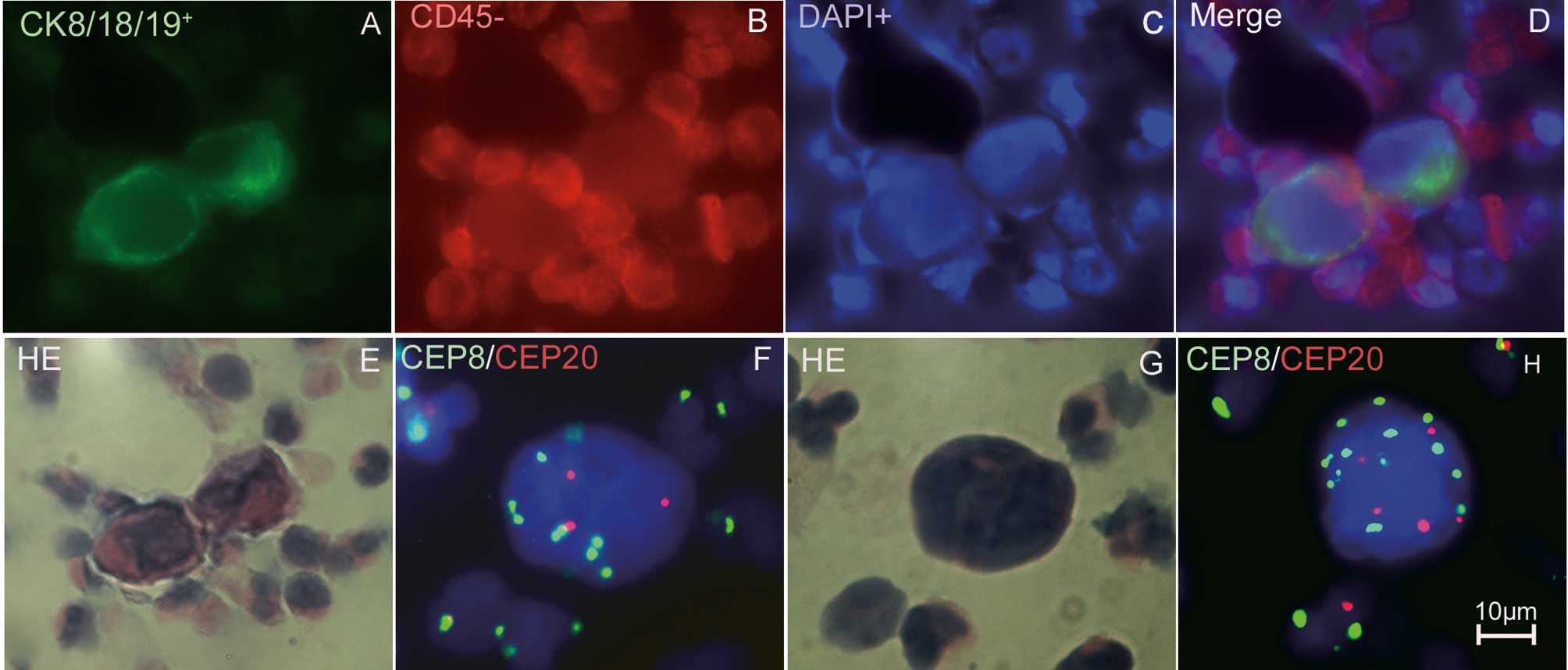
CTC identification
The key to successfully identifying CTCs is
differentiating them from other hematopoietic cells and squamous
cells. CTCs can be identified as malignant by cytomorphology,
tumor-specific antigen expression and aneusomy of the chromosomes
(47). The techniques used to
identify CTCs are broadly divided into cytometric- and nucleic
acid-based approaches (Table II).
Cytometric approaches use immunocytochemical methods to
characterize individual tumor cells. Nucleic acid-based approaches
detect DNA or RNA sequences that are differentially expressed in
tumor cells and normal controls (11). However, pseudogenes or non-specific
sequences may be identified using nucleic acid-based approaches.
When the efficacy of the therapy or the tumor burden is to be
evaluated, nucleic acid-based approaches may be a simple and
straightforward choice. If intact cellular morphology and genetic
phenotype are to be studied, cytometric approaches may be preferred
(48). FISH has been used to
directly identify circulating genetically abnormal cells (CACs) in
the peripheral blood of patients with non-small cell lung cancer.
Depending on the expression levels of abnormal biomarkers, up to 45
CACs per microliter were detected, compared to <10 CACs per
milliliter in most studies using immunomagnetic beads (22,49–53).
Peripheral blood-based membrane-array assays with a panel of
tumor-related mRNA markers (hTERT, CK-19, CEA and MUC1) were used
to identify CTCs in gastric cancer patients using a nucleic
acid-based approach (54). This
technique has a satisfactory level of sensitivity and specificity
(54). In vivo,
non-invasive label-free detection and eradication of circulating
metastatic melanoma cells using two-color photoacoustic flow
cytometry and a diode laser has also been attempted (55). Additionally, GFP-expressing
virus-based methods are remarkably simple and allow the precise
enumeration of viable CTCs (56).
Another approach used is fiber-optic array scanning technology
(FAST), which applies laser-printing techniques to the detection of
rare CTCs. The combination of FAST enrichment and automated digital
microscopy (ADM) imaging yields the level of performance required
for the reliable detection of metastatic colorectal cancer cells in
the blood (57,58). Further testing using clinical
samples and integration of all the modules into a single, fully
automated smart miniaturized system will enable minimally invasive
testing for the detection and characterization of CTCs (25).
 | Table II.Methods for CTC analysis. |
Table II.
Methods for CTC analysis.
| System | Blood volume per
test (ml) | Principle of
enrichment | Principle of
identification | Sensitivity | Recovery rate
(%) | Refs. |
|---|
| OncoQuick | 10–15 | Density
centrifugation and a porous barrier membrane | Cytometry or
RT-PCR | 1
CTC/9.5×104 WBC | 70–90 | 41,42 |
| ISET | 10 | Cell size | CK19 RT-PCR | 1
CTC/2×106 WBC | NS | 37,38 |
| MACS | 5–16 | Depletion of
leukocytes or enrichment of epithelial originated cells using
immunobeads | CK8/18+
cells | 1
CTC/1×106 WBC | NS | 36 |
| CellSearch
(Veridex) | 7.5 | Beads coated with
EpCAM |
CK8/CK18/CK19+ and
CD45− cells | 1
CTC/1×107 WBC | 85 | 45 |
| CTC microchip | 5 | Microposts coated
with EpCAM |
CK8/CK18/CK19+ and
CD45− cells | 1
CTC/1×107 WBC | >65 | 43 |
| FAST | | Beads coated with
EpCAM | CK+
cells |
10−6 | >86 | 57 |
CTCs and cancer stem cells
The cancer stem cell theory suggests that only a
small fraction of cancer cells are stem cells capable of
self-differentiation and self-replication. A few hundred cancer
stem cells (CSCs) were found to cause carcinogenesis in NOD/SCID
mice, whereas non-cancer stem cells did not (3). The proposed existence of rare CSCs
within an ordinary tumor cell population with limited proliferative
potential implies that such rare progenitors have the real ability
to metastasize (59). CD133,
CD44+/CD24−/low and CXCR4 have been proposed
as markers for CSCs in glioma, breast, colon, prostate, pancreatic
and esophageal cancer (60). CD133
mRNA detected in the peripheral blood of patients with colon cancer
is a predictor of poor prognosis (61).
CD44+/CD24−/low CTCs have also been
identified in the peripheral blood of patients with breast cancer.
These putative CSC marker-positive CTCs have been associated with
tumor metastasis (62). At
present, the relationship between CSCs and CTCs is not clear.
Further research is required to validate any relationship and their
clinical relevance.
Clinical relevance of CTCs
CTCs may predict tumor relapse, therapeutic efficacy
and/or prognosis (Table I). The
results of one study found that in case of lung cancer metastasis
to distant organs, the number of CTCs clearly increased (63). CTCs may also be surrogate markers
for early tumor metastasis (63).
In several carcinomas, peripheral blood CTCs were found to be a
predictor of poor prognosis (24,64).
Cristofanilli et al analyzed CTCs in the peripheral blood of
177 metastatic breast cancer patients enrolled in multi-center
double-blind prospective studies. The results showed that a
detection rate of ≥5 CTCs/7.5 ml peripheral blood indicated a worse
prognosis than a rate of <5 CTCs/7.5 ml. CTC dynamics clearly
reflected the efficacy of the chosen therapy (19). The basal level of CTCs is a good
prognostic indicator, and changes in CTC levels during treatment
may reflect the efficacy of the chosen therapy (16). A retrospective study revealed a
relationship between the overall survival rate of 37 prostate
cancer patients and CTC levels; the overall survival rate of
patients with ≥5 CTCs/7.5 ml peripheral blood was 0.7 years
compared to 4 years for patients with <5 CTCs/7.5 ml (P=0.002)
(65). In patients with breast
cancer, CTCs were still detectable in the peripheral blood after
the primary tumor was eradicated, and the risk of recurrence in
these patients was greater than for those with no detectable CTCs
(19). The detection of CK19
mRNA-positive CTCs in the peripheral blood of patients with stage I
or II breast cancer prior to adjuvant therapy was an independent
prognostic indicator of poor clinical outcome (66), mainly in those patients with
ER-negative, triple-negative and HER2-positive early-stage breast
cancer (67). CK20 mRNA-positive
CTCs detected within 24 h of primary colorectal cancer resection
were also found to be a strong predictor of recurrence (68).
The number of CTCs detected by the CellSearch system
before and during treatment was found to be an independent
predictor of progression-free survival and overall survival in
patients with metastatic colorectal cancer. This implies that CTCs
may provide prognostic information in addition to the results
obtained from imaging studies (51,69).
Detection of PSA-positive CTCs is a significant prognostic factor
for survival in patients with hormone refractory prostate cancer
(70). EGFR expression by CTCs in
patients with metastatic breast cancer was also used as a
predictive marker for targeted therapy (71). The greatest number of CTCs was
detected in patients with esophageal cancer immediately after
surgery and correlated with the rate of tumor relapse (72). Wild-type KRAS, detected in CTCs
from patients with metastatic colon cancer, strongly correlated
with their sensitivity to chemotherapy and with prognosis (73,74).
Apoptotic CTCs can be detected in the peripheral blood of patients
with prostate cancer after chemotherapy, which may reflect
treatment efficacy (75). The
presence of surviving CTCs is also an independent prognostic factor
in patients with T1G3 bladder cancer (76). Another study demonstrated that
TTF-1 mRNAexpressing CTCs may be a useful surrogate predictor of
disease progression before clinical symptoms are apparent in
non-small cell lung cancer (77).
However, in some solid tumors, the detection of CTCs
in the peripheral blood does not predict prognosis. For example,
circulating albumin mRNA failed to provide significant information
regarding the diagnosis and prognosis of hepatocellular carcinoma
(78), and the postoperative
detection of blood CTCs using CEA mRNA had no prognostic
significance in patients with colorectal cancer after surgical
resection (79). The use of CTCs
as prognostic indicators in some carcinomas is unreliable. This may
be due to the different methods used to detect them and the
different populations studied.
Advanced tools for tailored therapy?
Dynamic molecular analysis of CTCs may be helpful
for targeting therapy in individual patients. HER2 expression is
not increased in the primary tumor during the early stages of
breast cancer, but increased expression can be detected in CTCs in
advanced breast cancer. This may be why patients treated with
herceptin have a good prognosis (80). The molecular genetics of CTCs are
similar to those of the primary tumor. Therefore, CTCs may
represent the status of the primary tumor (81). Nearly 98% of patients with
HER2-positive primary and metastatic cancers had CTCs expressing
elevated levels of HER2. However, 33% of patients, in whom the
primary cancer was HER2-negative, had CTCs that were HER2-positive
(44). This suggests that HER2
expression by CTCs may provide the rationale for individually
targeted HER2 therapy (80,82,83).
Serial analysis of CTCs illustrates the molecular evolution of the
primary tumor during the course of treatment. It may also have
another advantage: even once the primary tumor is eradicated, CTCs
continue to provide a ‘real-time’ non-invasive method of cancer
cell genotyping.
CTC detection in peripheral blood is convenient,
rapid and reproducible. Analyzing the characteristics of CTCs may
help to evaluate the efficacy of therapy, provide a unique
diagnostic resource and predict prognosis (84). Enumeration and identification of
CTCs undergoing apoptosis may provide relevant information about
responses to therapy in prostate cancer patients (75). Although the size of the tumor and
the progress of the disease can be evaluated by radiography, its
sensitivity is limited. The opportunity for tumor eradication is
often lost due to the late detection of metastases by radiography
(85). CTC monitoring is an early
reproducible indication of disease status, superior to current
imaging methods. Moreover, CTC levels appear to be superior to
conventional imaging methods (even PET-CT) for evaluation of the
response to treatment (86).
Concluding remarks
Since the phenotype and genotype of metastatic
cancer cells are quite different from those of the primary tumor,
CTC levels provide a more accurate method of evaluating the
efficacy of chemotherapy and targeted therapy than analysis of the
primary tumor (80). When
significant CTC levels are confirmed, it may guide the treatment of
patients who need adjuvant therapy, as a reasonable estimate can be
made as to whether CTCs have been cleared from the peripheral
blood. Tumor malignancy is associated with complex signaling
pathways; therefore, it is desirable that CTCs be used as a tool to
forecast prognosis, preferably in combination with another index,
to comprehensively monitor their clinical relevance (2,55).
Detection, monitoring and molecular analysis of CTCs may provide a
non-invasive approach to the detection of early tumor dissemination
and the assessment of prognosis and appropriate treatment for
established cancers (1). As more
and more standardized and effective methods are established and the
molecular mechanisms involved in metastasis are elucidated, and as
more multi-center large sample clinical trials are validated, CTCs
may be used as a real-time tool for the tailored treatment of
cancer patients.
Acknowledgements
This study was supported by the
Jiangsu Provincial Health Department Science Foundation
(H200970).
References
|
1.
|
Maheswaran S and Haber DA: Circulating
tumor cells: a window into cancer biology and metastasis. Curr Opin
Genet Dev. 20:96–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pantel K, Alix-Panabieres C and Riethdorf
S: Cancer micro-metastases. Nat Rev Clin Oncol. 6:339–351. 2009.
View Article : Google Scholar
|
|
3.
|
Pantel K, Brakenhoff RH and Brandt B:
Detection, clinical relevance and specific biological properties of
disseminating tumour cells. Nat Rev Cancer. 8:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ashworth A: A case of cancer in which
cells similar to those in the tumours were seen in the blood after
death. Aust Med J. 14:146–149. 1869.
|
|
5.
|
Mocellin S, Keilholz U, Rossi CR and Nitti
D: Circulating tumor cells: the 'leukemic phase' of solid cancers.
Trends Mol Med. 12:130–139. 2006.
|
|
6.
|
Peach G, Kim C, Zacharakis E, Purkayastha
S and Ziprin P: Prognostic significance of circulating tumour cells
following surgical resection of colorectal cancers: a systematic
review. Br J Cancer. 102:1327–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Alunni-Fabbroni M and Sandri MT:
Circulating tumour cells in clinical practice: methods of detection
and possible characterization. Methods. 50:289–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Allan AL and Keeney M: Circulating tumor
cell analysis: technical and statistical considerations for
application to the clinic. J Oncol. 2010:4262182010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Panteleakou Z, Lembessis P, Sourla A, et
al: Detection of circulating tumor cells in prostate cancer
patients: methodological pitfalls and clinical relevance. Mol Med.
15:101–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Dotan E, Cohen SJ, Alpaugh KR and Meropol
NJ: Circulating tumor cells: evolving evidence and future
challenges. Oncologist. 14:1070–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ring A, Smith IE and Dowsett M:
Circulating tumour cells in breast cancer. Lancet Oncol. 5:79–88.
2004. View Article : Google Scholar
|
|
12.
|
Nagrath S, Sequist LV, Maheswaran S, et
al: Isolation of rare circulating tumour cells in cancer patients
by microchip technology. Nature. 450:1235–1239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sequist LV, Nagrath S, Toner M, Haber DA
and Lynch TJ: The CTC-chip: an exciting new tool to detect
circulating tumor cells in lung cancer patients. J Thorac Oncol.
4:281–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cristofanilli M: The biological
information obtainable from circulating tumor cells. Breast.
18(Suppl 3): 38–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Botteri E, Sandri MT, Bagnardi V, et al:
Modeling the relationship between circulating tumour cells number
and prognosis of metastatic breast cancer. Breast Cancer Res Treat.
122:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nole F, Munzone E, Zorzino L, et al:
Variation of circulating tumor cell levels during treatment of
metastatic breast cancer: prognostic and therapeutic implications.
Ann Oncol. 19:891–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Apostolaki S, Perraki M, Pallis A, et al:
Circulating HER2 mRNA-positive cells in the peripheral blood of
patients with stage I and II breast cancer after the administration
of adjuvant chemotherapy: evaluation of their clinical relevance.
Ann Oncol. 18:851–858. 2007. View Article : Google Scholar
|
|
18.
|
Cristofanilli M, Hayes DF, Budd GT, et al:
Circulating tumor cells: a novel prognostic factor for newly
diagnosed metastatic breast cancer. J Clin Oncol. 23:1420–1430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Takeuchi H and Kitagawa Y: Circulating
tumor cells in gastrointestinal cancer. J Hepatobiliary Pancreat
Surg. 17:577–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Xenidis N, Ignatiadis M, Apostolaki S, et
al: Cytokeratin-19 mRNA-positive circulating tumor cells after
adjuvant chemotherapy in patients with early breast cancer. J Clin
Oncol. 27:2177–2184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wu C, Hao H, Li L, et al: Preliminary
investigation of the clinical significance of detecting circulating
tumor cells enriched from lung cancer patients. J Thorac Oncol.
4:30–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wong SC, Chan CM, Ma BB, et al: Clinical
significance of cytokeratin 20-positive circulating tumor cells
detected by a refined immunomagnetic enrichment assay in colorectal
cancer patients. Clin Cancer Res. 15:1005–1012. 2009. View Article : Google Scholar
|
|
24.
|
Scher HI, Jia X, de Bono JS, et al:
Circulating tumour cells as prognostic markers in progressive,
castration-resistant prostate cancer: a reanalysis of IMMC38 trial
data. Lancet Oncol. 10:233–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Stakenborg T, Liu C, Henry O, et al:
Automated genotyping of circulating tumor cells. Expert Rev Mol
Diagn. 10:723–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Luzzi KJ, MacDonald IC, Schmidt EE, et al:
Multistep nature of metastatic inefficiency: dormancy of solitary
cells after successful extravasation and limited survival of early
micrometastases. Am J Pathol. 153:865–873. 1998. View Article : Google Scholar
|
|
27.
|
Chang YS, di Tomaso E, McDonald DM, Jones
R, Jain RK and Munn LL: Mosaic blood vessels in tumors: frequency
of cancer cells in contact with flowing blood. Proc Natl Acad Sci
USA. 97:14608–14613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shaffer DR, Leversha MA, Danila DC, et al:
Circulating tumor cell analysis in patients with progressive
castration-resistant prostate cancer. Clin Cancer Res.
13:2023–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Fehm T, Sagalowsky A, Clifford E, et al:
Cytogenetic evidence that circulating epithelial cells in patients
with carcinoma are malignant. Clin Cancer Res. 8:2073–2084.
2002.
|
|
30.
|
Husemann Y, Geigl JB, Schubert F, et al:
Systemic spread is an early step in breast cancer. Cancer Cell.
13:58–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Meng S, Tripathy D, Frenkel EP, et al:
Circulating tumor cells in patients with breast cancer dormancy.
Clin Cancer Res. 10:8152–8162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kallergi G, Mavroudis D, Georgoulias V and
Stournaras C: Phosphorylation of FAK, PI-3K, and impaired actin
organization in CK-positive micrometastatic breast cancer cells.
Mol Med. 13:79–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kallergi X, Kallergi G, Agelaki S, et al:
Phosphorylated EGFR and PI-3K/Akt signaling kinases are expressed
in circulating tumor cells of breast cancer patients. Breast Cancer
Res. 10:R802008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lurje G and Lenz HJ: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Lurje G, Schiesser M, Claudius A and
Schneider PM: Circulating tumor cells in gastrointestinal
malignancies: current techniques and clinical implications. J
Oncol. 2010:3926522010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Taubert H, Blumke K, Bilkenroth U, et al:
Detection of disseminated tumor cells in peripheral blood of
patients with breast cancer: correlation to nodal status and
occurrence of metastases. Gynecol Oncol. 92:256–261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Vona G, Sabile A, Louha M, et al:
Isolation by size of epithelial tumor cells: a new method for the
immunomorphological and molecular characterization of circulating
tumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Pinzani P, Salvadori B, Simi L, et al:
Isolation by size of epithelial tumor cells in peripheral blood of
patients with breast cancer: correlation with real-time reverse
transcriptase-polymerase chain reaction results and feasibility of
molecular analysis by laser microdissection. Hum Pathol.
37:711–718. 2006. View Article : Google Scholar
|
|
39.
|
Muller V, Alix-Panabieres C and Pantel K:
Insights into minimal residual disease in cancer patients:
implications for anti-cancer therapies. Eur J Cancer. 46:1189–1197.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Deng G, Herrler M, Burgess D, Manna E,
Krag D and Burke JF: Enrichment with anti-cytokeratin alone or
combined with anti-EpCAM antibodies significantly increases the
sensitivity for circulating tumor cell detection in metastatic
breast cancer patients. Breast Cancer Res. 10:R692008. View Article : Google Scholar
|
|
41.
|
Balic M, Dandachi N, Hofmann G, et al:
Comparison of two methods for enumerating circulating tumor cells
in carcinoma patients. Cytometry B Clin Cytom. 68:25–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Gertler R, Rosenberg R, Fuehrer K, Dahm M,
Nekarda H and Siewert JR: Detection of circulating tumor cells in
blood using an optimized density gradient centrifugation. Recent
Results Cancer Res. 162:149–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Stott SL, Lee RJ, Nagrath S, et al:
Isolation and characterization of circulating tumor cells from
patients with localized and metastatic prostate cancer. Sci Transl
Med. 2:25ra232010. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Flores LM, Kindelberger DW, Ligon AH, et
al: Improving the yield of circulating tumour cells facilitates
molecular characterisation and recognition of discordant HER2
amplification in breast cancer. Br J Cancer. 102:1495–1502. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Allard WJ, Matera J, Miller MC, et al:
Tumor cells circulate in the peripheral blood of all major
carcinomas but not in healthy subjects or patients with
nonmalignant diseases. Clin Cancer Res. 10:6897–6904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Mostert B, Kraan J, Bolt-de Vries J, et
al: Detection of circulating tumor cells in breast cancer may
improve through enrichment with anti-CD146. Breast Cancer Res
Treat. April 9–2010.(Epub ahead of print).
|
|
47.
|
Fehm T, Solomayer EF, Meng S, et al:
Methods for isolating circulating epithelial cells and criteria for
their classification as carcinoma cells. Cytotherapy. 7:171–185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Ring AE, Zabaglo L, Ormerod MG, Smith IE
and Dowsett M: Detection of circulating epithelial cells in the
blood of patients with breast cancer: comparison of three
techniques. Br J Cancer. 92:906–912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Katz RL, He W, Khanna A, et al:
Genetically abnormal circulating cells in lung cancer patients: an
antigen-independent fluorescence in situ hybridization-based
case-control study. Clin Cancer Res. 16:3976–3987. 2010. View Article : Google Scholar
|
|
50.
|
Maheswaran S, Sequist LV, Nagrath S, et
al: Detection of mutations in EGFR in circulating lung-cancer
cells. N Engl J Med. 359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Cohen SJ, Punt CJ, Iannotti N, et al:
Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Ntouroupi TG, Ashraf SQ, McGregor SB, et
al: Detection of circulating tumour cells in peripheral blood with
an automated scanning fluorescence microscope. Br J Cancer.
99:789–795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Leversha MA, Han J, Asgari Z, et al:
Fluorescence in situ hybridization analysis of circulating tumor
cells in metastatic prostate cancer. Clin Cancer Res. 15:2091–2097.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Wu CH, Lin SR, Yu FJ, et al: Development
of a high-throughput membrane-array method for molecular diagnosis
of circulating tumor cells in patients with gastric cancers. Int J
Cancer. 19:373–379. 2006.PubMed/NCBI
|
|
55.
|
Galanzha EI, Shashkov EV, Spring PM, Suen
JY and Zharov VP: In vivo, noninvasive, label-free detection and
eradication of circulating metastatic melanoma cells using
two-color photoacoustic flow cytometry with a diode laser. Cancer
Res. 69:7926–7934. 2009. View Article : Google Scholar
|
|
56.
|
Kojima T, Hashimoto Y, Watanabe Y, et al:
A simple biological imaging system for detecting viable human
circulating tumor cells. J Clin Invest. 119:3172–3181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Krivacic RT, Ladanyi A, Curry DN, et al: A
rare-cell detector for cancer. Proc Natl Acad Sci USA.
101:10501–10504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Marrinucci D, Bethel K, Lazar D, et al:
Cytomorphology of circulating colorectal tumor cells: a small case
series. J Oncol. 2010:8613412010. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Vermeulen L, Sprick MR, Kemper K, Stassi G
and Medema JP: Cancer stem cells – old concepts, new insights. Cell
Death Differ. 15:947–958. 2008.
|
|
61.
|
Lin EH, Hassan M, Li Y, et al: Elevated
circulating endothelial progenitor marker CD133 messenger RNA
levels predict colon cancer recurrence. Cancer. 110:534–542. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Theodoropoulos PA, Polioudaki H, Agelaki
S, et al: Circulating tumor cells with a putative stem cell
phenotype in peripheral blood of patients with breast cancer.
Cancer Lett. 288:99–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Tanaka F, Yoneda K, Kondo N, et al:
Circulating tumor cells as a diagnostic marker in primary lung
cancer. Clin Cancer Res. 15:6980–6986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Budd GT, Cristofanilli M, Ellis MJ, et al:
Circulating tumor cells versus imaging – predicting overall
survival in metastatic breast cancer. Clin Cancer Res.
12:6403–6409. 2006.
|
|
65.
|
Moreno JG, Miller MC, Gross S, Allard WJ,
Gomella LG and Terstappen LW: Circulating tumor cells predict
survival in patients with metastatic prostate cancer. Urology.
65:713–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Stathopoulou A, Vlachonikolis I, Mavroudis
D, et al: Molecular detection of cytokeratin-19-positive cells in
the peripheral blood of patients with operable breast cancer:
evaluation of their prognostic significance. J Clin Oncol.
20:3404–3412. 2002. View Article : Google Scholar
|
|
67.
|
Ignatiadis M, Xenidis N, Perraki M, et al:
Different prognostic value of cytokeratin-19 mRNA positive
circulating tumor cells according to estrogen receptor and HER2
status in early-stage breast cancer. J Clin Oncol. 25:5194–5202.
2007. View Article : Google Scholar
|
|
68.
|
Allen-Mersh TG, McCullough TK, Patel H,
Wharton RQ, Glover C and Jonas SK: Role of circulating tumour cells
in predicting recurrence after excision of primary colorectal
carcinoma. Br J Surg. 94:96–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Cohen SJ, Punt CJ, Iannotti N, et al:
Prognostic significance of circulating tumor cells in patients with
metastatic colorectal cancer. Ann Oncol. 20:1223–1229. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Halabi S, Small EJ, Hayes DF, Vogelzang NJ
and Kantoff PW: Prognostic significance of reverse transcriptase
polymerase chain reaction for prostate-specific antigen in
metastatic prostate cancer: a nested study within CALGB 9583. J
Clin Oncol. 21:490–495. 2003. View Article : Google Scholar
|
|
71.
|
Payne RE, Yague E, Slade MJ, et al:
Measurements of EGFR expression on circulating tumor cells are
reproducible over time in metastatic breast cancer patients.
Pharmacogenomics. 10:51–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Liu Z, Jiang M, Zhao J and Ju H:
Circulating tumor cells in perioperative esophageal cancer
patients: quantitative assay system and potential clinical utility.
Clin Cancer Res. 13:2992–2997. 2007. View Article : Google Scholar
|
|
73.
|
Yen LC, Yeh YS, Chen CW, et al: Detection
of KRAS oncogene in peripheral blood as a predictor of the response
to cetuximab plus chemotherapy in patients with metastatic
colorectal cancer. Clin Cancer Res. 15:4508–4513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Yang MJ, Chiu HH, Wang HM, et al:
Enhancing detection of circulating tumor cells with activating KRAS
oncogene in patients with colorectal cancer by weighted
chemiluminescent membrane array method. Ann Surg Oncol. 17:624–633.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Larson CJ, Moreno JG, Pienta KJ, et al:
Apoptosis of circulating tumor cells in prostate cancer patients.
Cytometry A. 62:46–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Gradilone A, Petracca A, Nicolazzo C, et
al: Prognostic significance of survivin-expressing circulating
tumour cells in T1G3 bladder cancer. BJU Int. 106:710–715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Yoon SO, Kim YT, Jung KC, Jeon YK, Kim BH
and Kim CW: TTF-1 mRNA-positive circulating tumor cells in the
peripheral blood predict poor prognosis in surgically resected
non-small cell lung cancer patients. Lung Cancer. May 13–2010.(Epub
ahead of print).
|
|
78.
|
Barbu V, Bonnand AM, Hillaire S, et al:
Circulating albumin messenger RNA in hepatocellular carcinoma:
results of a multicenter prospective study. Hepatology.
26:1171–1175. 1997.PubMed/NCBI
|
|
79.
|
Bessa X, Pinol V, Castellvi-Bel S, et al:
Prognostic value of postoperative detection of blood circulating
tumor cells in patients with colorectal cancer operated on for
cure. Ann Surg. 237:368–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Meng S, Tripathy D, Shete S, et al: HER-2
gene amplification can be acquired as breast cancer progresses.
Proc Natl Acad Sci USA. 101:9393–9398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Meng S, Tripathy D, Shete S, et al: uPAR
and HER-2 gene status in individual breast cancer cells from blood
and tissues. Proc Natl Acad Sci USA. 103:17361–17365. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Wulfing P, Borchard J, Buerger H, et al:
HER2-positive circulating tumor cells indicate poor clinical
outcome in stage I to III breast cancer patients. Clin Cancer Res.
12:1715–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Pestrin M, Bessi S, Galardi F, et al:
Correlation of HER2 status between primary tumors and corresponding
circulating tumor cells in advanced breast cancer patients. Breast
Cancer Res Treat. 118:523–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Slade MJ, Payne R, Riethdorf S, et al:
Comparison of bone marrow, disseminated tumour cells and
blood-circulating tumour cells in breast cancer patients after
primary treatment. Br J Cancer. 100:160–166. 2009. View Article : Google Scholar
|
|
85.
|
Smith SL and Rajan PS: Imaging of
pancreatic adenocarcinoma with emphasis on multidetector CT. Clin
Radiol. 59:26–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
De Giorgi U, Valero V, Rohren E, et al:
Circulating tumor cells and [18F]flurodeoxyglucose positron
emission tomography/computed tomography for outcome prediction in
metastatic breast cancer. J Clin Oncol. 27:3303–3311. 2009.
|