Introduction
Colorectal cancer is a leading cause of morbidity
and mortality in developed countries (1). In Japan, an increasingly Westernized
diet has led to a high incidence of colorectal cancer. Patients
with rectal cancers are known to have an increased rate of local
recurrence and decreased survival time compared to patients with
tumors of the colon, a result due primarily to the surgical
constraints imposed by the location of the rectum within the pelvis
(2).
Pre-operative chemoradiotherapy (CRT) is a
neoadjuvant therapy for locally advanced rectal cancer that reduces
the incidence of local recurrence and improves survival (3). Therefore, CRT is widely used in many
countries of the world. However, several tumors show a marked
response to CRT, whereas others do not. Furthermore, several
adverse events related to CRT, such as enteritis, anorexia,
cardiac/thromboembolic events, radiation dermatitis and hematologic
toxicity, were reported to occur at frequencies of 6–43% (4). Thus, pre-operative indicators of
chemoradiosensitivity are required to avoid unnecessary application
of pre-operative CRT, yet little is known about potential
biological markers that may be associated with response to
pre-operative CRT.
Recently, the discovery of rare subpopulations of
cancer stem cells has created a new focus in cancer research. The
heterogeneity of tumors can be explained by the concept of cancer
stem cells supported by anti-apoptotic signaling. There are a few
reports on cancer stem cells related to chemoradiation resistance
(5,6). Therefore, in this study we
investigated the factors, including cancer stem cell-related
factors, that influence the sensitivity of locally advanced rectal
cancer to pre-operative CRT using surgical resected specimens to
consider tumor heterogeneity.
Materials and methods
Patients
A total of 50 patients with locally advanced rectal
carcinoma were treated with pre-operative CRT and surgical
resection at the Department of Surgery I, Oita University Faculty
of Medicine, or associated institutions (Beppu Medical Center,
Nakatsu Municipal Hospital, Oita Prefectural Hospital and Nankai
Hospital) between January 2000 and May 2010. Tumors were located at
the middle or lower third of the rectum and were diagnosed as
clinical stage T2, T3 or T4, Nx and M0 (UICC TNM Classification of
Malignant Tumours, 2009). T stage was determined by computed
tomography (CT) scan or endoscopic ultrasonography. No distant
metastases were detected on plain chest X-rays or CT scans.
Thirty-nine patients were treated with pre-operative CRT and
another 11 patients were treated with pre-operative radiotherapy
(RT) alone. The total dose of radiation in most cases was 45 Gy
within 6 weeks, usually 1.5 Gy per treatment, five times per week.
The total dose range was 40–50 Gy. Several chemotherapy regimens
were used in the patients treated with CRT: TS-1 (80
mg/m2) in 21 patients, 5-fluorouracil (5-FU)-based in 5
patients, tegafur/uracil (UFT) and leucovorin or UFT alone in 8
patients, and tegafur in 5 patients. Curative surgery that included
total mesorectal excision was performed in all patients after an
interval of approximately 4 weeks following completion of
pre-operative treatment. Patient informed consent and approval of
the local ethics committee was obtained prior to the study.
Immunohistochemistry
A total of 12 biomarkers were chosen as candidate
predictive factors for the efficacy of pre-operative CRT (7–13).
These factors included tumor growth-related factors, epidermal
growth factor receptor (EGFR) and human epidermal growth factor
receptor-2 (HER2); cell cycle-related factors, p53, p21, Ki-67 and
Bcl-1; apoptosis-related factors, Bcl-2 and apoptosis
protease-activating factor-1 (APAF-1); tumor stroma-related
factors, vascular endothelial growth factor (VEGF) and macrophage
migration inhibitory factor (MIF); and cancer stem cell (tumor
initiating cell)-related factors, CD133 and CD24. Postoperative
resected specimens were used for immunohistochemistry.
Paraffin-embedded sections of tumor tissue from the
resected rectum were cut at a thickness of 4 μm, deparaffinized in
xylene and rehydrated. Endogenous peroxidase activity was blocked
with 3% hydrogen peroxidase for 10 min. For antigen retrieval,
sections were autoclaved at 121°C in 10 mM citrate buffer, pH 6.0,
for 10 min. Sections were then treated with primary antibodies.
Immunostaining was performed by the avidin-biotin-peroxidase
complex technique using a Histofine SAB-PO (Multi) kit (Nichirei
Co., Tokyo, Japan) and diaminobenzidine for the visualization of
the binding antibodies (14). The
following primary antibodies were used: EGFR (clone EGFR113, 1:100;
Lab Vision Inc., Fremont, CA, USA) (15); p53 (clone DO-7, 1:50;
DakoCytomation, Glostrup, Denmark); p21 (clone SX118, 1:40;
DakoCytomation); Ki-67 (clone MIB-1, 1:50; DakoCytomation); Bcl-1
(clone SP4; Nichirei Co.) (16);
Bcl-2 (clone 124, 1:40; DakoCytomation); APAF-1 (NCL-APAF-1, 1:20;
Novocastra, Newcastle, UK) (17);
VEGF (VEGF A-20, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA,
USA) (18); MIF (FL-115, 1:200;
Santa Cruz Biotechnology) (13);
CD133 (ab19898, 1:200; Abcam, Tokyo, Japan) (19); and CD24 (clone SN3b, 1:100; Lab
Vision Inc.) (20).
Immunohistochemistry for HER2 was performed with HercepTest
(DakoCytomation) (21). Negative
controls were treated identically, omitting the primary antibodies.
Tumor positivity for a given marker was evaluated using a
predetermined cut-off of 10% (the average of the percentage of
tumor cells stained in five fields at x100 magnification: ≤10%
tumor cell staining, negative; >10%, positive) according to
previous studies (7,8,22).
For Ki-67 immunoreactivity, staining was considered positive at
>60% (23). Staining was
assessed in the nucleus for p53, p21, Ki-67 and Bcl-1, and in the
cytoplasm for EGFR, APAF-1, VEGF, MIF, CD133 and CD24.
Immunoreactivity for Bcl-2 and HER2 expression was assessed in both
the cytoplasm and/or the cell membrane. Staining intensity was not
evaluated.
Classification of response to
pre-operative CRT
Tumor response to pre-operative CRT was evaluated
pathologically on postoperative specimens according to the
evaluation of the standard of therapeutic effect provided in the
General Rules for Clinical and Pathological Studies on Cancer of
the Colon, Rectum and Anus edited by the Japanese Society for
Cancer of the Colon and Rectum (24). According to these standards,
evaluation of the therapeutic effect was categorized according to
five grades: grade 0, absence of regressive changes; grade 1a,
regressive change of tumor <1/3; grade 1b, regressive change of
tumor <2/3; grade 2, regressive change of tumor >2/3; grade
3, absence of residual tumor cells. We considered grades 0 or 1a to
indicate low sensitivity and grades 1b, 2 or 3 to indicate high
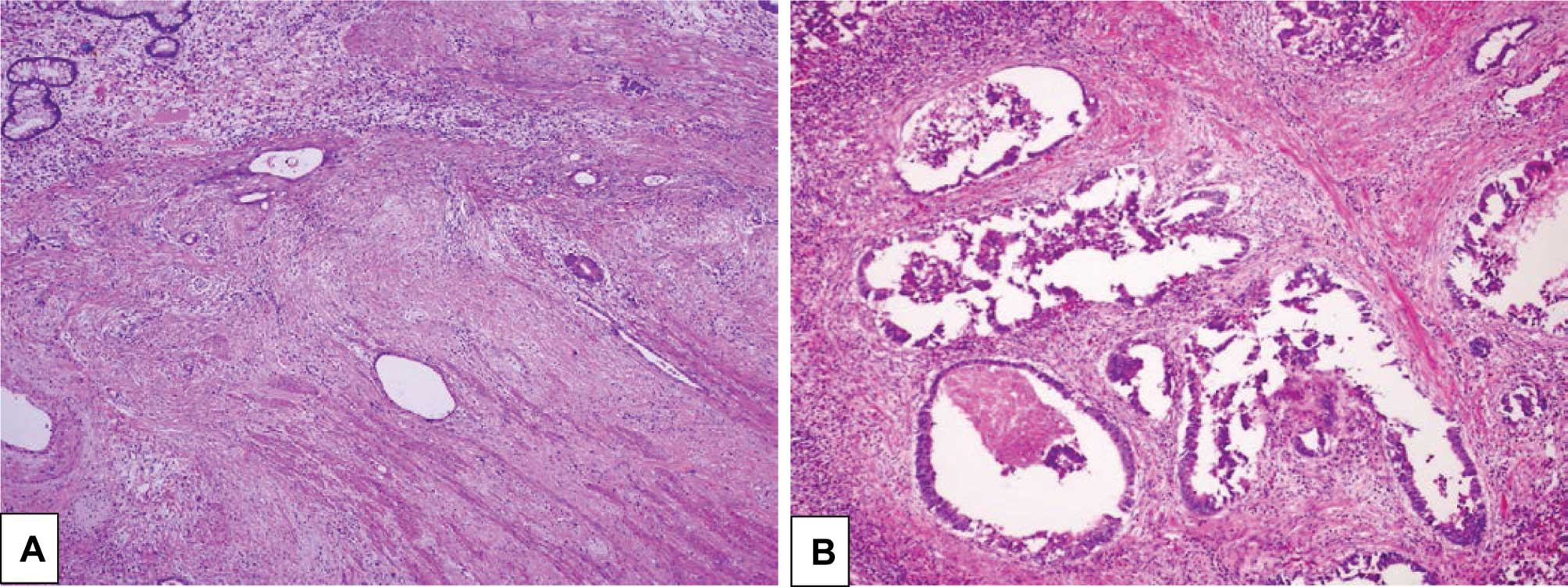
sensitivity to pre-operative CRT (Fig.
1).
Statistical analysis
For statistical comparisons of patient
characteristics between the two groups (low sensitivity and high
sensitivity), the Chi-square test, the Fisher's exact probability
test or the unpaired t-test was used. A value of P<0.05 was
considered statistically significant. All analyses were performed
with SPSS Software (version 11.0) (SPSS Japan Inc., Tokyo,
Japan).
Results
Patient and tumor characteristics
There were 37 (74%) men and 13 (26%) women included
in the study. The median age was 64 years (range 40–83).
Abdominoperineal resection was performed in 24 (48%) patients and a
sphincter-preserving operation was performed in 19 (38%) patients.
Macroscopic findings showed 82% of the tumors to be circumscribed
tumors and, histologically, most (85%) of the tumors were of the
well or moderately differentiated type. Lymph node metastasis was
observed in 12 (24%) patients. Vessel invasion was observed in 25
(50%) patients. On the basis of the classification of responses to
pre-operative CRT, 31 tumors showed high sensitivity and 19 tumors
showed low sensitivity to pre-operative CRT (Table I).
 | Table I.Patient and treatment
characteristics. |
Table I.
Patient and treatment
characteristics.
| Characteristic | No. of patients
(n=50) | % |
|---|
| Age (years) | | |
| Median | 64 | |
| Range | 40–83 | |
| Gender | | |
| Male | 37 | 74 |
| Female | 13 | 26 |
| Surgery | | |
| Total pelvic
exenteration | 1 | 14 |
| Abdominoperineal
resection | 24 | 48 |
|
Sphincter-preserving operation | 19 | 38 |
| Macropathology | | |
| Circumscribed | 41 | 82 |
| Infiltrative | 9 | 18 |
| Histologya | | |
| Well
differentiated | 9 | 19 |
| Moderately
differentiated | 31 | 66 |
| Poorly
differentiated | 3 | 6 |
| Mucinous | 4 | 9 |
| T-categorya | | |
| pT1 | 2 | 4 |
| pT2 | 8 | 17 |
| pT3 | 27 | 57 |
| pT4 | 10 | 21 |
| N-category | | |
| pN0 | 38 | 76 |
|
pN+ | 12 | 24 |
| Vessel
invasion | | |
| Negative | 25 | 50 |
| Positive | 25 | 50 |
| Tumor response (CRT
sensitivity) | | |
| High
sensitivity | 31 | 62 |
| Low
sensitivity | 19 | 38 |
Status of response to CRT according to
various clinical parameters
Gender, age, macropathology, location, histology,
N-category and surgery were not associated with tumor response
(Table II). Of the 10 patients
with pT1–2 tumors, 9 showed high sensitivity. The number of pT3–4
tumors showing high sensitivity was nearly equal to those showing
low sensitivity (P=0.034). Of the tumors negative for vessel
invasion, 21 of 25 showed high sensitivity, whereas 15 of 25 tumors
positive for vessel invasion showed low sensitivity (P=0.003).
 | Table II.Response according to various
clinical parameters. |
Table II.
Response according to various
clinical parameters.
| Parameter | High sensitivity
(n=31) | Low sensitivity
(n=19) | P-value |
|---|
| Gender | | | 0.481 |
| Male | 24 | 13 | |
| Female | 7 | 6 | |
| Age (years) | | | 0.635 |
| Median | 64 | 65 | |
| Range | 44–82 | 40–83 | |
| Macropathology | | | 0.715 |
|
Circumscribed | 26 | 15 | |
| Infiltrative | 5 | 4 | |
| Location | | | 0.273 |
| Upper | 4 | 5 | |
| Lower | 27 | 14 | |
| Histologya | | | 0.102 |
| Well/moderate
differentiation | 26 | 14 | |
| Poor/mucinous
differentiation | 2 | 5 | |
| T-categorya | | | 0.034 |
| pT1/2 | 9 | 1 | |
| pT3/4 | 19 | 18 | |
| N-category | | | 0.764 |
| pN0 | 24 | 14 | |
| pN1,2 | 7 | 5 | |
| Vessel
invasion | | | 0.003 |
| Negative | 21 | 4 | |
| Positive | 10 | 15 | |
| Surgery | | | 0.464 |
| LAR/Lap. LAR | 13 | 6 | |
| APR/Lap. APR | 18 | 13 | |
Response rates according to various
pathological parameters
Factors related to tumor growth, the cell cycle,
apoptosis and tumor stroma were not associated with tumor response
(Table III). Only factors related
to cancer stem cells (tumor-initiating cells) were associated with
tumor response. A significant association was found between the
resistance of the tumor to treatment and negative CD133 status
(P=0.003), and there was a significant statistical correlation
between the resistance of the tumor to treatment and positive CD24
status (P=0.029). In the high-sensitivity tumors, 3 tumors that had
complete pathologic tumor regression were excluded from the
pathological study (histology and T-category in Tables I and II) and immunohistochemical analysis since
the resected specimens did not contain cancer cells (Fig. 2).
 | Table III.Response according to various
pathological parameters. |
Table III.
Response according to various
pathological parameters.
| Biomarker | High sensitivity
(n=28) | Low sensitivity
(n=19) | P-value |
|---|
| HER2 | | | 1.000 |
| + | 1 | 0 | |
| − | 27 | 19 | |
| EGFR | | | 0.453 |
| + | 4 | 5 | |
| − | 24 | 14 | |
| VEGF | | | 0.119 |
| + | 21 | 18 | |
| − | 7 | 1 | |
| MIF | | | 0.770 |
| + | 13 | 8 | |
| − | 15 | 11 | |
| p53 | | | 0.137 |
| + | 24 | 19 | |
| − | 4 | 0 | |
| p21 | | | 0.143 |
| + | 5 | 7 | |
| − | 23 | 12 | |
| Ki-67 | | | 0.739 |
| + | 19 | 12 | |
| − | 9 | 7 | |
| Bcl-1 | | | 1.000 |
| + | 7 | 4 | |
| − | 21 | 15 | |
| Bcl-2 | | | 0.435 |
| + | 16 | 13 | |
| − | 12 | 6 | |
| APAF-1 | | | 0.119 |
| + | 21 | 18 | |
| − | 7 | 1 | |
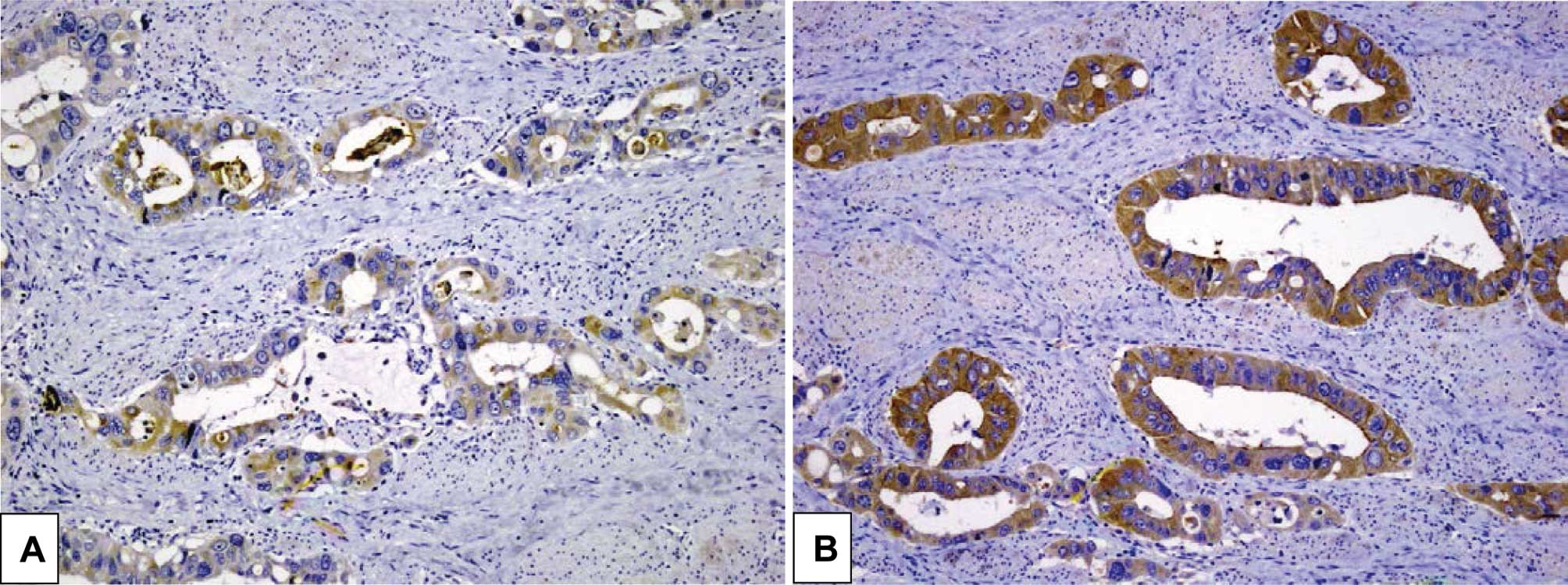
| CD133 | | | 0.003 |
| + | 2 | 9 | |
| − | 26 | 10 | |
| CD24 | | | 0.029 |
| + | 14 | 16 | |
| − | 14 | 3 | |
Response rates based on combinations of
CD133 and CD24
When both CD133 and CD24 were positive, 9 of 10
(90%) tumors showed low sensitivity, whereas when both CD133 and
CD24 were negative, 3 of 16 (19%) tumors showed low sensitivity
(Table IV). Co-overexpression of
CD133 and CD24 was associated with low sensitivity
(CD133+ and CD24+ vs. others, P=0.001).
Negative expression of both CD133 and CD24 was associated with high
sensitivity (CD133− and CD24− vs. others,
P=0.030).
 | Table IV.Response according to combinations of
CD133 and CD24. |
Table IV.
Response according to combinations of
CD133 and CD24.
| Case | High sensitivity
(n=28)
| Low sensitivity
(n=19)
|
|---|
| No. | % | No. | % |
|---|
| CD133+
and CD24+a | 1 | 10 | 9 | 90 |
| CD133+
and CD24− | 1 | 100 | 0 | 0 |
| CD133−
and CD24+ | 13 | 65 | 7 | 35 |
| CD133−
and CD24−b | 13 | 81 | 3 | 19 |
Discussion
The present study demonstrated that
co-overexpression of cancer stem cell-related factors, CD133 and
CD24, was significantly associated with locally advanced rectal
cancer exhibiting low sensitivity to pre-operative CRT. This result
suggests that these two biomarkers may influence sensitivity to
pre-operative CRT.
In this study, we used resected specimens from
patients who had been treated with pre-operative CRT. For
identifying factors which predict the efficacy of CRT before
treatment, the use of pre-treatment biopsy specimens is advisable.
However, there is heterogeneity in the tumor (5). Therefore, biopsy specimens were not
used, and resected specimens were used to investigate the entire
tumor specimen.
For the evaluation of CD133 and CD24 expression,
immunostaining was classified using the 10% cut-off scoring system.
Although one report set the cut-off value to 50%, we adopted the
standard system as it has been widely used in many studies.
Expression of CD133 and CD24 was distributed evenly within the
resected tumors. In the localization of staining, membranous
expression of CD24 without cytoplasmic positivity was detected, but
we did not include it as being indicative of positive
expression.
The concept of cancer stem cells which has been
proposed in the field of blood cancer (25) has been adjusted to address solid
tumors, such as those of colorectal cancer (26). The fundamental cancer stem cell
concept assumes that cancer cells exhibit a hierarchy, as do normal
cells, and that a small fraction of cancer cells are maintained as
‘cancer stem cells’, which have the ability of self-renewal and
differentiation (27). Cancer stem
cells have recently been proposed to be the cancer-initiating cells
that are responsible for tumorigenesis and for contributing to drug
resistance in cancer (28).
Although a comparatively large number of studies have been reported
concerning cancer stem cells and resistance to either chemotherapy
or radiotherapy in various cancers, there are few studies available
concerning cancer stem cells and resistance to CRT (5).
CD133 and CD24 have been reported as cancer stem
cell markers of colorectal cancer in previous studies (26,29,30).
CD133 is a 5-transmembrane glycoprotein of 865 amino acids with a
total molecular weight of 120 kDa. CD133 antigen expression has
been found in such various undifferentiated cells as hematopoietic
stem cells (31) and fetal brain
stem cells (32). In cancer cells,
CD133 has been found to be expressed on cancer stem or
tumor-initiating cells in cancers, such as leukemia (33), brain tumors (34) and colorectal cancer. CD24 consists
of a small protein core comprising 27 amino acids, which is
extensively glycosylated and is bound to the cell membrane via a
phosphatidylinositol anchor (35).
Several reports have shown that CD24 is expressed in several solid
tumors, such as those of small-cell lung cancer and neuroblastoma
(36,37), but not in those of colorectal
cancer.
Recently, positive clinical studies on the
effectiveness of pre-operative CRT on locally advanced rectal
cancer have been reported (38).
However, pre-operative CRT is not effective in all cases and,
actually, cases in which no antineoplastic effect was obtained also
exist. Since the treatment period for pre-operative CRT is
approximately 10 weeks, patients who obtain no response to CRT lose
valuable time during which they could have been treated more
effectively. Thus, it is necessary to investigate factors which
influence the efficacy of pre-operative CRT.
The results of the present study suggest that the
presence of CD133 and CD24 expression is associated with the
efficacy of pre-operative CRT. Assuming that CD133 and CD24 are
predictive factors of the sensitivity to pre-operative CRT,
patients with both CD133+ and CD24+ are
expected to have low sensitivity to CRT. So, it may be recommended
that such patients undergo surgery without first undergoing CRT.
However, since patients with both CD133− and
CD24− are expected to have high sensitivity to CRT, it
may be necessary to aggressively treat these patients first with
pre-operative CRT.
In conclusion, the present study shows that the
overexpression of cancer stem cell-related factors, CD133 and CD24,
is associated with the sensitivity of locally advanced rectal
cancer to pre-operative CRT. Further prospective studies are
required to establish a new therapeutic system that appropriately
uses pre-operative CRT for the benefit of patients with locally
advanced rectal cancer. Our group is presently conducting a
prospective study using biopsy specimens from pre-therapeutic
tumors (UMIN003398). This retrospective study provides valuable
information for realization of the ongoing prospective study.
Acknowledgements
The authors thank Dr Koshi Mimori
(Department of Surgery and Molecular Oncology, Medical Institute of
Bioregulation, Kyushu University); Dr Yoichi Muto, Dr Tetsuya
Kusumoto and Dr Toshifumi Matsumoto (Department of Surgery, Beppu
Medical Center); Dr Masahito Ikeda, Dr Fumiaki Kishihara and Dr
Akio Shiromizu (Department of Surgery, Nakatsu Municipal Hospital);
Dr Hisanobu Sakata and Dr Kyuzo Fujii (Department of Surgery, Oita
Prefectural Hospital); Dr Takayuki Kamegawa and Dr Akio Morimoto
(Department of Surgery, Nankai Hospital); and Dr Akira Matsumoto
(Department of Radiology, Oita University Faculty of Medicine) for
their invaluable assistance in this study. They also thank Nagako
Katsuki, Mayumi Takeda, Emi Aono and Tomoka Sato for the excellent
technical support.
References
|
1.
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
2.
|
Wolpin BM, Meyerhardt JA, Mamon HJ and
Mayer RJ: Adjuvant treatment of colorectal cancer. CA Cancer J
Clin. 57:168–185. 2007. View Article : Google Scholar
|
|
3.
|
Sauer R, Becker H, Hohenberger W, et al:
Pre-operative versus postoperative chemoradiotherapy for rectal
cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Calvo FA, Serrano FJ, Diaz-González JA, et
al: Improved incidence of pT0 downstaged surgical specimens in
locally advanced rectal cancer (LARC) treated with induction
oxaliplatin plus 5-fluorouracil and pre-operative chemoradiation.
Ann Oncol. 17:1103–1110. 2006. View Article : Google Scholar
|
|
5.
|
Ishii H, Iwatsuki M, Ieta K, et al: Cancer
stem cells and chemoradiation resistance. Cancer Sci. 99:1871–1877.
2008. View Article : Google Scholar
|
|
6.
|
Haraguchi N, Inoue H, Tanaka F, et al:
Cancer stem cells in human gastrointestinal cancers. Hum Cell.
19:24–29. 2006. View Article : Google Scholar
|
|
7.
|
Zlobec I, Vuong T and Compton CC: The
predictive value of apoptosis protease-activating factor 1 in
rectal tumors treated with pre-operative, high-dose-rate
brachytherapy. Cancer. 106:284–286. 2005. View Article : Google Scholar
|
|
8.
|
Negri FV, Campanini N, Camisa R, et al:
Biological predictive factors in rectal cancer treated with
pre-operative radiotherapy or radiochemotherapy. Br J Cancer.
98:143–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zlobec I, Vuong T, Compton CC, et al:
Combined analysis of VEGF and EGFR predicts complete tumour
response in rectal cancer treated with pre-operative radiotherapy.
Br J Cancer. 98:450–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: CD133 expression is an independent prognostic marker for
low survival in colorectal cancer. Br J Cancer. 99:1285–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Haraguchi N, Ohkuma M, Sakashita H, et al:
CD133+CD24+ population efficiently enriches
colon cancer initiating cells. Ann Surg Oncol. 15:2927–2933.
2008.
|
|
12.
|
Choi D, Lee HW, Hur KY, et al: Cancer stem
cell markers CD133 and CD24 correlate with invasiveness and
differentiation in colorectal adenocarcinoma. World J
Gastroenterol. 15:2258–2264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
He XX, Chen K, Yang J, et al: Macrophage
migration inhibitory factor promotes colorectal cancer. Mol Med.
15:1–10. 2009.PubMed/NCBI
|
|
14.
|
Tanahashi J, Daa T, Gamachi A, et al:
Human intestinal spirochetosis in Japan; its incidence,
clinicopathologic features, and genotypic identification. Mod
Pathol. 21:76–84. 2008.
|
|
15.
|
Sato JD, Kawamoto T, Le AD, Mendelsohn J,
Polikoff J and Sato GH: Biological effects in vitro of monoclonal
antibodies to human epidermal growth factor receptors. Mol Biol
Med. 1:511–529. 1983.PubMed/NCBI
|
|
16.
|
Cheuk W, Wong KO, Wong CS and Chan JK:
Consistent immunostaining for cyclin D1 can be achieved on a
routine basis using a newly available rabbit monoclonal antibody.
Am J Surg Pathol. 28:801–807. 2004. View Article : Google Scholar
|
|
17.
|
Paik SS, Jang KS, Song YS, et al: Reduced
expression of Apaf-1 in colorectal carcinoma correlates with tumor
progression and aggressive phenotype. Ann Surg Oncol. 14:3453–3459.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rosmorduc O, Wendum D, Corpechot C, et al:
Hepatocellular hypoxia-induced vascular endothelial growth factor
expression and angiogenesis in experimental biliary cirrhosis. Am J
Pathol. 155:1065–1073. 1999. View Article : Google Scholar
|
|
19.
|
Dubrovska A, Kim S, Salamone RJ, et al:
The role of PTEN/Akt/PI3K signaling in the maintenance and
viability of prostate cancer stem-like cell populations. Proc Natl
Acad Sci USA. 106:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kim KH, Choi JS, Kim JM, et al: Enhanced
CD24 expression in endometrial carcinoma and its expression pattern
in normal and hyperplastic endometrium. Histol Histopathol.
24:309–316. 2009.PubMed/NCBI
|
|
21.
|
Press MF, Hung G, Godolphin W and Slamon
DJ: Sensitivity of HER-2/neu antibodies in archival tissue samples:
potential source of error in immunohistochemical studies of
oncogene expression. Cancer Res. 54:2771–2777. 1994.
|
|
22.
|
Yada K, Shibata K, Matsumoto T, Ohta M,
Yokoyama S and Kitano S: Protease-activated receptor-2 regulates
cell proliferation and enhances cyclooxygenase-2 mRNA expression in
human pancreatic cancer cells. J Surg Oncol. 89:79–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sadahiro S, Suzuki T, Maeda Y, et al:
Predictors of tumor down-sizing and regression with pre-operative
radiotherapy alone and with concomitant tegafur/uracil (UFT) for
resectable advanced rectal adenocarcinoma. Hepatogastroenterology.
54:1107–1112. 2007.
|
|
24.
|
Japanese Society for Cancer of the Colon
and Rectum: General Rules for Clinical and Pathological Studies on
Cancer of the Colon, Rectum and Anus: The 7th edition, revised
version. Tokyo: Kanehara; 2009
|
|
25.
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ieta K, Tanaka F, Haraguchi N, et al:
Biological and genetic characteristics of tumor-initiating cells in
colon cancer. Ann Surg Oncol. 15:638–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Weichert W, Denkert C, Burkhardt M, et al:
Cytoplasmic CD24 expression in colorectal cancer independently
correlates with shortened patient survival. Clin Cancer Res.
11:6574–6581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Yin AH, Miraglia S, Zanjani ED, et al:
AC133, a novel marker for human hematopoietic stem and progenitor
cells. Blood. 90:5002–5012. 1997.PubMed/NCBI
|
|
32.
|
Uchida N, Buck DW, He D, et al: Direct
isolation of human central nervous system stem cells. Proc Natl
Acad Sci USA. 97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Bhatia M: AC133 expression in human stem
cells. Leukemia. 15:1685–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
35.
|
Fischer GF, Majdic O, Gadd S and Knapp W:
Signal transduction in lymphocytic and myeloid cells via CD24, a
new member of the phosphoinositol-anchored membrane molecules. J
Immunol. 144:638–641. 1990.PubMed/NCBI
|
|
36.
|
Jackson D, Waibel R, Weber E, Bell J and
Stahel RA: CD24, a signal-transducing molecule expressed on human B
cells, is a major surface antigen in small-cell lung carcinomas.
Cancer Res. 52:5264–5270. 1992.
|
|
37.
|
Akashi T, Shirasawa T and Hirokawa K: Gene
expression of CD24 core polypeptide molecule in normal rat tissues
and human tumor cell lines. Virchows Arch. 425:399–406. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Roh MS, Colangelo LH, O'Connell MJ, et al:
Pre-operative multimodality therapy improves disease-free survival
in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol.
27:5124–5130. 2009. View Article : Google Scholar : PubMed/NCBI
|