Introduction
With its first description in 1925, Wolbach and Howe
implicated vitamin A and its derivatives (retinoids) in epithelial
development and tumorigenesis (1).
The activity of vitamin A, apart from that involved in vision, is
mediated by retinoids. Many years later, De Luca further implicated
the retinoids in differentiation and embryogenesis (2) by showing their effects on limb
development, epithelial integrity and tumorigenesis. Since they are
involved in numerous life processes (embryogenesis, cell growth,
cell differentiation and cell death), retinoids are essential for
life. One of their most important actions is their antitumor
activity, whereby they inhibit tumor growth and promote apoptosis
(3,4). This has led to their therapeutic
application against cancer, for example in the treatment of acute
promyelocytic leukemia (5,6).
Retinoids cross the cell membrane through
hydrophobic interactions and/or endocytosis in order to bind to
their specific receptors: the retinoic acid receptors (RARs)
(7) and rexinoid receptors (RXRs)
(8–10). When retinoids are present in cells,
they bind the RAR/RXR heterodimer, which acts as a transcription
factor. The retinoid-receptor complex binds to the retinoic acid
response element sequence, located near the promoter of target
genes, to induce or inhibit transcription (3).
The RAR family comprises three members: α, β and γ.
RARβ expression is frequently reduced in tumor cells, probably due
to the hypermethylation of its promoter (11,12),
and in association with tumor progression in different organs or
with pathologies in head and neck tissues (13), basal skin cells (14), breast (15), lung (16), esophagus (17), prostate (18), thyroid (19), larynx (20), endometrium (12) and oral tissues (21). All of these studies have concluded
that RARβ should be considered a tumor suppressor (22). However, studies on RARα and γ
expression have reported conflicting results, depending on the
pathology and the technique used.
To the best of our knowledge, the expression of the
RARs has not been reported in patients with colorectal cancer
(CRC). In France, CRC is the second most common cancer in men and
the third most common in women (23). This pathology is classified as the
second leading cause of cancer-related death in industrialized
countries (National Cancer Institute data), and the 5-year survival
rate is very low. CRC development is characterized by four tumor
stages, defined by the International Pathology Tumor Node
Metastasis (pTNM) classification system (24), which is used as the diagnostic
system upon which patient treatment is based. However, few proteins
predictive of response have been identified.
RARs are implicated in homeostasis and, in
particular, in epithelial morphology, which led us to hypothesize
that the expression of these receptors is implicated in CRC
development. Since few predictive proteins are available for CRC,
the aim of this study was to examine the cellular distribution of
the three RARs by immunohistochemical analysis of normal and
pathological human colon tissues from different tumor stages. Their
expression was compared to the cell proliferation rate, which is
known to be associated with tumor growth, and was detected by
immunostaining for Ki-67. The results revealed the importance of
RAR signaling in the progression of CRC. Correlations with tumor
grade, therapeutic response and survival were established.
Materials and methods
Patients and tissue samples
All cases of histologically confirmed CRC were
included, regardless of whether the patients had received
chemotherapy. Based on the Helsinki protocol, the exclusion
criteria included juvenile patients, pregnant or breast-feeding
women, rectal or colonic lesions that were not histologically
confirmed to be CRCs, patients in whom follow-up was impossible and
insufficient or unexploitable tissue due to inadequate
preservation.
Archived formalin-fixed paraffin-embedded blocks of
colon tissues were obtained from the Pathology Department of the
Limoges Teaching Hospital. The specimens were from consecutive
patients who underwent elective resection for CRC between January
2006 and December 2007. Eighty patients (37 women and 43 men) with
a mean age of 71 years (range 41–93) were included prospectively.
The first follow-up evaluation was made on October 31, 2008, with a
median follow-up time of 24 months (range 11–32). The second
follow-up evaluation was made on December 15, 2010, with a median
follow-up time of 46 months (range 25–66). The tumors were graded
according to the pTNM international classification (24). Forty patients had local disease
(stage I, T1/2-N0, n=6; stage II, T3/4-N0, n=34), 26 had regional
lymph-node involvement (stage III, any T-N1/2) and 14 had advanced
disease (stage IV, any T, any N, presence of metastasis).
Histological slides of the primary tumor were reviewed to identify
the normal-appearing areas adjacent to the tumor sites and the
tumor areas, excluding the central tumor zone, which was usually
necrotic. The tissue blocks were sectioned (4-μm thick) and stained
with H&E saffran (HES) for pathological diagnosis, TNM grading
and immunostaining.
Histologically normal colon tissues from 10 patients
who had been treated for benign pathologies, such as idiopathic
chronic constipation (n=7) or diverticulosis (n=3), constituted the
control group and were used to determine the constitutional
expression.
Clinical and pathological parameters
Clinical, paraclinical (biological and imaging) and
histological parameters were collected by Michelle Nouaille and
technicians at the Pathology Department, Limoges Teaching Hospital,
at the time of patient admission. The patients all underwent a
uniform postoperative follow-up by the same team: they were
examined within 1 month of resection, then every 3–4 months for the
first year, every 6 months for the next 3 years and then at
gradually increasing intervals. A clinical examination and
quantification of serum carcinoembryonic antigen (CEA) were
performed at each visit. Computed tomographic (CT) scans were
performed every 6–12 months. A full colonoscopy was performed 1
year after surgery, then once every 3–5 years. Positron emission
tomographic (PET) scans were selectively performed when
abnormalities or axial imaging raised the possibility of
recurrence. Local recurrence was defined as the first clinical,
radiological and/or pathological evidence of a tumor of the same
histological type within the colon. Distant recurrence was defined
as clinical, radiological and/or pathological evidence of systemic
disease at sites including, but not limited to, the liver, lungs,
peritoneum and para-aortic region. Recurrence-free survival and
disease-specific survival were analyzed. ‘Evolution’ was defined as
disease progression without adjuvant therapy, ‘chemoresistance’ as
disease progression during or after adjuvant therapy, and
‘remission’ as the absence of clinical signs of disease
progression.
Antibodies
The antibodies used were rabbit polyclonal
antibodies raised against a peptide mapping to the C-terminus of
human RARα (sc-551; Santa Cruz Biotechnology, Le-Perrayen-Yvelines,
France), human RARβ (sc-552) or human RARγ (sc-550). The
commercially available antibody for Ki-67 (M7240; DakoCytomation
SA, Trappes, France), which recognizes a 395-kDa nuclear protein
expressed during cell-cycle phases (G1, S, G2 and M) (25) was used. For the isotypic controls,
we used immunoglobulin G (IgG) (rabbit, I8140 and mouse, I8765;
Sigma, St. Quentin Fallavier, France).
Control of antibody specificity
Total proteins from the WiDr cell line (American
Type Culture Collection) were extracted using a lysis buffer
following the manufacturer's recommendations (Cell Signaling
Technology, Ozyme, St. Quentin Yvelines, France) in order to
perform Western blotting. The results showed that RAR proteins
stained at the expected molecular weights, without non-specific
binding, as determined with isotypic controls for the three
anti-RAR antibodies (data not shown).
Immunohistochemistry
Ki-67, RARα and RARγ were immuno-histochemically
detected in paraffin-embedded tissues using the BenchMark
technology (Ventana Medical Systems, Illkirch, France). The pathway
RARα and RARγ staining module was used according to the Ki-67
protocol according to the manufacturer's instructions. The
processing of the bar-code-labeled slides was fully automated and
included the following steps: baking the slides, solvent-free
deparaffinization and antigen retrieval in CC1 cell-conditioning
buffer (30 min at 95°C). The samples were incubated with the
primary antibody previously diluted in diluent solution (1:50 for
Ki-67, 1:100 for RARα and 1:150 for RARγ) for 32 min at 37°C.
Horseradish peroxidase (26)-coupled secondary antibody was added
(8 min at 37°C), then the proteins were detected with the
chromogenic substrate diaminobenzidine (DAB) (8 min at 37°C). The
tissue sections were also counterstained with hematoxylin (12 min
at 37°C) and a bluing reagent (4 min at 37°C) to increase the
contrast. The slides were mounted with a non-aqueous mounting
medium.
For RARβ immunohistochemistry, paraffin sections
were deparaffinized in toluene and alcohol, and rehydrated with
phosphate-buffered saline (PBS). Before staining, the sections were
subjected to steam heat antigen retrieval in citrate buffer (200 μM
citric acid, 9.8 mM sodium citrate, pH 7.0) for 5 min. This step
was repeated four times in a microwave oven (750 W). After washing
in PBS, the slides were incubated for 10 min with 5%
H2O2 in methanol to inhibit endogenous
peroxidases. Non-specific sites were blocked with PBS-3% bovine
serum albumin (BSA) for 30 min. The sections were then incubated
with the primary antibody (1:500) in PBS-3% BSA for 1 h at room
temperature. After washing, the epitopes were labeled with the
anti-rabbit HRP Envision™ plus system and visualized with liquid
DAB (Dako SA). The sections were couterstained and examined with a
Leica microscope, and the images were captured with a Zeiss
camera.
To test the specificity of the signals, negative
control experiments were performed either by omitting the primary
antibody, by substituting the primary antibody with non-immune
serum, or by omitting both the primary and secondary antibodies. No
staining was observed in any of the negative controls (data not
shown).
Quantification of immunostaining
Photomicrographs of each slide were captured with a
Zeiss microscope with a magnification of x200. The most
homogeneously stained tumor (T) and non-tumor (NT) areas on each
slide were selected for quantification. Immunoreactivity was scored
by a staining index based on the percentage of positive cells, by a
semi-quantitative estimate, as follows: (−; 0), tissue with
negative staining; (+; 1), tissue with staining in 25–49% of cells;
(++; 2), tissue with staining in 50–74% of cells; (+++; 3), tissue
with staining in ≥75% of cells. ‘Overexpression’ was defined as a
staining index of ≥75%. The results were expressed as the mean of
three independent quantifications made by different
individuals.
Statistical analysis
The overall variations in the staining percentages
for the RARs and Ki-67 and their relationships to tumor grade were
evaluated by analysis of variance (ANOVA) using SYSTAT 12.0 (SPSS,
2007). Tukey's post hoc test was used to assess the
significance of the differences between the stages, and P-values
<0.05 were considered significant. Correlations between the
parameters were visualized by cluster analysis using Spearman's ϱ
as the measure of similarity, using PAST 1.83 (27). Survival curves were constructed
using the free-access software of the Dartmouth-Hitchcock Norris
Cotton Cancer Center (http://biostat.hitchcock.org/BSR/Analytics/CompareTwoSurvivalDistributions.asp).
Results
Baseline characteristics and overall
survival
The overall 2-year survival rate was 70%, probably
due to the age of the patients and the high proportion of
advanced-stage cases. At the time of analysis, patient survival was
100% for stage I, 75% for stage II, 65% for stage III and 47% for
stage IV. Nine patients (8 with stage II and 1 with stage III) died
of causes not related to CRC (cardiac or neurological etiologies).
Adjuvant chemotherapy was administered for stages III and IV.
Twenty patients received no adjuvant therapy (16 stage III and 4
stage IV) due to postoperative death (n=3), age >85 years (n=14)
and/or patient refusal (n=3). Cancer progression occurred in 11/20
patients and cancer recurrence in 9/11 patients during adjuvant
chemotherapy.
Two years later, at the second evaluation, 77
patients had continued with the follow-up (3 had been lost). At
this time, the overall 4-year survival rate was 49%; patient
survival was 100% for stage I, 48% for stage II, 54% for stage III
and 23% for stage IV. Apart from 5 stage II patients who died due
to unrelated causes, 10 patients (5 in stage II, 1 in stage III and
4 in stage IV) succumbed to CRC during the interval between the
first and the second evaluations.
Control of RAR expression in normal
prostate
The use of antibodies against RARs for
immunohistochemistry of chemically fixed tissues was previously
tested in prostate tissue (18).
When different antibody dilutions (1:50 to 1:500) were tested, the
results obtained in normal prostate tissue were reproducible, with
localization patterns similar to those described by Richter et
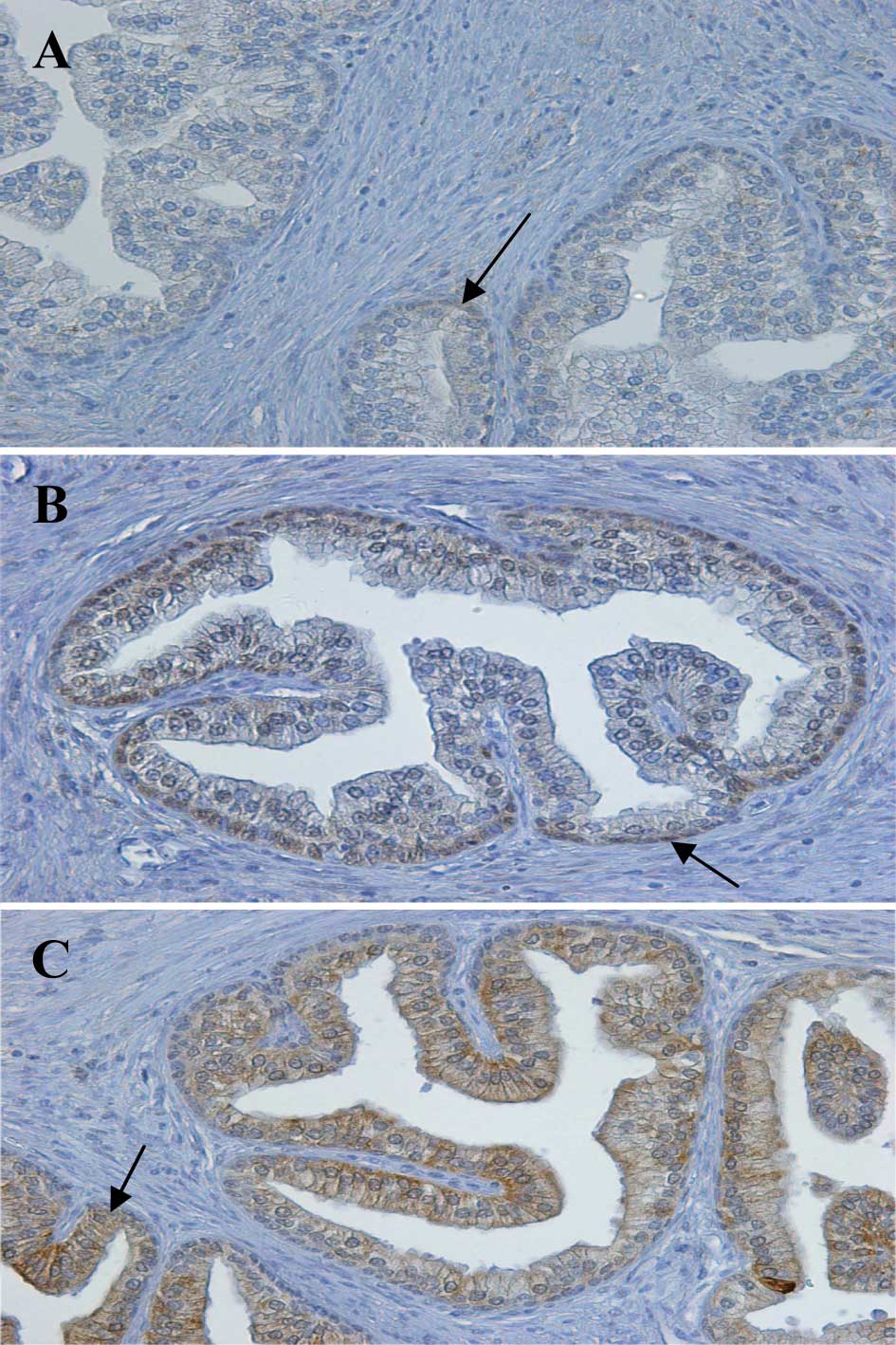
al (18). As shown in Fig. 1 (arrows); for RARα, homogeneous
staining in the cytoplasm with little nuclear staining was noted;
for RARβ, the presence of staining in the basal nuclei was noted;
for RARγ, homogeneous staining in the epithelial cytoplasm with
little nuclear staining was observed. Since these results confirmed
the specificity of the anti-RAR antibodies, they were used on the
CRC tissues.
Ki-67 and RAR expression in different
stages of CRC
The constitutional expression of the proteins was
first evaluated by immunohistochemistry in the normal control
group, then examined in the adjacent NT tissue of each patient, for
use as an internal control. The Ki-67 and RAR staining profiles in
the NT tissues were identical to those observed in the control
group. Finally, the expression of the RARs was examined in the T
and NT areas in the specimens from patients with different stages
of CRC.
Random Ki-67 staining was detected in the nuclei of
all the cells, located both inside and outside the T fields, with
some differences in the percentages of labeled cells among patients
(data not shown). However, ANOVA between the groups of different
stages revealed no statistically significant differences
(P>0.05).
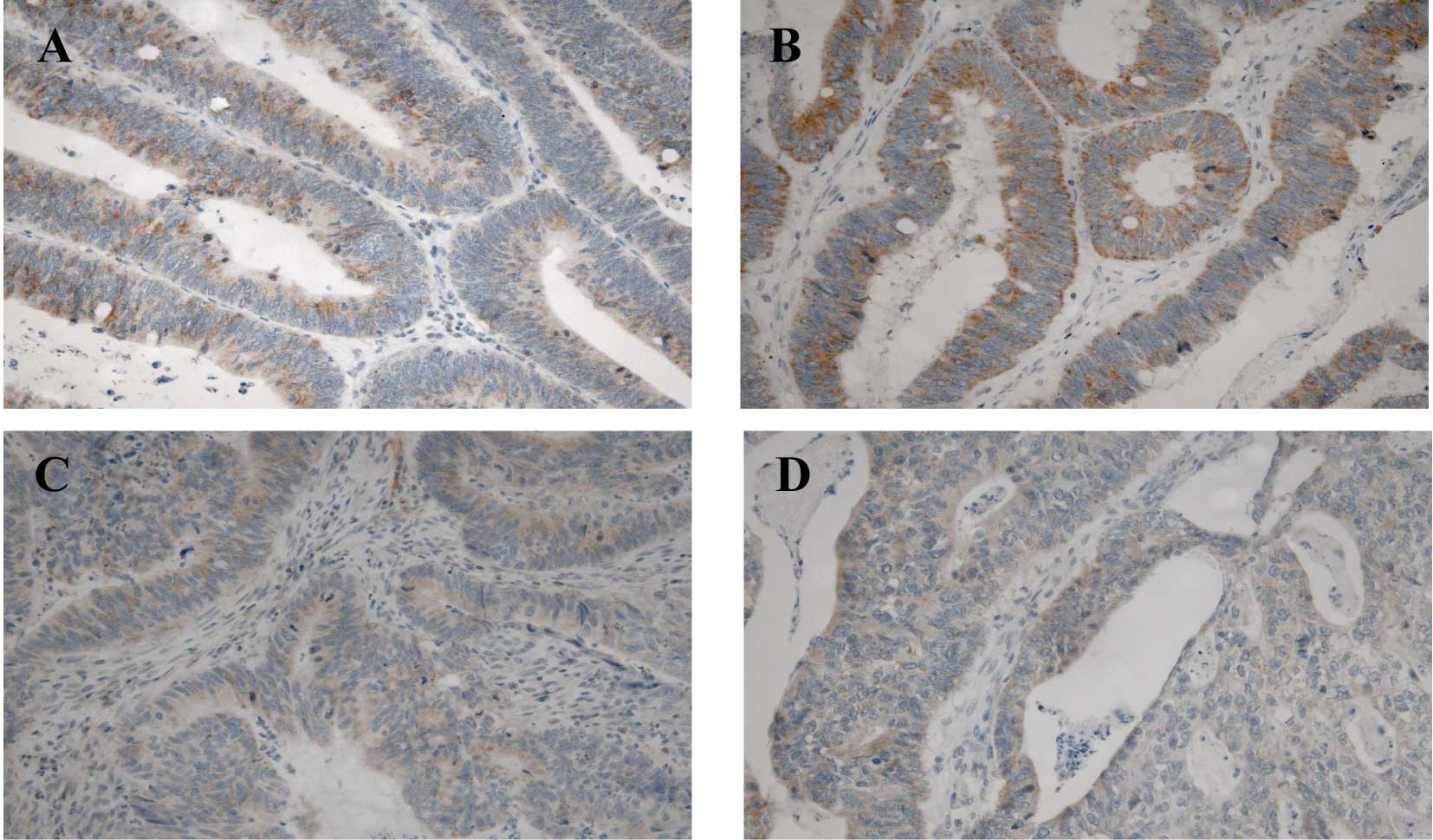
RARα staining was uniformly detected in the
cytoplasm of the epithelial cells in the NT and T tissues (Fig. 2). Of the 80 patients analyzed, all
expressed this receptor in the NT tissues (50–75% of cells), as did
in the control group (data not shown). In the T tissues, only 6
(7.5%) (stage II, n=1; stage III, n=3 and stage IV, n=2) showed no
expression, 11 (13.75%) showed weak expression, 20 (25%) showed
moderate expression and most (n=43; 53.75%) showed strong RARα
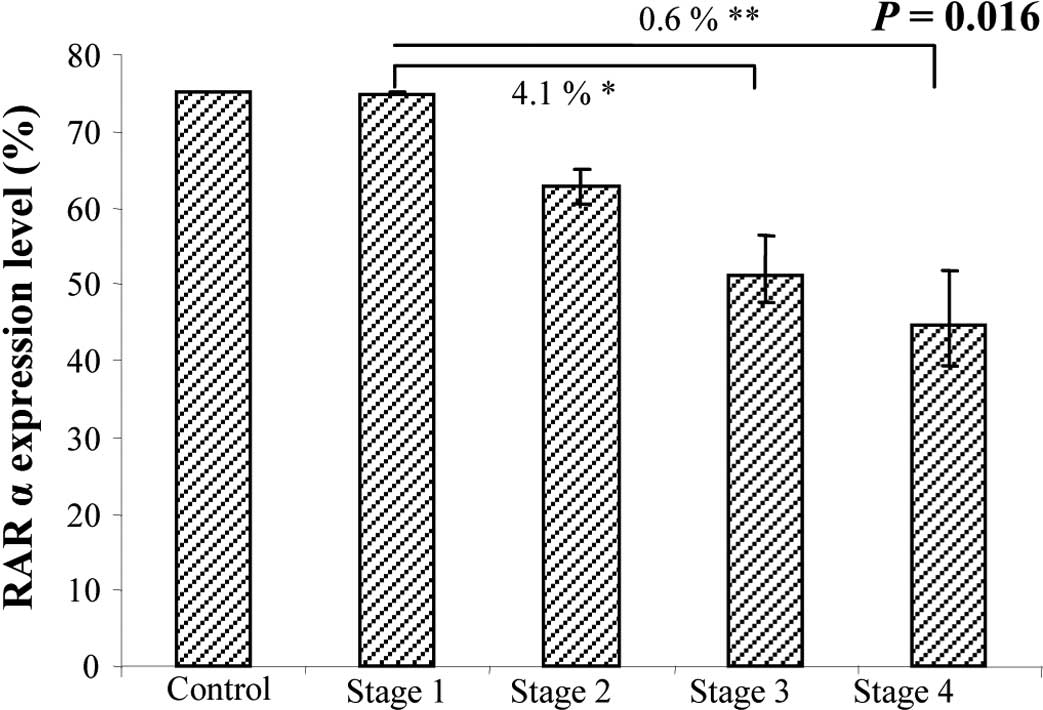
expression. At the inital evaluation, a statistically significant
difference between stages was detected with ANOVA (P=0.016)
(Fig. 3). Indeed, RARα expression
in the T tissues was directly correlated with tumor stage, as it
was predominantly expressed in the early rather than the late
stages of CRC, when its expression decreased. Reinforcing this
result, this tendency was maintained at the second evaluation
(P=0.0018)
RARβ staining was restricted to the mucous membrane
and was uniform in the cytoplasm of epithelial cells in the NT and
T tissues. In the NT tissues, RARβ was expressed as a mean of 71%,
comparable to that observed in the normal control group (72.5%). In
the T tissues, 15 patients (18.75%) (stage II, n=7; stage III, n=4;
and stage IV, n=4) showed no expression, 4 (5%) showed weak
expression, 6 (7.5%) showed moderate expression and most (55;
68.75%) showed strong RARβ expression. No statistically significant
differences were observed between the CRC stages as analyzed by
ANOVA (P>0.4).
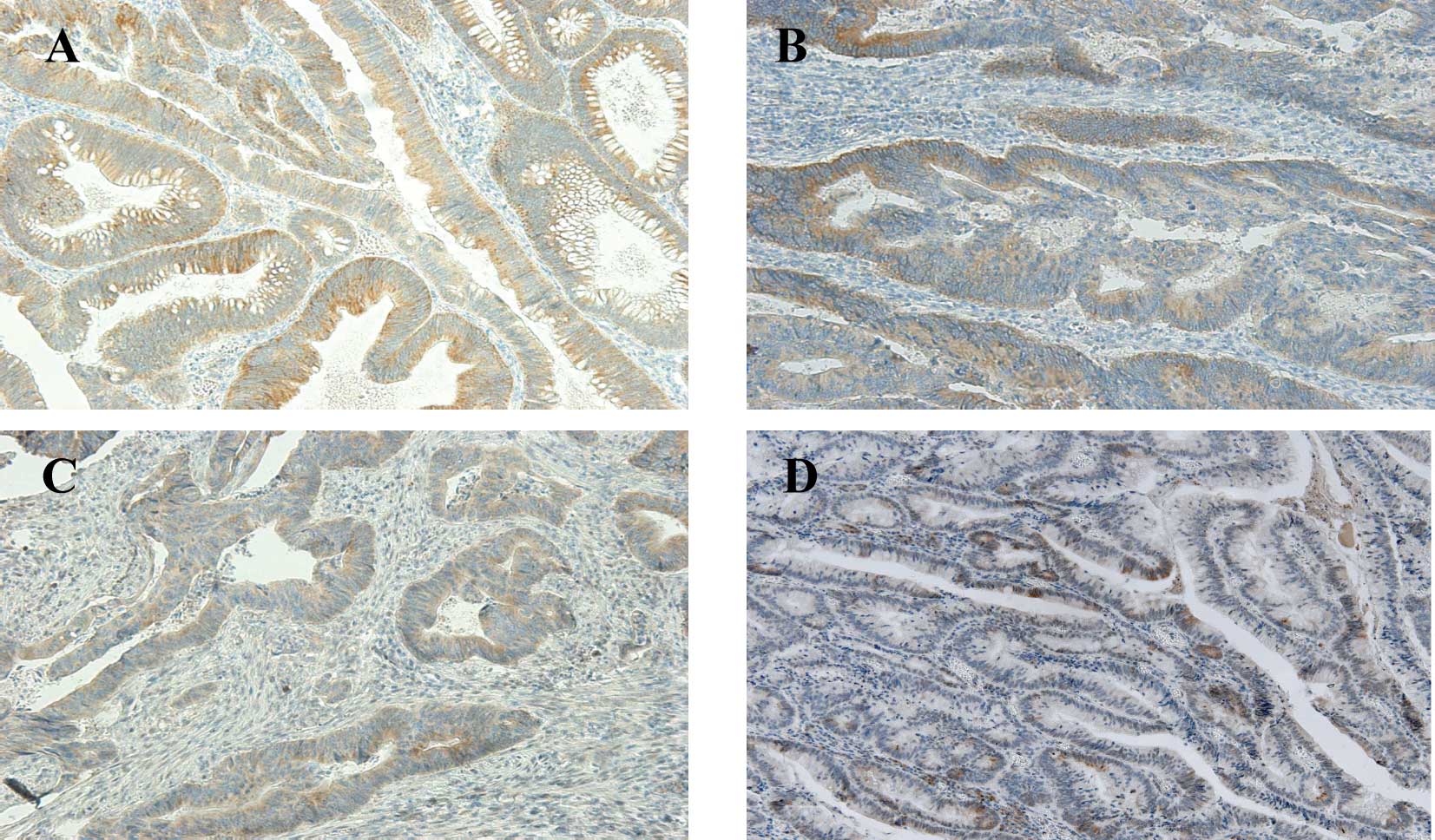
RARγ staining was very similar to that observed for
RARβ, as it was predominant in the cytoplasm of epithelial cells in
both the NT and T tissues (Fig.
4). As with the other RARs, the majority of patients expressed
RARγ in the NT tissues (50–75% of cells), similar to the normal
control group (data not shown). In the T tissues, only 1 (1.25%)
(stage II) showed no expression, 10 (12.5%) showed weak expression,
19 (23.75%) showed moderate expression and most (50; 62.5%) showed
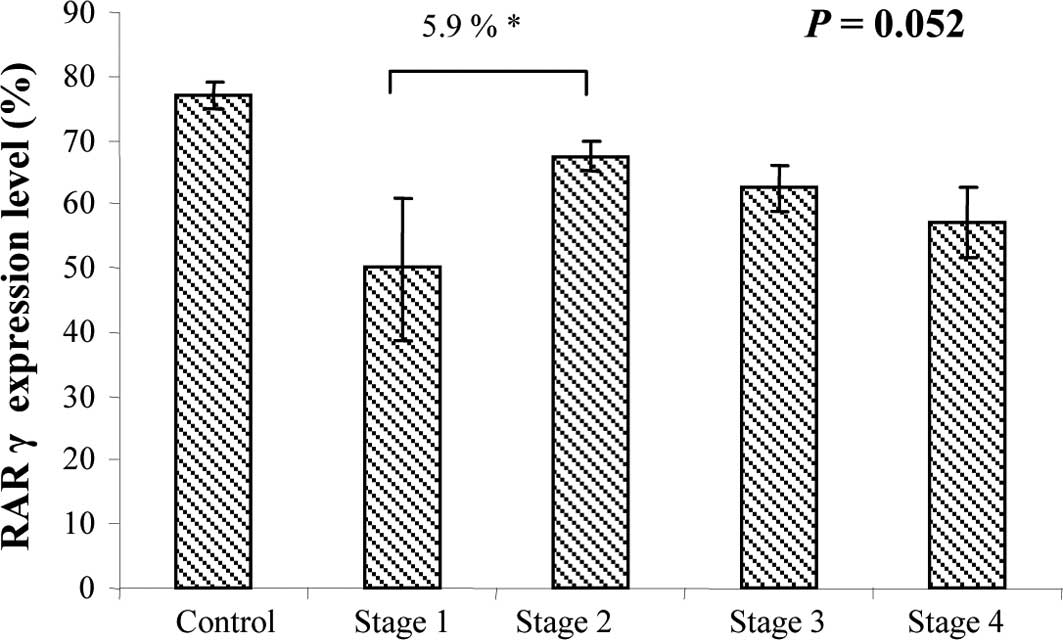
strong RARγ expression. RARγ expression tended to differ at each
CRC stage when assessed by ANOVA (P=0.052) (Fig. 5). However, this value was probably
attributable to the small number of patients in stage I (only 6
patients).
Correlation with patient outcome
RARα expression in the T tissues was positively
correlated with CRC stage [correlation coefficient (r)=0.0011] and
remission (r=0.027). However, it was negatively correlated with
disease evolution (r=0.012), chemoresistance (r=0.024) and death
(r=0.0047). RARβ expression may be considered a marker of CRC
development, as it decreased in conjuction with disease progression
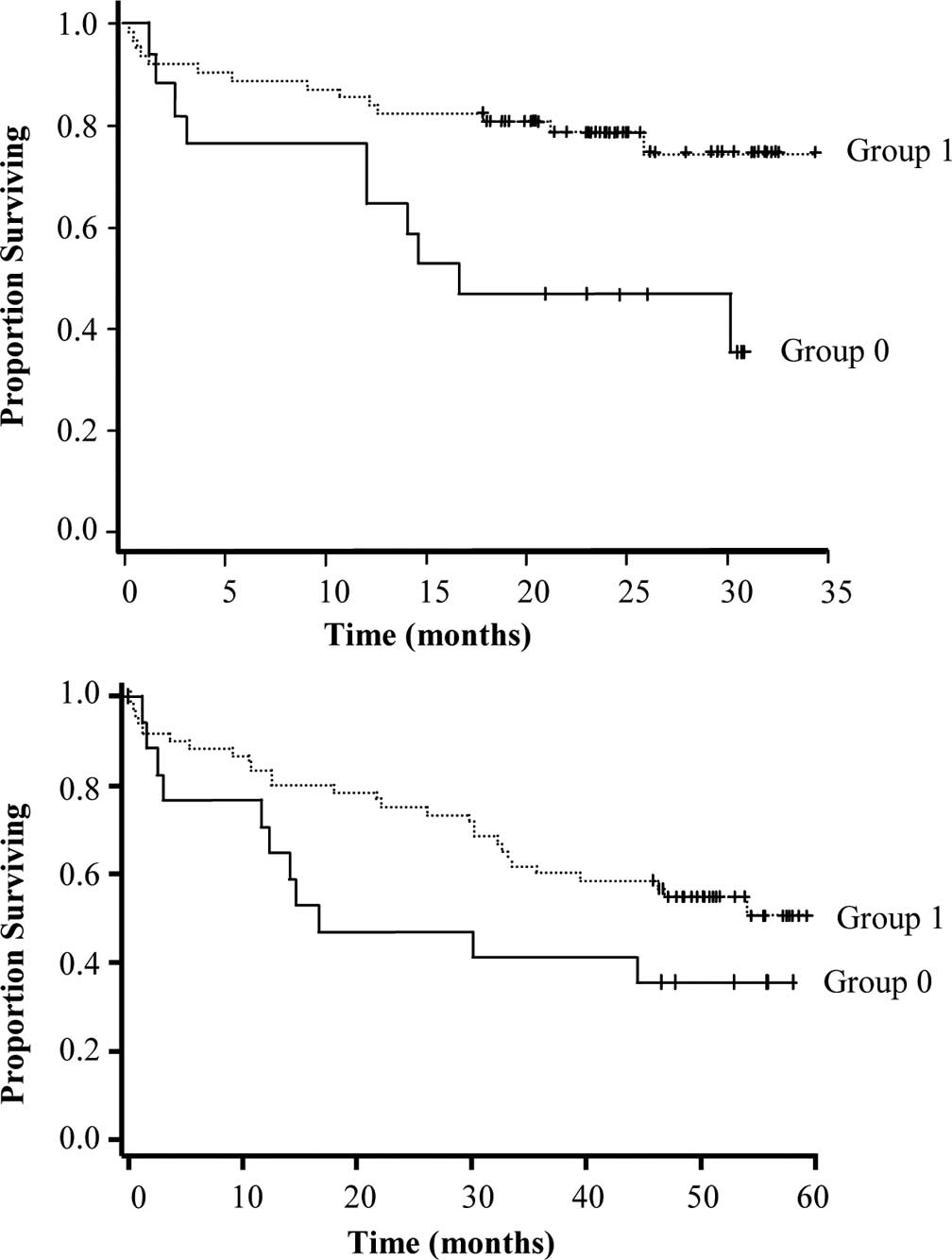
(confirmed by ANOVA at the two evaluations). Moreover, strong RARβ
expression in the T tissues was associated with a more favorable
survival probability (P=0.0072 at the first and P=0.1 at the second
evaluation) (Fig. 6A and B).
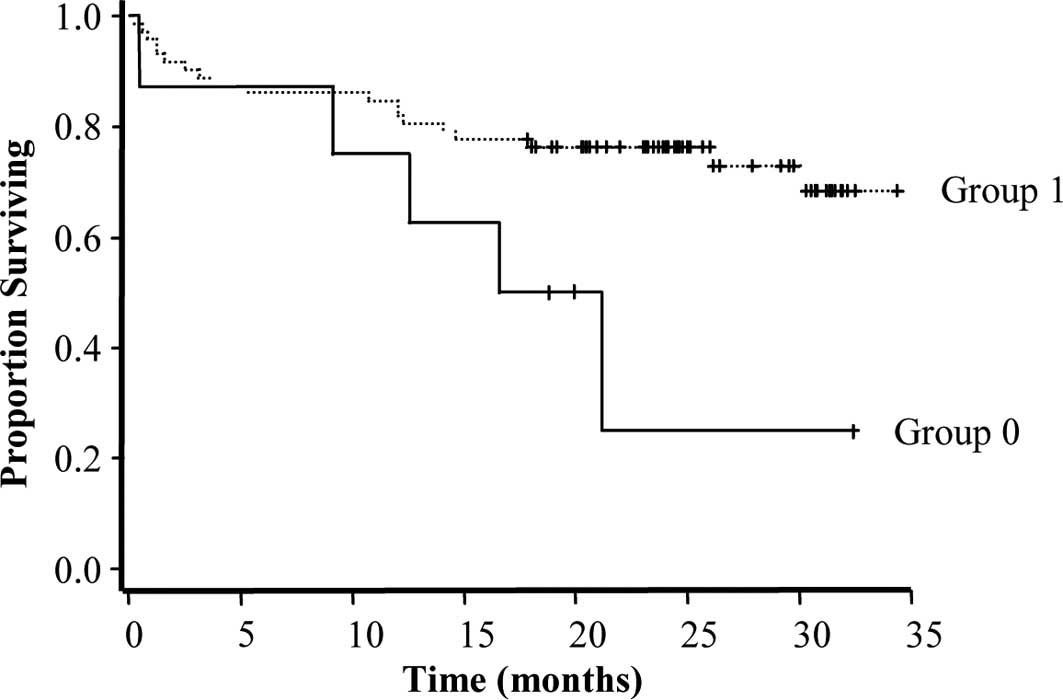
RARβ expression in the NT tissues may serve as a
marker of a more favorable prognosis, as its expression was
positively associated with remission (r=0.021) and negatively
associated with disease evolution (r=0.022), chemoresistance
(r=0.016) and death (r=0.033). Although RARβ was also expressed in
the NT tissues of the patients as the internal control group, its
high expression was linked to a longer survival (P=0.04) (Fig. 7).
RARγ expression in the NT tissues was negatively
associated with disease evolution (r=0.033). Moreover, its
expression in the T tissues was negatively associated with
chemoresistance (r=0.014). Based on these results, RARγ may be used
as an indicator of a more favorable prognosis for CRC, although no
significant association with survival probability was found
(P>0.05).
Discussion
In the present study, the expression of the
proliferation marker Ki-67 and the three RARs in different stages
of CRC, including the rare analysis of stage I (which is rarely
operated on), was analyzed by immunohistochemistry in both the T
and NT areas (the latter representing the tumor environment) of
each patient.
Ki-67 immunoreactivity was present in the NT and T
tissues of all of the patients analyzed, but no correlation with
tumor grade or other parameters was established. Strong Ki-67
reactivity was found in the T tissues, which confirmed the high
proliferative activity of CRC, but the proliferation rate was not
an indicator of disease progression, as observed for prostate
cancer (28).
RARβ expression is commonly lost in various tumor
types (12–20). In this study, RARβ expression in
the T tissues was not an indicator of tumor progression. Different
RARβ isoforms, with varying biological functions, have been
identified. Two known RARβ promoters and alternative splicing give
rise to three major isoforms in humans (β1,
β2 and β4). RARβ2 is the most
abundant, and the term RARβ used in the literature frequently
refers to this isoform. The loss of RARβ2 expression
during cancer development is associated with tumorigenesis and
retinoid resistance. The induction of its expression suppresses
carcinogenesis. RARβ4 expression is also increased in
various types of cancer, but induction of its expression increases
the growth of tumor cells that do not express RARβ2
(29). In the present study,
expression of RARβ was examined without distinguishing its
different isoforms, which explains our results. Evaluating the
specific expression of the various RARβ isoforms in different CRC
stages and in pre-cancerous stages is of interest. RARβ expression
in the NT tissues was the most significant finding of the study, as
it was correlated with remission. Therefore, as a positive marker,
RARβ may be an indicator of patient response to treatment and a
prognostic marker of a beneficial clinical outcome.
RARα expression in the T tissues was lower than that
in the NT tissues and decreased from the early to late CRC stages
(first follow-up, P=0.016; second follow-up, P=0.0018), as has been
shown in head and neck tumors (13), carcinogenesis of the endometrium
(12) and breast tumors (30). Its expression was also positively
associated with remission, which reinforces the hypothesis that
RARα is a marker of disease progression.
Finally, RARγ expression in the T tissues decreased
progressively with tumor stage (P=0.052), which suggests that RARγ
may serve as an indicator of CRC tumor progression, as previously
found in the carcinogenesis of the endometrium (12) and oral lesions (21). Its expression in the T tissues was
also negatively correlated with chemoresistance. Therefore, weak
RARγ expression or its loss in T tissues may be an indicator of a
poor clinical outcome. In parallel, its expression was negatively
correlated with disease progression in the NT tissues.
Collectively, these results suggest that RARγ may be as a suitable
indicator of treatment response for CRC.
Altered RAR expression is associated with the
tumorigenic transformation of cells. Retinoids are potentially
important due to their multi-target actions, and promising results
have been obtained in different in vitro studies
demonstrating the inhibition of cell growth, increased cell
differentiation and the induction of apoptosis (3). Although in vitro growth
inhibition of human CRC cells by retinoids or their analogues has
been documented (31–33), prompting initial enthusiasm, the
findings concerning their therapeutic efficacy in vivo
remain controversial. Conflicting results have emerged, and
retinoid resistance has been reported (34). The adverse effects of retinoids are
also considerable; therefore, they must be used with caution.
Further studies are warranted to clarify the mechanisms of
retinoids and to improve their clinical usefulness, in particular
in CRC.
In this patient cohort comprising 80 patients living
in the Limousin region of France, Ki-67 and RAR expression was
evaluated in tissues from patients with different stages of CRC by
immunohistochemical analysis. The relationships found provide
information complementary to the pTNM international classification.
The mechanisms implied by the changes in RAR expression are not
currently well defined. Further investigations are required to
better understand the roles of retinoids in CRC carcinogenesis, in
particular the corroboration of these results by a study on RXR
expression. It may be useful to examine RAR expression in
pre-cancerous patient tissues in order to improve patient care and
the treatment of CRC, the second most common cause of death by
cancer in industrialized countries.
Acknowledgements
This study was supported in part by
the University of Limoges, La Ligue Contre le Cancer and the Région
Limousin (to A.P.). H.A. is the recipient of a grant from the
Conseil Régional du Limousin. We express our gratitude to Professor
Descottes (deceased), Professor Valleix and Professor Gainant
(Heads of the Departments of Surgery), to the technical experts of
the Pathology Department, Limoges Teaching Hospital, for the
helpful assistance with the immunohistochemistry, to ‘La
Tumorothèque du Limousin’ for providing the samples and to Dr J.
Cook-Moreau for the editorial assistance.
References
|
1.
|
Wolbach B and Howe PR: Tissue changes
following deprivation of fat soluble A vitamin. J Exp Med.
42:753–777. 1925. View Article : Google Scholar
|
|
2.
|
De Luca LM: Retinoids and their receptors
in differentiation, embryogenesis, and neoplasia. FASEB J.
5:2924–2933. 1991.PubMed/NCBI
|
|
3.
|
Altucci L and Gronemeyer H: The promise of
retinoids to fight against cancer. Nat Rev Cancer. 1:181–193. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mongan NP and Gudas LJ: Diverse actions of
retinoid receptors in cancer prevention and treatment.
Differentiation. 75:853–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Asou N: All-trans retinoic acid in the
treatment of acute promyelocytic leukemia. Intern Med. 46:91–93.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Degos L and Wang ZY: All trans retinoic
acid in acute promyelocytic leukemia. Oncogene. 20:7140–7145. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nagy L, Thomazy VA, Shipley GL, et al:
Activation of retinoid X receptors induces apoptosis in HL-60 cell
lines. Mol Cell Biol. 15:3540–3551. 1995.PubMed/NCBI
|
|
8.
|
Germain P, Staels B, Dacquet C, Spedding M
and Laudet V: Overview of nomenclature of nuclear receptors.
Pharmacol Rev. 58:685–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Germain P, Chambon P, Eichele G, et al:
International Union of Pharmacology. LX Retinoic acid receptors.
Pharmacol Rev. 58:712–725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Germain P, Chambon P, Eichele G, et al:
International Union of Pharmacology. LXIII Retinoid X receptors.
Pharmacol Rev. 58:760–772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sun SY: Retinoic acid receptor beta and
colon cancer. Cancer Biol Ther. 3:87–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Li R, Saito T, Tanaka R, et al:
Hypermethylation in promoter region of retinoic acid receptor-beta
gene and immunohistochemical findings on retinoic acid receptors in
carcinogenesis of endometrium. Cancer Lett. 219:33–40. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xu XC, Ro JY, Lee JS, Shin DM, Hong WK and
Lotan R: Differential expression of nuclear retinoid receptors in
normal, premalignant, and malignant head and neck tissues. Cancer
Res. 54:3580–3587. 1994.PubMed/NCBI
|
|
14.
|
Kamradt J and Reichrath J: Expression of
retinoic acid receptor proteins in basal cell carcinomas: an
immunohistochemical analysis. J Histochem Cytochem. 44:1415–1420.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Widschwendter M, Berger J, Daxenbichler G,
et al: Loss of retinoic acid receptor beta expression in breast
cancer and morphologically normal adjacent tissue but not in the
normal breast tissue distant from the cancer. Cancer Res.
57:4158–4161. 1997.PubMed/NCBI
|
|
16.
|
Picard E, Seguin C, Monhoven N, et al:
Expression of retinoid receptor genes and proteins in
non-small-cell lung cancer. J Natl Cancer Inst. 91:1059–1066. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhang W, Rashid A, Wu H and Xu XC:
Differential expression of retinoic acid receptors and p53 protein
in normal, premalignant, and malignant esophageal tissues. J Cancer
Res Clin Oncol. 127:237–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Richter F, Joyce A, Fromowitz F, et al:
Immunohistochemical localization of the retinoic acid receptors in
human prostate. J Androl. 23:830–838. 2002.PubMed/NCBI
|
|
19.
|
Haugen BR, Larson LL, Pugazhenthi U, et
al: Retinoic acid and retinoid X receptors are differentially
expressed in thyroid cancer and thyroid carcinoma cell lines and
predict response to treatment with retinoids. J Clin Endocrinol
Metab. 89:272–280. 2004. View Article : Google Scholar
|
|
20.
|
Karamouzis MV, Sotiropoulou-Bonikou G,
Vandoros G, Varakis I and Papavassiliou AG: Differential expression
of retinoic acid receptor beta (RARβ) and the AP-1 transcription
factor in normal, premalignant and malignant human laryngeal
tissues. Eur J Cancer. 40:761–773. 2004.
|
|
21.
|
Ralhan R, Chakravarti N, Kaur J, et al:
Clinical significance of altered expression of retinoid receptors
in oral precancerous and cancerous lesions: Relationship with cell
cycle regulators. Int J Cancer. 118:1077–1089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Alvarez S, Germain P, Alvarez R,
Rodriguez-Barrios F, Gronemeyer H and de Lera AR: Structure,
function and modulation of retinoic acid receptor beta, a tumor
suppressor. Int J Biochem Cell Biol. 39:1406–1415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bossard N, Veltenc M, Remonteta L, et al:
Survival of cancer patients in France: A population-based study
from The Association of the French Cancer Registries (FRANCIM). Eur
J Cancer. 43:149–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Greene FL, Page DL, Fleming I, et al: AJCC
(American Joint Committee on Cancer) Cancer Staging Manual. 6th
edition. Springer-Verlag; New York: pp. 1132002
|
|
25.
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
26.
|
Du L, Wang H, He L, et al: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hammer Ø, Harper DAT and Ryan PD: PAST:
Paleontological statistics software package for education and data
analysis. Palaeontologia Electronica. 4:92001.
|
|
28.
|
Gyftopoulos K, Perimenis P,
Sotiropoulou-Bonikou G, Sakellaropoulos G, Varakis I and Barbalias
GA: Immunohistochemical detection of retinoic acid receptor-alpha
in prostate carcinoma: correlation with proliferative activity and
tumor grade. Int Urol Nephrol. 32:263–269. 2000. View Article : Google Scholar
|
|
29.
|
Xu XC: Tumor-suppressive activity of
retinoic acid receptor-beta in cancer. Cancer Lett. 253:14–24.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Van der Leede BM, Geertzema J, Vroom TM,
et al: Immunohistochemical analysis of retinoic acid receptor-alpha
in human breast tumors: retinoic acid receptor-alpha expression
correlates with proliferative activity. Am J Pathol. 148:1905–1914.
1996.PubMed/NCBI
|
|
31.
|
Lee MO, Han SY, Jiang S, Park JH and Kim
SJ: Differential effects of retinoic acid on growth and apoptosis
in human colon cancer cell lines associated with the induction of
retinoic acid receptor beta. Biochem Pharmacol. 59:485–496. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Nicke B, Kaiser A, Wiedenmann B, Riecken
EO and Rosewicz S: Retinoic acid receptor alpha mediates growth
inhibition by retinoids in human colon carcinoma HT29 cells.
Biochem Biophys Res Commun. 261:572–577. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kim EJ, Kang YH, Schaffer BS, Bach LA,
MacDonald RG and Park JH: Inhibition of Caco-2 cell proliferation
by all-trans retinoic acid: role of insulin-like growth factor
binding protein-6. J Cell Physiol. 190:92–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Freemantle SJ, Spinella MJ and Dmitrovsky
E: Retinoids in cancer therapy and chemoprevention: promise meets
resistance. Oncogene. 22:7305–7315. 2003. View Article : Google Scholar : PubMed/NCBI
|