Contents
Introduction
Cancer cachexia
Nutritional support for cancer patients
Conclusion
Introduction
Malnutrition is commonly observed in cancer patients
and adversely affects the quality of life (QoL) and survival of
these patients. It is caused by a variety of factors, including
decreased food intake, adverse effects from anticancer treatment
and wasteful metabolic processes (1). Over the past two decades, there have
been major advances in the methods and techniques used in the
dietary therapy of patients with cancer and other diseases. Enteral
nutrition is developing rapidly as endoscopic techniques have made
it simpler to place feeding tubes, and a variety of enteral
nutrition solutions are commercially available. Enteral nutrition
is an effective way to deliver nutrients when patients are unable
to ingest food because of neurologic disorders or structural
abnormalities in the upper gastrointestinal tract, including the
oropharynx, esophagus and stomach. The role of enteral nutrition as
an adjuvant to anticancer therapy has not been fully evaluated.
Glutamine, n-3 polyunsaturated fatty acids (FAs) and
probiotics/prebiotics are therapeutic factors that potentially
modulate gastrointestinal (GI) toxicity related to cancer
treatments (2).
Total parenteral nutrition (TPN) is an effective
method of delivering nutrients into the blood stream. It has been
proven to be life-saving for patients with chronic severe
gastrointestinal insufficiency (such as short bowel or radiation
enteritis), whose cancer is cured or non-progressive. As an
adjuvant to chemotherapy, TPN does not appear to be useful, unless
there are prolonged periods of gastrointestinal toxicity (as in the
case with bone marrow transplantation) that severely limit oral
intake and absorption (3).
Perioperative parenteral nutrition is only recommended in
malnourished patients when enteral nutrition is not feasible. In
non-surgical well-nourished oncologic patients, routine parenteral
nutrition is not recommended since it has been proven to offer no
advantage and is associated with increased morbidity. A benefit,
however, has been reported in patients undergoing hematopoietic
stem cell transplantation (HSCT). Short-term parenteral nutrition
is commonly accepted in patients with acute gastrointestinal
complications from chemotherapy and radiotherapy, and long-term
(home) parenteral nutrition may sometimes be a life-saving maneuver
in patients with subacute/chronic radiation enteropathy (4).
Weight loss, decreased appetite and difficulty in
the consumption of food are common features of the terminal phase
of cancer. Some patients also become physically unable to take in
sufficient nutrition, or eating may become painful, time-consuming
or otherwise burdensome. Difficulty eating may be self-limited,
such as temporary nausea or illness, or may be expected to last the
rest of the lifespan, such as untreatable gastrointestinal
obstruction. Treatment of underlying symptoms or conditions,
changes in diet and nutritional supplements may be helpful in
certain situations, and appetite stimulants may increase intake,
body weight and QoL, but they do not affect the prognosis in the
terminally ill (5).
Cancer cachexia
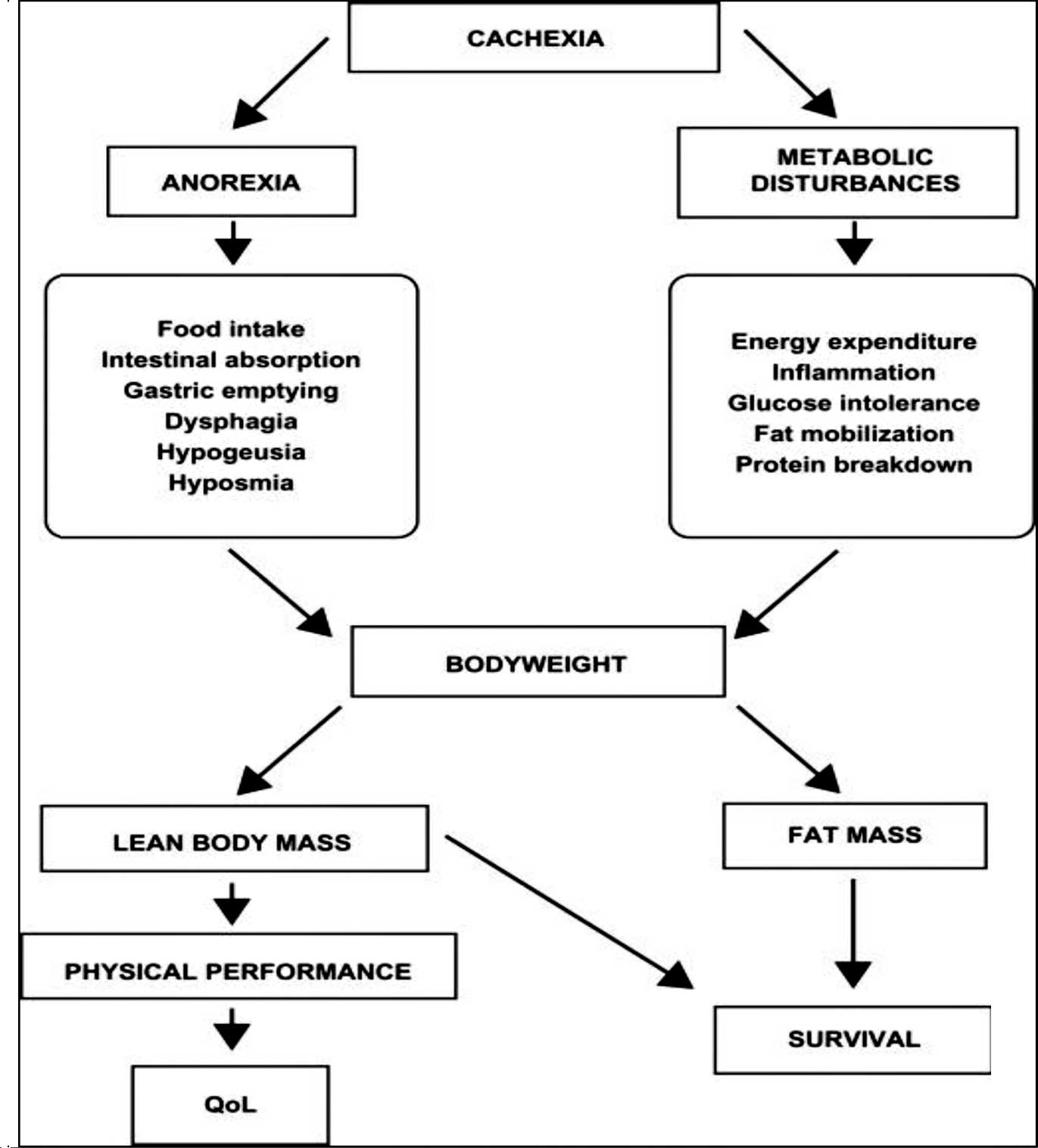
Cancer cachexia is a complex syndrome characterized
by a chronic, progressive, involuntary weight loss which is poorly
or only partially responsive to standard nutritional support and it
is often associated with anorexia, early satiety and asthenia. It
is usually attributable to two main components: a decreased
nutrient intake (which may be due to critical involvement of the
gastrointestinal tract by the tumor, or to cytokines and similar
anorexia-inducing mediators); and metabolic alterations due to the
activation of systemic proinflammatory processes (4).
Metabolic derangements may result in insulin
resistance, increased lipolysis and normal or increased lipid
oxidation with loss of body fat, increased protein turnover with
loss of muscle mass and an increase in the production of acute
phase proteins. The systemic inflammatory reaction that develops
with many cancers is an important cause of loss of appetite
(anorexia) and weight (6). The
syndrome of decreased appetite, weight loss, metabolic alterations
and an inflammatory state is therefore referred to as cancer
cachexia or cancer anorexia-cachexia syndrome. These
cytokine-induced metabolic alterations appear to prevent cachectic
patients from regaining body cell mass during nutritional support,
are associated with a reduced life expectancy and are not relieved
by exogenous nutrients alone (7–9).
Cancer patients traditionally have been regarded as
hypermetabolic; however, a heterogeneous picture of energy
expenditure has been described, with resting energy expenditure
ranging from less than 60% to more than 150% of that predicted
(10). Although cancer patients
often have reduced food intake (due to systemic effects of the
disease, local tumor effects, psychological effects or adverse
effects of treatment), alterations in nutrient metabolism and
resting energy expenditure may also contribute to the nutritional
status (11). Whereas resting
energy expenditure is increased, total energy expenditure may be
unchanged due to a decrease in physical activity. Thus, overall
energy balance may be maintained by a concomitant reduction in
activity, while this decreased physical activity may be a
reflection of a reduced QoL (12).
Cachexia cannot be easily differentiated from
under-nutrition due to simple starvation. Both cachectic and
undernourished patients exhibit a loss of body weight and may be
anorectic; however, simply undernourished patients show a tendency
to save their protein mass, they decrease their resting energy
expenditure and they respond quite well to nutritional support if
their general status is not compromised in an irreversible way
(Fig. 1) (13).
Anticancer treatments can also be a major cause of
malnutrition. Chemotherapy causes nausea, vomiting, abdominal
cramping and bloating, mucositis, paralytic ileus and even
malabsorption. Despite the recent advent of antiemetic drugs, such
as the setrons, and the optimization of their administration
schedule, vomiting remains an important cause of malnutrition in
cancer. Various antineoplastic agents, such as fluorouracil,
adriamycin, methotrexate and cisplatin, induce severe
gastrointestinal complications (14).
Health-related QoL is a multidimensional concept
which quantifies the psychological, physical and social effects of
an illness and its therapy. In cancer patients, the health status
is well reflected in the measured QoL, which is largely influenced
by nutritional aspects. The evaluation of QoL assesses patient
well-being by taking into account physical, psychological and
social conditions (15). Cancer
and its treatment result in severe biochemical and physiological
alterations associated with a deterioration of QoL. These metabolic
changes lead to decreased food intake and promote wasting. Above
and beyond the physical and the metabolic effects of cancer,
patients also suffer from psychological distress, including
depression. Depending on the type of cancer treatment (either
curative or palliative), the clinical condition of the patient and
nutritional status, adequate and patient-tailored nutritional
intervention should be prescribed (diet counseling, oral
supplementation, enteral or total parenteral nutrition). Such an
approach, which should be started as early as possible, reduces or
even reverses their poor nutritional status, improves their
performance status and consequently their QoL. Nutritional
intervention accompanying curative treatment has an additional and
specific role, which is to increase the tolerance and response to
oncology treatment, decrease the rate of complications and possibly
reduce morbidity by optimizing the balance between energy
expenditure and food intake. In palliative care, nutritional
support aims at improving patient QoL by controlling symptoms, such
as nausea, vomiting and pain related to food intake, and by
postponing loss of autonomy. Assessment of QoL should be considered
in the evaluation of any nutritional support to optimize its
benefits in regards to the needs and expectations of the patient
(16).
Interactions between QoL and nutritional
status of the patients
The inadequate nutritional status and cancer
anorexia-cachexia syndrome related to it are clinically relevant
since the response to antineoplastic measures, such as radiation
and chemotherapy, may be diminished, their side effects aggravated
and patient QoL and prognosis negatively affected. Therefore,
supportive nutritional care of oncological patients is of central
importance (17). An impaired
nutritional status is associated with reduced QoL, lower activity
level, increased treatment-related adverse reactions, reduced tumor
response to treatment and reduced survival. However, a cause-effect
relationship is yet to be established (18).
A link between QoL and nutritional status is
supported by evidence that an insufficient nutritional status is
frequently related to reduced QoL (19,20).
Similarly, food intake – one of the major determinants of
nutritional status – appears to influence QoL, as a correlation
between them exists (21).
Moreover, a low QoL is associated with nutrition-related symptoms
and weight loss (22).
Influence of nutritional intervention on
oncology treatment
In curative oncology treatment, nutritional
intervention aims to reduce the number of complications and to
shorten the recovery phase. The probability of developing
malnutrition is increased after curative treatment as aggressive
therapeutic strategies are frequently used. A prolonged therapy
based on patient response may further aggravate the risk for
impaired nutritional status, although it should be acknowledged
that tumor site and inherent nutritional risk of the treatment also
contribute to the deterioration of the nutritional status.
Nevertheless, alleviation of nutrition-related symptoms and signs
may contribute to the well being of the patient (23,24).
In palliative oncology treatment, the aim of
nutritional intervention is to sustain or enhance recovery of
patient performance in everyday life, their well-being and their
QoL. Palliative cancer treatment is also a system of care that
strives to relieve the suffering of patients with progressive
cancer. Given the intractable symptoms with which certain
malignancies manifest, palliative care offers a practical approach
towards improving patient QoL. However, there is an array of
ethical issues associated with this treatment strategy, such as
particular methods of pain relief, a reliable assessment of
suffering, autonomy and multi-specialist care (25). The development of palliative care
in terms of recognizing the needs of the dying has becoming a
nursing and medical speciality. The involvement of the World Health
Organization (WHO) in palliative care and the continuous
development of treatment modalities available to cancer patients
creates the expectation that outcomes for the patient should also
be positively influenced (26,27).
Palliative care is focused on maintaining adequate hydration,
alleviating or controlling symptoms (e.g., nausea and vomiting) and
maintaining body weight and composition. When selecting the type of
nutritional intervention, for instance oral nutritional
supplementation (ONS), enteral nutrition or parenteral nutrition,
the wishes of the patient and their family must be considered
(28).
Nutritional support for cancer patients
The purpose of nutritional assessment is to identify
the subset(s) of patient who may benefit from dietary counseling by
a dietitian, to determine the severity and cause(s) of
malnutrition, to identify patients at risk of complications of
chemotherapy, radiation therapy or surgery, and to assess the
efficacy of nutritional support. The nutritional parameters and
indices should have sufficient sensitivity and specificity to
reliably reflect the course of malnutrition during the disease,
from baseline at diagnosis to remission or cure, through each
specific therapeutic intervention. Nutritional assessment must be
combined with a careful evaluation of performance status and QoL,
so that nutritional management is correctly adapted to the
patient's real needs and entails a minimum of constraints. Ideally,
nutritional support should benefit the patient without feeding the
tumor or, better, while starving the tumor. Drastic restriction of
the amount of protein in food inhibits tumor growth in most animal
models, but limitation of protein intake is also detrimental to a
malnourished host. The stimulation of tumor growth by enteral or
parenteral nutrition has never been clearly demonstrated in humans.
In vivo evaluation of tumor growth is technically difficult,
and most studies rely on data gathered in very small populations
(14,23,29).
Enteral nutrition in non-surgical cancer
patients
Enteral nutrition (EN) by means of ONS and tube
feeding (TF) offers the possibility of increasing or ensuring
nutrient intake in cases where normal food intake is inadequate.
There are no data from controlled studies to suggest a
cancer-specific enteral formula. Standard formulas are recommended
for EN of cancer patients. General information about enteral
nutrition in shown in Table I
(30).
 | Table I.Summary of information concerning
enteral nutrition: Non-surgical oncology. |
Table I.
Summary of information concerning
enteral nutrition: Non-surgical oncology.
| Subject |
Recommendations |
|---|
| General | Nutritional
assessment of cancer patients should be performed frequently, and
nutritional intervention initiated early when deficits are
detected. |
| General
indications | There are no
reliable data that show any effect of enteral nutrition on tumor
growth. Such theoretical considerations should, therefore, have no
influence on the decision to feed a cancer patient. Start
nutritional therapy if undernutrition already exists or if it is
anticipated that the patient will be unable to eat for >7 days.
Start enteral nutrition if an inadequate food food intake (<60%
of estimated energy expenditure for >10 days) is anticipated. It
should substitute the difference between actual intake and
calculated requirements. |
| Perioperative | In weight-losing
patients, due to insufficient nutritional intake, enteral nutrition
should be provided to improve or maintain nutritional status.
Patients with severe nutritional risk may benefit from nutritional
support 10–14 days prior to major surgery, even when surgery has to
be delayed. |
| During radiotherapy
or radiochemotherapy | Use of intensive
dietary advice and oral nutritional supplements is advised to
increase dietary intake and prevent therapy-associated weight loss
and interruption of radiation therapy. |
| During
chemotherapy | Routine enteral
nutrition is not indicated during radiation therapy. Routine
enteral nutrition during chemotherapy has no effect on tumor
response to chemotherapy or on chemotherapy-associated unwanted
effects and, therefore, is not considered useful. |
| During stem cell
transplantation | Routine use of
enteral nutrition is not recommended. |
| In incurable
patients | When oral intake is
decreased, parenteral nutrition may be preferred to tube feeding in
certain situations (i.e., increased risk of haemorrhage and
infections associated with enteral tube placement in
immunocompromised and thrombocytopenic patients). Enteral nutrition
may be provided in order to minimize weight loss, as long as the
patient consents and the dying phase has not started. When the end
of life is very close, most patients only require minimal amounts
of food and little water to reduce thirst and hunger. Small amounts
of fluid may also help to avoid states of confusion induced by
dehydration. |
| Application | Subcutaneously
infused fluids in hospital or at home may be helpful and may also
provide a vehicle for the administration of drugs. The enteral
route is preferable whenever feasible. Administration of
pre-operative enteral nutrition is preferable before admission to
the hospital. |
| Route | Use of tube feeding
is advocated when an obstructing head or neck or esophageal cancer
interferes with swallowing, or when severe local mucositis is
expected. |
| During radiotherapy
or radiochemotherapy | Tube feeding is
either delivered via transnasal or percutaneous routes. Because of
radiation-induced oral and esophageal mucositis, a percutaneous
gastrostomy may be preferred. |
| Type of formula
General | Use of standard
formulae is advised. Regarding n-3 fatty acids, randomized clinical
trial evidence is contradictory/controversial and at present it is
not possible to reach any definite conclusion with regard to
improved nutritional status/physical function. It is unlikely that
n-3 fatty acids prolong survival in advanced cancer. |
| Perioperative | Use of
pre-operative enteral nutrition preferably with immunomodulating
substrates (arginine, n-3 FAs, nucleotides) is advisable for 5–7
days in all patients undergoing major abdominal surgery independent
of their nutritional status. |
| During stem cell
transplantation | Enteral
administration of glutamine or eicosapentanoic acid is not
recommended due to inconclusive data. |
| Drug treatment | In the presence of
systemic inflammation, pharmacological efforts are recommended in
addition to nutritional interventions to modulate the inflammatory
response. In cachectic patients, steroids or progestins are
recommended in order to enhance appetite, modulate metabolic
derangements and prevent impairment of QoL. Administration of
steroids for short-term periods should be carried out only weighing
their benefits against their adverse side effects. Consider the
risk of thrombosis during progestin therapy. |
The therapeutic goal for cancer patients is the
improvement of function and outcome by i) preventing and treating
undernutrition; ii) enhancing antitumor treatment effects; iii)
reducing adverse effects of antitumor therapies; and iv) improving
QoL (30).
Nutritional therapy should be initiated when
undernutrition already exists or when it is anticipated that the
patient may be unable to eat for more than 7 days. EN should also
be initiated when an inadequate food intake (<60% of estimated
energy expenditure) is anticipated for more than 10 days. It should
substitute the difference between actual intake and calculated
requirements.
According to recent guidelines, initiating EN in
patients is indicated upon decreased oral intake (31,32).
When nutritional intake is chronically reduced, then a
corresponding weight loss and a worsening of prognosis are
anticipated. To determine a reduced intake of normal food, a simple
24 h recall is usually adequate. If this proves difficult in
individual cases, it may be appropriate to ask the patient whether
his/her nutritional intake is less than 50% (low intake) or less
than 25% (minimal intake) of their usual intake before the onset of
the disease. In patients who are losing weight due to insufficient
nutritional intake, EN should be provided to improve or maintain
nutritional status (31). This may
also contribute to the maintenance of QoL (30).
Omega-3 fatty acids
Omega-3 (n-3) FAs are long-chain polyunsaturated FAs
with a final carbon-carbon double bond in the n-3 position, the
third bond, from the methyl end of the chain. Omega-6 (n-6) FAs
have a similar structure with the first double bond 6 carbons from
the methyl end of the chain. Humans are unable to desaturate the
n-3 or n-6 double bond and as such this makes both compounds
‘essential FAs’ which can only be obtained from dietary sources.
Omega-6 fatty acid is consumed as linoleic acid or arachidonic acid
found in meats and vegetable oils (safflower, corn and soybean
oil). The principal dietary source of n-3 FAs is from oily
cold-water fish namely eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA). General interest has focused on the
properties of n-3 FAs. Both omega-3 and omega-6 FAs are used as
substrates for the production of eicosanoids which are a class of
compounds, including prostaglandins (PGs), thromboxanes and
leukotrienes intimately involved in immunomodulation, inflammation
and tumor formation. Eicosanoids produced using n-6 FAs
(arachidonic acid) as a substrate stimulate inflammation and tumor
angiogenesis, whereas eicosanoids produced from n-3 FAs, EPA and
DHA are anti-inflammatory and do not stimulate angiogenesis
(33,34). The administration of fish oil
reduces production of cytokines, such as IL-6, TNF and IL-1, in
healthy subjects (35).
The potential benefit of n-3 FA supplementation is
the effect of these fats on cachexia. Anti-inflammatory agents,
such as fish oil, in combination with nutritional supplementation
may reverse features of cachexia (36). Pancreatic cancer patients often
develop debilitating cachexia. Certain studies have shown weight
gain and improved QoL after daily supplementation of the diet with
an energy- and protein-dense (610 kcal, 32.2 g protein) liquid
supplement containing 2.2 g EPA and 0.96 g DHA for 8 weeks
(37). Patients who consumed a
supplement that contained energy and protein, but did not contain
the n-3 FAs, did not gain weight.
The effects of omega-3 FAs on improvement in
appetite and body weight have been studied in cancer patients
(30). Takatsuka et al
(38) studied 16 consecutive
patients, 7 of whom received 1.8 g/day EPA orally from 30 days
before until 180 days after allogeneic HSCT. EPA was found to lower
the levels of prostanoids and cytokines. In addition, complications
of HSCT were less and the survival rate was significantly higher in
the group treated with EPA. In a short-term (2-week)
placebo-controlled trial, Bruera et al (39) studied 60 weight-losing cancer
patients and found no effect of 1.8 g EPA/day on appetite,
tiredness, nausea, well-being, caloric intake and nutritional
status or function. However, it was indicated that ingested EPA
accumulates in tissues over time, and 2 weeks may be too short to
induce clinically measurable effects. In a clinical study, a group
of 18 weight-losing patients with advanced pancreatic cancer
received oral fish-oil preparations providing approximately 2.2 g
of EPA and 1.4 g of the related docosahexanoic acid daily. Before
treatment, all patients experienced weight loss at a median rate of
2.9 kg/month. After 3 months of supplementation, the patients'
weights stabilized, with less than half of the patients continuing
to lose weight. There was no change in the percentage of total body
water during the study, suggesting that patients were not simply
retaining fluid (40).
The use of EPA-containing protein- and energy-dense
ONS (EPA-ONS) was shown to reduce weight loss, increase lean body
mass (LBM), improve functional capacity, nutritional status and
QoL, however not to any greater degree than conventional
supplements (41). Intention to
treat patients indicated that, at the mean dose taken, enrichment
with n-3 FAs did not provide a therapeutic advantage and that both
supplements were equally effective in arresting weight loss.
Post hoc dose-response analysis suggests that if taken in
sufficient quantity, only the n-3 FA-enriched energy- and
protein-dense supplement results in net gain of weight, lean tissue
and improved QoL. (42). Read
et al (43) indicated that
after an average amount of 408 ml/day EPA for 3 weeks, a
significant increase was noted in mean patient weight (2.5 kg)
(p=0.03), and LBM was maintained. In the summary of this study, it
was stated that dietary counseling by suitably qualified dietitians
and the administration of EPA in patients with advanced colerectal
cancers receiving chemotherapy, help to maintain weight and
possibly improve symptom control, nutritional status and QoL.
In summary, multiple mechanisms play a role in the
suppression of tumor growth by n-3 FAs. Some of the mechanisms may
play a more dominant role in particular tumor types, i.e.,
alteration of estrogen is likely to be more important for
suppression of breast than of esophageal cancer. Pre-clinical
studies indicate that n-3 FAs should be beneficial for cancer
treatment. Mechanistic studies indicate feasible mechanisms for the
influence of n-3 FAs on tumor growth, survival and response to
chemotherapy. A limited number of clinical studies indicate that
n-3 FAs may be beneficial when consumed before chemotherapy. It
seems important to commence human trials using an (n-3) fatty acid
as a supplement to standard chemotherapy (2,44,45).
Glutamine
Glutamine is a non-essential amino acid with a
special role in metabolism and nutrition. A number of key functions
of glutamine in metabolism are shown in Table II (46). Being a precursor for nucleotide
synthesis, glutamine availability is a key factor in cell growth.
In the human body, glutamine availability is crucial for intestinal
mucosal cells and also for immune-competent cells. When glutamine
is used as an energy source, a high metabolic flow rate is present;
furthermore a small fraction of that flux will be sufficient to
dramatically increase the nucleotide synthesis rate. Therefore,
many cells use glutamine as an energy substrate. It has been
demonstrated that stressed cells have a particular preference for
glutamine as an energy source. The role of glutamine for
inter-organ transport is also well established (47).
 | Table II.Glutamine functions. |
Table II.
Glutamine functions.
| Precursor for
DNA/RNA |
| Constituent for
proteins |
| Energy substrate
for immunocompetent cells and enterocytes |
| Substrate for
gluconeogenesis |
| Precursor for
glutamate in the brain – glutamate is an important excitatory
neurotransmitter in the brain |
| Pathway for
glutamate transport out of the brain |
| Via glutamate a
precursor for glutathione, which is an antioxidant |
| Substrate for renal
ammoniagenesis and acid-base regulation |
An increasing number of clinical investigations have
focused on the supplementation of specialized enteral and
parenteral nutrition with the amino acid glutamine to improve the
efficacy of nutritional support (48,49).
Endogenous production of glutamine may become
insufficient during critical illness. The shortage of glutamine is
reflected as a decrease in plasma concentration, which is a
prognostic factor for poor outcome in sepsis. Since glutamine is a
precursor for nucleotide synthesis, rapidly dividing cells are most
likely to suffer from a shortage. Therefore, exogenous glutamine
supplementation is necessary (50). In particular, when i.v. nutrition
is administered, extra glutamine supplementation becomes critical,
as most current formulations for i.v. use do not contain any
glutamine for technical reasons. The major portion of endogenously
produced glutamine comes from skeletal muscle. For patients who
must remain for a long time in the intensive care unit (ICU), the
muscle mass decreases rapidly, which leaves a tissue of diminishing
size to maintain the export of glutamine. The metabolic and
nutritional adaptation in long-staying ICU patients has been poorly
studied and is one of the fields that requires more scientific
evidence for clinical recommendations (47). To date, there is evidence to
support the clinical use of glutamine supplementation in critically
ill, in hematology and in oncology patients. Strong evidence is
presently available for i.v. glutamine supplementation to
critically ill patients on parenteral nutrition (51). This must be regarded as the
standard of care. For patients on enteral nutrition, more evidence
is required. Concerning the administration of glutamine, there are
good arguments to use the measurement of plasma glutamine
concentration for guidance. This may provide an indication for
treatment as well as proper dosing. Most patients will have a
normalized plasma glutamine concentration by adding 20–25 g/24 h.
Furthermore, there are no reported adverse or negative effects
attributable to glutamine supplementation (52).
Glutamine behaves as an essential amino acid in
clinical settings where there is marked metabolic stress, such as
that which occurs after HSCT (53). Glutamine supplementation in animals
was found to reduce bacterial translocation and mucosal damage
after chemotherapy or radiotherapy, making supplementation an
attractive option for reducing post-transplant complications
(54,55). There has also been a lack of an
objective tool for measuring mucosal barrier injury (MBI), not only
of the mouth, but also of the digestive tract as a whole, despite
the fact that MBI is the most frequent cause of morbidity
associated with the myeloablative-conditioning treatment to prepare
for an HSCT (56). Oral mucositis
is relatively easy to recognize, whereas the detection of
intestinal mucosal injury has relied essentially on non-specific
symptoms, such as nausea, vomiting, diarrhea and abdominal cramps,
which affect almost every HSCT recipient and do not necessary
reflect MBI (57).
Oral and i.v. glutamine appear to have differing
effects. There is no apparent reason why this should be the case.
Glutamine (i.v.), when administered with TPN, produces improvement
in gut function and less gut atrophy, suggesting that i.v.
glutamine has local effects on the gut mucosa. Oral glutamine
appears to have no effect on mortality, infections, time to
neutrophil recovery or relapses. Oral glutamine may reduce
mucositis (decreased average score and fewer days of opioids and
trends for less days of mucositis and less severe mucositis) and
Graft-vs.-host-disease (GVHD) (which may be due to mucositis being
a risk factor for GVHD) (58,59).
The administration of enteral or parenteral
glutamine at doses of up to 40 g/day appears safe according to
studies of patients receiving BMT and high-dose chemotherapy
published to date (60). It is
possible that glutamine is utilized as a growth factor in
interactions between malignant tumors and chemotherapeutic drugs
(61). Thus, carefully planned
pharmacological studies may be required when large doses of
glutamine are administered to patients receiving cytotoxic drugs.
However, the available clinical data in cancer patients does not
suggest that glutamine-supplemented nutrition enhances or induces
tumor growth or worsens clinical outcomes. However, further study
of long-term outcomes with other novel forms of therapy, is
indicated to complement primarily short-term safety data available
to date. Glutamine deserves further study to elucidate its
interactions with methotrexate and to investigate its effects on
autologous HSCT patients. Supplementation of glutamine should be
considered in the design of future randomized, controlled clinical
trials and in the metabolic support of individuals undergoing
marrow transplantation and cancer (60,62).
Probiotics and prebiotic
oligosaccharides
Diarrhea is a common complication of enteral
nutrition in cancer patients. For many years, fiber was extensively
investigated for its role in preventing diarrhea; however, a more
recent focus has been the investigation of specific fiber blends,
including soluble fibers and prebiotics, for which there is now
considerable quality evidence. Enteral nutrition may result in
deleterious effects on gastrointestinal microbiota, including
reductions in bifidobacteria and key butyrate producers. Their
modulation by prebiotics has been confirmed in studies on healthy
individuals, but convincing evidence in acutely ill patients is
required (63).
The pathogenesis of diarrhea involves antibiotic
prescription, enteropathogenic colonization and abnormal colonic
responses, all of which involve an interaction with colonic
microbiota. Alterations in the colonic microbiota have been
identified in patients receiving enteral tube feeding, and these
changes may be associated with the incidence of diarrhea.
Preventing negative alterations in the colonic microbiota has
therefore been investigated as a method of reducing the incidence
of diarrhea. Probiotics and prebiotics may be effective because of
their functions in modulating both the structure and composition as
well as activities of both mucosa and microflora suppression of
enteropathogenic colonization (stimulation of immune function and
modulation of colonic metabolism) (2,64).
Randomized controlled trials of probiotics have produced
contrasting results, although Saccharomyces boulardii has
been shown to reduce the incidence of diarrhea in patients in the
ICU receiving enteral tube feeding. Prebiotic
fructo-oligosaccharides have been shown to increase the
concentration of fecal bifidobacteria in healthy subjects consuming
an enteral formula, although this finding has not yet been
confirmed in patients receiving enteral TF. Furthermore, there are
no clinical trials investigating the effect of a prebiotic alone on
the incidence of diarrhea. Further trials of the efficacy of
probiotics and prebiotics, alone and in combination, in preventing
diarrhea in this patient group are warranted (64).
Probiotics and prebiotics may beneficially affect a
series of GI functions by modulating both the structure and
composition, as well as activities of both mucosa and microflora
(64). Potential targets and
expected benefits have been identified as reduced risk for
metabolic syndrome and prevention of colorectal cancer (65).
The potential effects of probiotics and prebiotics
in cancer are as follows (64,66–68).
i) Reversing the disruption of micro-biota and improving resistance
to colonization by pathogens: Gut microflora themselves act as
barriers against invasion by pathogens. Chemotherapy profoundly
disturbs floral balance in a manner that potentiates colonization
by pathogens, and an efficient probiotic or prebiotic may favorably
modify these changes, inhibiting overgrowth of potential pathogens
that cause secondary infectious diarrhea following chemotherapy.
ii) Beneficially modulating the immune system: Probiotics
extensively influence intestinal innate/adaptive immunity and
barrier function. Probiotics and prebiotics may also directly or
indirectly modulate the inflammatory cytokine network. These
probiotics and prebiotics appear to enhance the gut barrier and
reduce inflammatory injury by rebalancing the
proinflammatory/anti-inflammatory cytokine network. iii) Enhancing
production of short-chain FAs (SCFAs): Fermentation of
oligofructose and insulin increases production of SCFAs, primarily
acetate, butyrate and propionate, in the gut. SCFAs, the main
energy source for colonic mucosal enterocytes, play a central
metabolic role in maintaining the epithelial cell barrier and in
repairing mechanisms that are likely important for the prevention
or resolution of inflammation. iv) Drug metabolism by intestinal
microflora contributes to the pharmacological profile of various
drugs: Probiotics and prebiotics may modulate the pharmacokinetics
of anticancer drugs by altering the composition and metabolic
activity of the microflora. More research is required to determine
probiotic and prebiotic modulation of bacterial enzyme activity
during chemotherapy.
Enteral nutrition in surgical cancer
patients
During the last few years, the importance of the
correct nutritional assessment as a part of the therapeutic process
of human pathologies has a greatly increased relevance. Still more
in oncology, such a relationship between nutritional assessment and
a beneficial result of the therapeutic treatment has a fundamental
importance (69). There is a clear
correlation between the degree of malnutrition and increased risk
of perioperative complications in cancer patients undergoing
surgery. It is known from various studies that the value of a
variety of nutrition status parameters for predicting risk of
surgical complications is important (70). Nutrition support therapy should not
be used routinely in patients undergoing major cancer operations
(71). For surgical patients,
practical information, such as weight loss or subjective global
assessment, would provide a better basis for deciding whether or
not to delay surgery. At least 10 days of nutritional support is
recommended in severely malnourished patients before major
digestive surgery. In non-severely malnourished patients,
pre-operative oral immunonutrition is associated with a 50%
decrease in post-operative complications. The benefit of
immune-enhancing diets in severely malnourished patients remains to
be proven. For patients undergoing radiochemotherapy, dietary
counseling is proposed. In cases of severely malnourished patients
or if dietary counseling suffers a setback, EN should be
recommended (72).
Total parenteral nutrition in
surgical/non-surgical cancer patients
The maintenance or improvement of QoL, and the
increase in the effectiveness of antitumor therapy and a reduction
in side effects are further objectives in regards to cancer
patients. Indications for TPN in tumor patients are essentially
identical to those in patients with benign illnesses, with
preference given to oral or enteral nutrition when feasible. A
combined nutritional concept is preferred when oral or enteral
nutrition, particularly exclusive artificial nutrition, is
administered. The use of TPN as a general accompaniment to
radiotherapy or chemotherapy is not indicated, but TPN is indicated
in chronic severe radiogenic enteritis or after allogenic
transplantation with pronounced mucositis or GVHD (73). General information regarding TPN
application in oncology patients is shown in Table III (4).
 | Table III.Summary of information regarding
total parenteral nutrition. |
Table III.
Summary of information regarding
total parenteral nutrition.
| Subject |
Recommendations |
|---|
| General | Nutritional
assessment of all cancer patients should begin with tumor diagnosis
and be repeated at every visit in order to initiate nutritional
intervention early, before the general status is severely
compromised. |
| Indications | Total daily energy
expenditure in cancer patients may be assumed to be similar to
healthy subjects, or 20–25 kcal/kg/day for bedridden and 25–30
kcal/kg/day for ambulatory patients. Therapeutic goals for TPN in
cancer patients are the improvement of function and outcome by:
-
- preventing and treating
undernutrition/cachexia,
-
- enhancing compliance with antitumor
treatments,
-
- controlling certain adverse effects of antitumor
therapies,
-
- improving QoL.
|
| Nutritional
provision | TPN is ineffective
and probably harmful in non-aphagic oncological patients in whom
there is no gastrointestinal reason for intestinal failure. TPN is
recommended in patients with severe mucositis or severe radiation
enteritis. Supplemental TPN is recommended in patients when
inadequate food and enteral intake (<60% of the estimated energy
expenditure) is anticipated for >10 days. TPN is not recommended
if oral/enteral nutrient intake is adequate. In the presence of
systemic inflammation, it is extremely difficult to achieve whole
body protein anabolism in cancer patients. In this situation, in
addition to nutritional interventions, pharmacological efforts are
recommended to modulate the inflammatory response. |
| Perioperative
care | Preliminary data
suggest a potential positive role of insulin (Grade C). There are
no data on n-3 FAs. Peri-operative TPN is recommended in
malnourished candidates for artificial nutrition, when EN is not
possible. |
| During non-surgical
therapy | Routine use of TPN
during chemotherapy, radiotherapy or combined therapy is not
recommended. |
| Incurable
patients | When patients are
malnourished or are facing a period longer than 1 week of
starvation and enteral nutritional support is not feasible, TPN is
recommended. In intestinal failure, long-term TPN should be offered
when i) enteral nutrition is insufficient, ii) expected survival
due to tumor progression is longer than 2–3 months), iii) it is
expected that PN can stabilize or improve performance status and
QoL, and iv) the patient desires this mode of nutritional
support. |
| Hematopoietic stem
cell transplantation (HSCT) | There is probable
benefit in providing incurable cancer patients with weight loss and
reduced nutrient intake with ‘supplemental’ TPN. In HSCT patients,
TPN should be reserved for those with severe mucositis, ileus or
intractable vomiting. No clear recommendation can be made as to the
time of introduction of TPN in HSCT patients. Its withdrawal should
be considered when patients are able to tolerate approximately 50%
of their requirements enterally. HSCT patients may benefit from
glutamine-supplemented TPN. |
| Tumor growth | Although TPN
supplies nutrients to the tumor, there is no evidence that this has
deleterious effects on the outcome. This consideration should
therefore have no influence on the decision to feed a cancer
patient when TPN is clinically indicated. |
The treatment aims for TPN in cancer patients
(73) are as follows. i) TPN
should stabilize the nutritional state and prevent or reduce
progressive weight loss; ii) TPN should maintain or improve the
QoL; iii) TPN may increase the effectivity and reduce the side
effects of anticancer therapies.
The majority of cancer patients requiring long-term
TPN are cachectic and hypophagic because of (subacute) intestinal
obstruction due to peritoneal carcinomatosis. This condition is
often associated with expansion of extracellular water volume, and
an overzealous administration of glucose may easily precipitate a
peritoneal effusion which consequently forces withdrawal of the
intravenous nutrition (74).
In patients who are losing weight mainly because of
an insufficient nutritional intake, artificial nutritional support
should be provided to maintain nutritional status or at least
prevent further nutritional deterioration. This may also contribute
to the maintenance of QoL. Any such improvement in the nutritional
status is usually modest and is most expected when weight loss is
mainly due to hypophagia. In the presence of systemic inflammation,
however, it appears to be extremely difficult to achieve whole body
protein anabolism in cancer patients. In this situation, in
addition to nutritional interventions, pharmacological efforts are
recommended to modulate the inflammatory response (75). Patients who received the planned
amounts of energy and nitrogen (given by vein when necessary) were
found to exibit improved energy balance, increased body fat and
greater maximum exercise capacity, in addition to prolonged
survival when compared to patients randomized to support without
TPN (76).
Enteral and parenteral nutrition confer a number of
risks, including the physiologic stress and discomfort associated
with the placement of a feeding tube or central line and
complications involved in the placement or in nutrition. Infection
is the most common complication of both types of nutritional
support and occurs frequently with parenteral nutrition because of
the high nutrient value of the infusion. Although usually easily
treated, these infections often require hospitalization and
insertion of a new catheter, and may lead to complications, such as
subacute bacterial endocarditis. Other common complications of
enteral and parenteral nutrition include metabolic problems, such
as hyperglycemia and fluid, and electrolyte imbalances, diarrhea
from enteral feeding and hepatic abnormalities from parenteral
feeding. In the terminally ill, both types of nutrition cause fluid
overload, worsening edema or shortness of breath (77).
Conclusion
In conclusion, enteral or parenteral nutrition
support decreases the catabolic rate of the patients, helping them
withstand the side effects of the therapeutic measures, but do not
reverse to anabolism. Terminally ill cancer patients who are
refractory to the different therapeutic measures require palliative
care. Nutrition is a basic human right and is conceived by the
patient and his family, as well as by the medical community and
human society, to be vital for survival. However, a group of
patients exist who, although they are not candidates for
antineoplastic therapy, do remain in good physical and mental
condition with expected lifespans of 3 months or more, and who are
suffering from conditions, such as intestinal obstruction or
fistulas, which makes the preferred route of EN impossible. In this
specific patient group, palliative parenteral nutrition should be
considered. The decision should be taken after careful
multidisciplinary discussion. The patient and caregivers should be
aware that this is not a cancer-specific treatment and probably
will not prolong the patient's life.
References
|
1.
|
Jones L, Watling RM, Wilkins S and Pizer
B: Nutritional support in children and young people with cancer
undergoing chemotherapy. Cochrane Database Syst Rev. 7:1–50.
2010.PubMed/NCBI
|
|
2.
|
Xue H, Sawyer MB, Wischmeyer PE and
Baracos VE: Nutrition modulation of gastrointestinal toxicity
related to cancer chemotherapy: from preclinical findings to
clinical strategy. J Parenter Enteral Nutr. 35:74–90. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shike M: Nutrition therapy for the cancer
patient. Hematol Oncol Clin North Am. 10:221–234. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bozzetti F, Arends J, Lundholm K,
Micklewright A, Zurcher G and Muscaritoli M: ESPEN Guidelines on
parenteral nutrition: non-surgical oncology. Clin Nutr. 28:445–454.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Morss S: Enteral and parenteral nutrition
in terminally ill cancer patients: a review of the literature. Am J
Hosp Palliat Care. 23:369–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Muscaritoli M, Bossola R, Bellantone R and
Fanelli FR: Therapy of muscle wasting in cancer: what is the
future? Curr Opin Clin Nutr Metab Care. 7:459–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Simons JPFHA, Schols AM, Buurman WA and
Wouters EF: Weight loss and low body cell mass in males with lung
cancer: relationship with systemic inflammation, acute-phase
response, resting energy expenditure, and catabolic and anabolic
hormones. Clin Sci. 97:215–223. 1999. View Article : Google Scholar
|
|
8.
|
Barber MD: The pathophysiology and
treatment of cancer cachexia. Nutr Clin Pract. 17:203–209. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tisdale MJ: Cachexia in cancer patients.
Nat Rev Cancer. 11:862–871. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Arends J: Metabolism in cancer patients.
Anticancer Res. 30:1863–1868. 2010.
|
|
11.
|
Van Cutsem E and Arends J: The causes and
consequences of cancer-associated malnutrition. Eur J Oncol Nurs.
9(Suppl. 2): 51–63. 2005.PubMed/NCBI
|
|
12.
|
Jatoi A, Daly BD, Hughes VA, Dallal GE,
Kehayias J and Roubenoff R: Do patients with nonmetastatic
non-small cell lung cancer demonstrate altered resting energy
expenditure? Ann Thorac Surg. 72:348–351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Argilés JM, Olivan M, Busquets S and
López-Soriano FJ: Optimal management of cancer anorexia-cachexia
syndrome. Cancer Manag Res. 22:27–38. 2010.
|
|
14.
|
Nitenberg G and Raynard B: Nutritional
support of the cancer patient: issues and dilemmas. Crit Rev Oncol
Hematol. 34:137–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Drudge-Coates L: Improving management of
patients with advanced cancer. Patient Prefer Adherence. 2:415–424.
2010. View Article : Google Scholar
|
|
16.
|
Marín Caro MM, Laviano A and Pichard C:
Nutritional intervention and quality of life in adult oncology
patients. Clin Nutr. 26:289–301. 2007.PubMed/NCBI
|
|
17.
|
Ströhle A, Zänker K and Hahn A: Nutrition
in oncology: the case of micronutrients (Review). Oncol Rep.
24:815–828. 2010.PubMed/NCBI
|
|
18.
|
Marín Caro MM, Laviano A and Pichard C:
Impact of nutrition on quality of life during cancer. Curr Opin
Clin Nutr Metab Care. 10:480–487. 2007.PubMed/NCBI
|
|
19.
|
Tian J and Chen JS: Nutritional status and
quality of life of the gastric cancer patients in Changle County of
China. World J Gastroenterol. 11:1582–1586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gupta D, Lis CG, Granick J, Grutsch JF,
Vashi PG and Lammersfeld CA: Malnutrition was associated with poor
quality of life in colorectal cancer: a retrospective analysis. J
Clin Epidemiol. 59:704–709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ravasco P, Monteiro-Grillo I, Vidal PM and
Camilo ME: Cancer: disease and nutrition are key determinants of
patients' quality of life. Support Care Cancer. 12:246–252.
2004.
|
|
22.
|
Petruson KM, Silander EM and Hammerlid EB:
Quality of life as predictor of weight loss in patients with head
and neck cancer. Head Neck. 27:302–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Bower M, Jones W, Vessels B, Scoggins C
and Martin R: Role of esophageal stents in the nutrition support of
patients with esophageal malignancy. Nutr Clin Pract. 25:244–249.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Marín Caro MM, Gómez Candela C, Castillo
Rabaneda R, Lourenço Nogueira T, García Huerta M and Loria Kohen V:
[Nutritional risk evaluation and establishment of nutritional
support in oncology patients according to the protocol of the
Spanish Nutrition and Cancer Group]. Nutr Hosp. 23:458–468.
2008.
|
|
25.
|
Mudigonda T and Mudigonda P: Palliative
cancer care ethics: principles and challenges in the Indian
setting. Indian J Palliat Care. 16:107–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maree JE and Wright SC: Palliative care: a
positive outcome for cancer patients? Curationis. 31:43–49. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Granda-Cameron C, Viola SR, Lynch MP and
Polomano RC: Measuring patient-oriented outcomes in palliative
care: functionality and quality of life. Clin J Oncol Nurs.
12:65–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wiseman M: The treatment of oral problems
in the palliative patient. J Can Dent Assoc. 72:453–458.
2006.PubMed/NCBI
|
|
29.
|
Koretz RL: Should patients with cancer be
offered nutritional support: Does the benefit outweigh the burden?
Eur J Gastroenterol Hepatol. 19:379–382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Arends J, Bodoky G, Bozzetti F, et al:
ESPEN Guidelines on enteral nutrition: non-surgical oncology. Clin
Nutr. 25:245–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
ASPEN Board of Directors: Clinical
Guidelines Task Force, Nutrition assessment – adults. J Parenter
Enteral Nutr. 26:9–12. 2002.
|
|
32.
|
Sax HC and Souba WW: Enteral and
parenteral feedings. Guidelines and recommendations. Med Clin North
Am. 77:863–880. 1993.PubMed/NCBI
|
|
33.
|
Hardman WE: Omega-3 FA to augment cancer
therapy. J Nutr. 132(Suppl. 11): 35082002.PubMed/NCBI
|
|
34.
|
Hardman WE: N-3 fatty acids and cancer
therapy. J Nutr. 134(Suppl. 12): 3427–3430. 2004.PubMed/NCBI
|
|
35.
|
Caughey GE, Mantzioris E, Gibson RA,
Cleland LG and James MJ: The effect on human tumor necrosis factor
alpha and interleukin 1beta production of diets enriched in or fish
oil. Am J Clin Nutr. 63:116–119. 1996.PubMed/NCBI
|
|
36.
|
Barber MD: Cancer cachexia and its
treatment with fish-oil-enriched nutritional supplementation.
Nutrition. 17:751–755. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Moses AW, Slater C, Preston T, Barber MD
and Fearon KC: Reduced total energy expenditure and physical
activity in cachexia patients with pancreatic cancer can be
modulated by an energy and protein dense oral supplement enriched
with n-3 fatty acids. Br J Cancer. 90:996–1002. 2004. View Article : Google Scholar
|
|
38.
|
Takatsuka H, Takemoto Y and Iwata N: Oral
eicosapentaenoic acid for complications of bone marrow
transplantation. Bone Marrow Transplant. 28:769–774. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Bruera E, Strasser F and Palmer JL: Effect
of fish oil on appetite and other symptoms in patients with
advanced cancer and anorexia/cachexia: a double-blind,
placebo-controlled study. J Clin Oncol. 21:129–134. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Wigmore SJ, Ross JA, Falconer JS, Plester
CE, Tisdale MJ, Carter DC and Fearon KC: The effect of
polyunsaturated fatty acids on the progress of cachexia in patients
with pancreatic cancer. Nutrition. 12(Suppl. 1): 27–30. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Bauer JD and Capra S: Nutrition
intervention improves outcomes in patients with cancer cachexia
receiving chemotherapy – a pilot study. Support Care Cancer.
213:270–274. 2005.PubMed/NCBI
|
|
42.
|
Fearon KC, von Meyenfeldt MF, Moses AG, et
al: Effect of a protein and energy dense n-3 fatty acid-enriched
oral supplement on loss of weight and lean tissue in cancer
cachexia: a randomised double blind trial. Gut. 52:1479–1486. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Read JA, Beale PJ, Volker DH, Smith N,
Childs A and Clarke SJ: Nutrition intervention using an
eicosapentaenoic acid (EPA)-containing supplement in patients with
advanced colorectal cancer. Effects on nutritional and inflammatory
status: a phase II trial. Support Care Cancer. 15:301–307. 2007.
View Article : Google Scholar
|
|
44.
|
Spencer L, Mann C, Metcalfe M, Webb M,
Pollard C and Spencer D: The effect of omega-3 FAs on tumour
angiogenesis and their therapeutic potential. Eur J Cancer.
45:2077–2086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Fritsche KL: Are omega-3 fatty acids
effective in enhancing tumoricidal cell activity? J Nutr.
135:2916–2917. 2005.
|
|
46.
|
Oudemans-van Straaten HM, Bosman RJ,
Treskes M, van der Spoel HJ and Zandstra DF: Plasma glutamine
depletion and patient outcome in acute ICU admissions. Intensive
Care Med. 27:84–90. 2001.PubMed/NCBI
|
|
47.
|
Berg A, Rooyackers O, Norberg A and
Wernerman J: Elimination kinetics of L-alanyl-L-glutamine in ICU
patients. Amino Acids. 29:221–228. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Darmaun D: Role of glutamine depletion in
severe illness. Diabetes Nutr Metab. 13:25–30. 2000.PubMed/NCBI
|
|
49.
|
Ziegler TR, Bazargan N and Galloway JR:
Glutamine enriched parenteral nutrition; saving nitrogen and saving
money? Clin Nutr. 19:375–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Novak F, Heyland DK, Avenell A, Drover JW
and Su X: Glutamine supplementation in serious illness: a
systematic review of the evidence. Crit Care Med. 30:2022–2029.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Goeters C, Wenn A, Mertes N, et al:
Parenteral L-alanyl-L-glutamine improves 6-month outcome in
critically ill patients. Crit Care Med. 30:2032–2037. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Wernerman J: Clinical use of glutamine
supplementation. J Nutr. 138:2040–2044. 2008.PubMed/NCBI
|
|
53.
|
Crowther M, Avenell A and Culligan DJ:
Systematic review and meta-analyses of studies of glutamine
supplementation in haematopoietic stem cell transplantation. Bone
Marrow Transplant. 44:413–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Ziegler TR: Glutamine supplementation in
cancer patients receiving bone marrow transplantation and high dose
chemotherapy. J Nutr. 131:2578–2584. 2001.PubMed/NCBI
|
|
55.
|
Blijlevens NMA, Donnelly JP and De Pauw
BE: Mucosal barrier injury: biology, pathology, clinical
counterparts and consequences of intensive treatment for
haematological malignancy: an overview. Bone Marrow Transplant.
25:1269–1278. 2000. View Article : Google Scholar
|
|
56.
|
Elting LS, Cooksley C, Chambers M, Cantor
SB, Manzullo E and Rubenstein EB: The burdens of cancer therapy:
clinical and economic outcomes of chemotherapy-induced mucositis.
Cancer. 98:1531–1539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Sonis ST, Oster G, Fuchs H, et al: Oral
mucositis and the clinical and economic outcomes of hematopoietic
stem-cell transplantation. J Clin Oncol. 19:2201–2205.
2001.PubMed/NCBI
|
|
58.
|
Kuskonmaz B, Yalcin S, Kucukbayrak O, et
al: The effect of glutamine supplementation on hematopoietic stem
cell transplant outcome in children: a case-control study. Pediatr
Transplant. 12:47–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Aquino VM, Harvey AR, Garvin JH, et al: A
double-blind randomized placebo-controlled study of oral glutamine
in the prevention of mucositis in children undergoing hematopoietic
stem cell transplantation: a pediatric blood and marrow transplant
consortium study. Bone Marrow Transplant. 36:611–616. 2005.
View Article : Google Scholar
|
|
60.
|
Ziegler TR: Glutamine supplementation in
bone marrow transplantation. Br J Nutr. 87(Suppl. 1): 9–15. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Wilmore DW, Schloerb PR and Ziegler TR:
Glutamine in the support of patients following bone marrow
transplantation. Curr Opin Clin Nutr Metab Care. 2:323–327. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Arfons LM and Lazarus HM: Total parenteral
nutrition and hematopoietic stem cell transplantation: an expensive
placebo? Bone Marrow Transplant. 36:281–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Whelan K and Schneider SM: Mechanisms,
prevention, and management of diarrhea in enteral nutrition. Curr
Opin Gastroenterol. 27:152–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Whelan K: Enteral-tube-feeding diarrhoea:
manipulating the colonic microbiota with probiotics and prebiotics.
Proc Nutr Soc. 66:299–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
De Preter V, Hamer HM, Windey K and
Verbeke K: The impact of pre- and/or probiotics on human colonic
metabolism: does it affect human health? Mol Nutr Food Res.
55:46–57. 2011.
|
|
66.
|
Pagnini C, Corleto VD, Hoang SB, Saeed R,
Cominelli F and Delle Fave G: Commensal bacteria and ‘oncologic
surveillance’: suggestions from an experimental model. J Clin
Gastroenterol. 42:S193–S196. 2008.
|
|
67.
|
Pagnini C, Saeed R, Bamias G, Arseneau KO,
Pizarro TT and Cominelli F: Probiotics promote gut health through
stimulation of epithelial innate immunity. Proc Natl Acad Sci USA.
107:454–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Roberfroid M, Gibson GR, Hoyles L,
McCartney AL, Rastall R and Rowland I: Prebiotic effects: metabolic
and health benefits. Br J Nutr. 104(Suppl. 2): 1–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Santacroce L, Leone D, Valenzano A,
Luperto P, Bottalico L and Losacco T: Nutritional problems in the
surgical patients with head and neck tumours. Literature review and
personal experience. Clin Ter. 156:227–230. 2005.(In Italian).
|
|
70.
|
Huhmann MB and August DA: Nutrition
support in surgical oncology. Nutr Clin Pract. 24:520–526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Huhmann MB and August DA: Review of
American Society for Parenteral and Enteral Nutrition (ASPEN)
clinical guidelines for nutrition support in cancer patients:
nutrition screening and assessment. Nutr Clin Pract. 23:182–188.
2008. View Article : Google Scholar
|
|
72.
|
Senesse P, Assenat E, Schneider S,
Chargari C, Magné N and Azria D: Nutritional support during
oncologic treatment of patients with gastrointestinal cancer: Who
could benefit? Cancer Treat Rev. 34:568–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Arends J, Zuercher G, Dossett A, Fietkau
R, Hug M and Schmid I: Non-surgical oncology – Guidelines on
arenteral nutrition. Ger Med Sci. 18:1–14. 2009.
|
|
74.
|
Pironi L, Joly F, Forbes A, et al:
Long-term follow-up of patients on home parenteral nutrition in
Europe: implications for intestinal transplantation. Gut. 60:17–25.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Orrevall Y, Tishelman C, Herrington MK and
Permert J: The path from oral nutrition to home parenteral
nutrition: a qualitative interview study of the experiences of
advanced cancer patients and their families. Clin Nutr.
23:1280–1287. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Braunschweig C, Liang H and Sheean P:
Indications for administration of parenteral nutrition in adults.
Nutr Clin Pract. 19:255–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Dy SM: Enteral and parenteral nutrition in
terminally ill cancer patients: a review of the literature. Am J
Hosp Palliat Care. 23:369–377. 2006. View Article : Google Scholar : PubMed/NCBI
|