Introduction
In colonic carcinogenesis, a serrated neoplastic
pathway has been recommended as an alternative pathway distinct
from the adenoma-carcinoma sequence. A hyperplastic polyp has been
regarded as a benign lesion without malignant potential, whereas a
tubular adenoma (TA) is a precursor of colorectal cancer in the
adenoma-carcinoma sequence. Recently, polyps with serrated
structures have been classified into subgroups, including
hyperplastic polyps, traditional serrated adenomas and sessile
serrated adenomas (SSAs) (1–3).
SSA is a new entity identified by Torlakovic et
al in 1996 (4).
Endoscopically, SSAs are flat, pale and large in size (5,6),
which are different from the endoscopic features of hyperplastic
polyps. The histological appearance of SSAs is distinguished from
that of hyperplastic polyps on the basis of their abnormal
architectural features, such as branching of crypts, dilatation of
the base of the crypts, growth of crypts parallel to the muscularis
mucosa and surface villosity or papillarity (6,7).
Recent studies have shown that SSAs are more likely to occur in the
proximal colon and show a high frequency of BRAF mutations, DNA
methylation of the promoter region of the mismatch repair gene,
such as hMLH1 and CpG island methylator phenotype (CIMP) (8–12).
Since SSA has characteristics similar to colon cancer with
microsatellite instability (MSI), it has attracted considerable
attention as a possible precursor lesion to MSI cancers (13).
Clinicopathological findings suggest that MSI
cancers and SSAs are frequently located in the proximal colon
(14,15). Recent studies have shown distinct
characteristics of MSI cancers emerging from the proximal and
distal colon (16,17). Compared to distal MSI cancers, the
proximal forms show female predominance, a more aggressive
differentiation and a significantly higher frequency of hMLH1
methylations. The reason for the occurrence of these differences
has not been elucidated. Since the location determines the
characteristics of MSI cancers, precursors should also share
location-associated features.
This study was designed to investigate the
location-associated genetic and epigenetic features of SSA to
evaluate its role as a precursor of MSI cancers. TA and colon
cancer were also investigated for comparison.
Materials and methods
This study was approved by the Jichi Medical
University Institutional Review Board prior to commencement. SSAs,
TAs and colorectal cancer tissues were prospectively collected from
patients at the Jichi Medical University Hospital and the Jichi
Medical University Saitama Medical Center. SSAs and TAs were
obtained endoscopically and classified into two categories
according to their location, i.e., proximal or distal. Colon cancer
tissues were obtained from patients who underwent surgical
treatment. For all lesions, a part of the fresh tissue was
immediately frozen for genetic analysis, and the remaining tissue
was used for histological analysis. Proximal lesions were defined
as those proximal to the splenic flexure, whereas distal lesions
were defined as those distal to the splenic flexure. All colon
cancer tissues were collected from the proximal colon.
SSA was diagnosed by five architectural features:
basal crypt serration, basal dilatation of crypts, crypts that run
horizontal to the basement membrane, crypt branching and surface
villosity or papillarity (6,7).
When the endoscopically resected polyp had two or more of the
above-mentioned features, it was diagnosed as SSA. The lesions
showing typical histological features of so called ‘traditional
serrated adenoma’ (2) were
excluded from analysis.
In total, 30 SSAs, 22 TAs and 66 colon cancer
tissues were evaluated for their genetic and epigenetic
features.
BRAF and KRAS mutation analysis
BRAF (T1799A) and KRAS mutations were studied by
direct sequencing after polymerase chain reaction (PCR)
amplification of exon 15 of the BRAF gene and codon 12 and
13 of the KRAS gene. For detection of the BRAF mutation,
genomic DNA obtained from fresh frozen samples was amplified using
the following primers: forward, TCATAATGCTTGCTCTGATAGGA and
reverse, GGCCAAAAATTTAATCAGTGGA. For the detection of the KRAS
mutation, the following primers were used: forward,
CTGAAAATGACTGAATATAAACTTGT and reverse, ATA TGCATATTAAAACAAGATTTACC
as previously described (18,19).
PCR products were purified on a YM-30 Microcon column (Millipore)
and sequenced using the BigDye terminator v3.1 cycle sequencing kit
on ABI Prism 3100 (both from Applied Biosystems, Tokyo, Japan).
MSI analysis
Genomic DNA was extracted from fresh frozen samples
using the EZ1 DNA tissue kit (Qiagen, Tokyo, Japan) and was
amplified by PCR using the monomorphic markers BAT25 and BAT26 as
previously described (20). PCR
products were analyzed by Gene Scan using ABI Prism 3100, and the
sample was scored showing MSI if there were additional peaks in the
PCR products, or otherwise scored as microsatellite stable
(MSS).
hMLH1 methylation and CIMP
Combined bisulfite restriction analysis was
performed to assess gene methylation using primers that were
designed to amplify the regions around the transcription sites of
the target genes (21). Bisulfite
modification was performed according to the manufacturer’s
instructions using the Epitect Bisulfite kit (Qiagen). Genomic DNA
(1 μg) was used for conversion with the bisulfite reagent. The
mismatch repair gene hMLH1 and five MINT loci (MINT1, MINT2,
MINT12, MINT25 and MINT31) were examined as previously described
(22–24). The primer sequences, annealing
temperatures and restriction enzymes utilized were identical to
those previously described (24).
After digestion, products were electrophoresed on 2% agarose gels
and stained with ethidium bromide. Methylation density was
confirmed using the image analysis program ImageJ, and positive
methylation was defined when the methylation-sensitive restriction
enzyme digested ≥10% of the DNA (24). All samples with methylation at
three or more loci were designated as CIMP-positive.
Statistical analysis
Differences between the groups were evaluated using
the Student’s t-test, Chi-square test or Fisher’s exact test.
Statistical significance tests were two-tailed, and p<0.05 was
considered statistically significant. Analysis was performed using
SPSS software (version 17; SPSS Japan Inc., Tokyo, Japan).
Results
Sessile serrated adenoma
The location-associated clinical and molecular
features of SSAs were evaluated (Table
I). Patient age, gender and polyp size were not significantly
different between the proximal and distal SSAs. The BRAF mutation
was found in 86.4% of the proximal SSAs and 37.5% of the distal
SSAs (p=0.007). However, the KRAS mutation was found only in the
distal SSAs (p=0.015). BRAF and KRAS mutations were mutually
exclusive. Epigenetic analysis demonstrated no hMLH1 methylation in
both SSAs and, hence, both were MSS. However, CIMP was observed in
50% of the proximal SSAs, whereas no distal SSA showed CIMP.
 | Table I.Clinical and molecular features of the
proximal and distal sessile serrated adenoma (SSA) cases. |
Table I.
Clinical and molecular features of the
proximal and distal sessile serrated adenoma (SSA) cases.
| Proximal SSA
(n=22) | Distal SSA (n=8) | p-value |
|---|
| Patient age (years)
(mean ± SD) | 60.9±9.2 | 59.1±11.5 | 0.672 |
| Gender
(male/female) | 14/8 | 4/4 | 0.678 |
| Size (mm) (mean ±
SD) | 12.5±5.3 | 14.0±6.9 | 0.664 |
| BRAF (V600E) | 86.4% (19/22) | 37.5% (3/8) | 0.007 |
| KRAS (codon
12/13) | 0.0% (0/22) | 25.0% (2/8) | 0.015 |
| hMLH1
methylation | 0.0% (0/22) | 0.0% (0/8) | - |
| MSI | 0.0% (0/21) | 0.0% (0/8) | - |
| CIMP | 50.0% (11/22) | 0.0% (0/8) | 0.012 |
Ttubular adenoma
Location-associated features of TAs were
investigated (Table II). Patient
age, gender and polyp size were not significantly different between
the two locations. The BRAF mutation, hMLH1 methylation, MSI and
CIMP were not found in either the proximal or distal TAs. The KRAS
mutation was found in 36.4% of the proximal TAs and in 40% of the
distal TAs, showing no significant difference (p=0.864).
 | Table II.Comparison of the clinical and
molecular features between the proximal and distal tubular adenoma
(TA) cases. |
Table II.
Comparison of the clinical and
molecular features between the proximal and distal tubular adenoma
(TA) cases.
| Proximal TA
(n=12) | Distal TA (n=10) | p-value |
|---|
| Patient age (years)
(mean ± SD) | 65.1±8.2 | 58.1±9.8 | 0.068 |
| Gender
(male/female) | 8/4 | 5/5 | 0.666 |
| Size (mm) (mean ±
SD) | 18.5±16.0 | 19.1±17.9 | 0.934 |
| BRAF (V600E) | 0.0% (0/12) | 0.0% (0/10) | - |
| KRAS (codon
12/13) | 36.4% (4/11) | 40.0% (4/10) | 0.864 |
| hMLH1
methylation | 0.0% (0/12) | 0.0% (0/10) | - |
| MSI | 0.0% (0/12) | 0.0% (0/10) | - |
| CIMP | 0.0% (0/12) | 0.0% (0/10) | - |
Proximal colon cancer
Proximal colon cancer was investigated for
comparison to proximal SSA. The proximal colon cancer samples were
divided according to the presence of MSI. In this study, 24 colon
cancer tissues showed MSI and 42 were MSS. The clinical and
molecular features of the MSI and MSS cancers were compared
(Table III). Patient age and
gender were not significantly different between both types of
cancers. Mucinous or poorly differentiated cancer was more
frequently noted in the MSI cancers (p=0.007). The BRAF mutation
was found in 58.3% of the MSI cancers and in 9.5% of the MSS
cancers (p<0.001). In contrast, the KRAS mutation was more
frequently present in the MSS than in the MSI cancers (38.8 and
8.3%, respectively; p=0.01). Epigenetic analysis showed that hMLH1
methylation was frequently present in the MSI cancers (50%), and no
methylation was found in the MSS cancers (p<0.001). Furthermore,
CIMP was frequently found in the MSI cancers (66.7%), whereas it
was relatively rare in the MSS cancers (11.9%) (p<0.001).
 | Table III.Comparison of the clinical and
molecular features between the proximal MSI and MSS cancers. |
Table III.
Comparison of the clinical and
molecular features between the proximal MSI and MSS cancers.
| MSI cancer
(n=24) | MSS cancer
(n=42) | p-value |
|---|
| Patient age (years)
(mean ± SD) | 68±8.8 | 66.9±13.9 | 0.704 |
| Gender
(male/female) | 8/16 | 19/23 | 0.438 |
| Stage (-I/-II) | 5/19 | 8/31 | 0.861 |
| Mucinous/poorly
differentiated | 37.5% (9/24) | 7.7% (3/39) | 0.007 |
| BRAF (V600E) | 58.3% (14/24) | 9.5% (4/42) | <0.001 |
| KRAS (codon
12/13) | 8.3% (2/24) | 38.1% (16/42) | 0.01 |
| hMLH1
methylation | 50.0% (12/24) | 0.0% (0/42) | <0.001 |
| CIMP | 66.7% (16/24) | 11.9% (5/42) | <0.001 |
Next, MSI cancers were divided into two groups based
on the presence or absence of the BRAF mutation (Table IV). Patient age and gender were not
significantly different between the MSI cancers with or without the
BRAF mutation. Although the frequency of hMLH1 methylation was not
statistically different, CIMP was more frequently observed in
BRAF-mutated MSI cancers (85.7%) than in the BRAF wild-type MSI
cancers (40%) (p=0.032). A high frequency of CIMP was found only in
the proximal SSAs and the proximal MSI cancers harboring the BRAF
mutation.
 | Table IV.Comparison of MSI cancers with or
without BRAF mutation. |
Table IV.
Comparison of MSI cancers with or
without BRAF mutation.
| BRAF (+)
(n=14) | BRAF (−)
(n=10) | p-value |
|---|
| Patient age (mean ±
SD) | 62.4±14.6 | 71.9±11.6 | 0.070 |
| Female | 71.4% (10/14) | 60% (6/10) | 0.441 |
| KRAS | 0.0% (0/14) | 20% (2/10) | 0.163 |
| hMLH1 | 57.1% (8/14) | 40% (4/10) | 0.680 |
| CIMP | 85.7% (12/14) | 40% (4/10) | 0.032 |
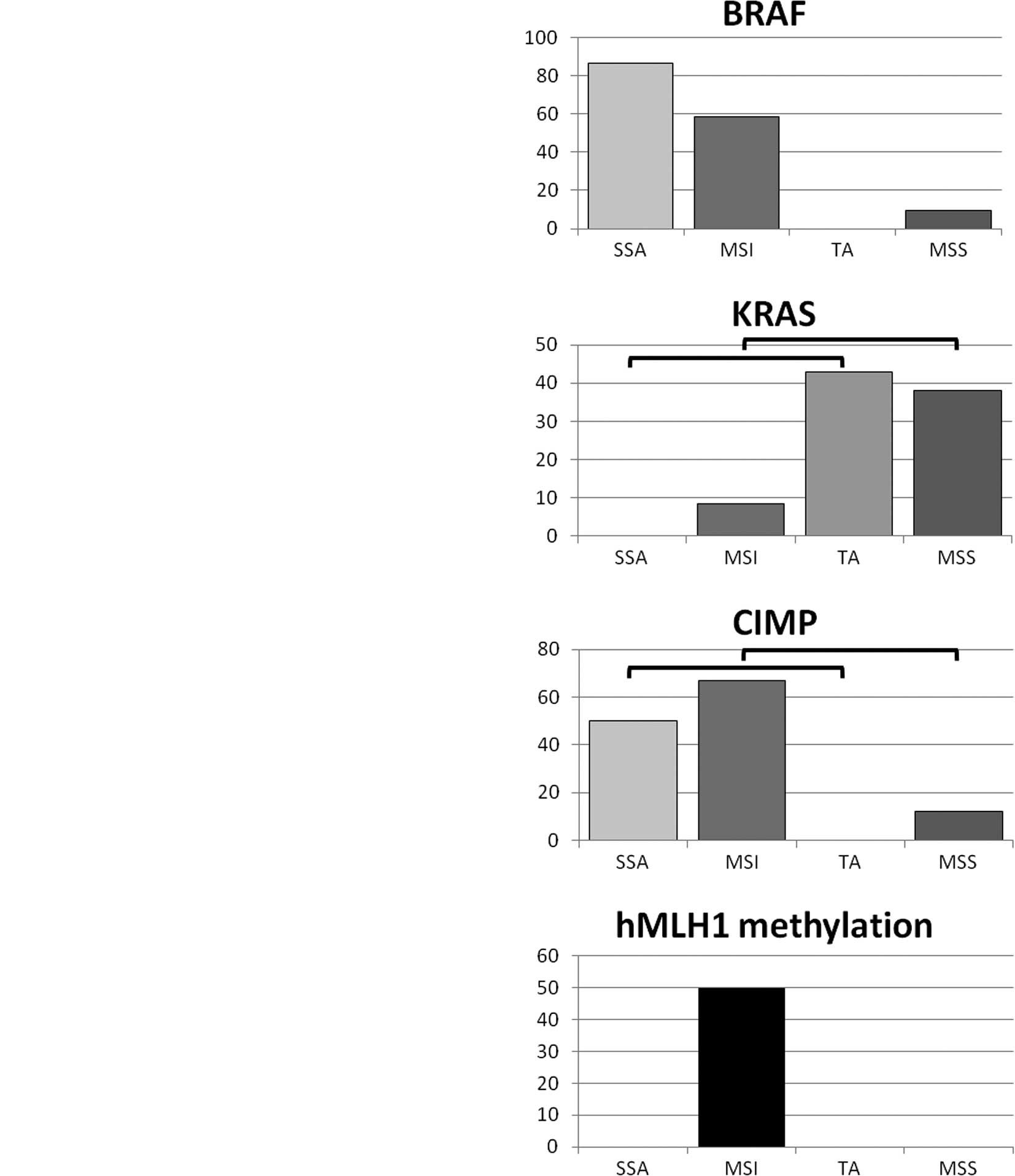
Comparison of genetic and epigenetic
features of the proximal SSAs, TAs, MSI cancers and MSS
cancers
Genetic and epigenetic features and differences were
compared between the proximal SSAs, TAs, MSI cancers and MSS
cancers (Fig. 1). The BRAF
mutation was more frequently found in the SSAs and MSI cancers than
in the TAs and MSS cancers. On the other hand, the KRAS mutation
was more frequently found in the TAs and MSS cancers than in the
SSAs and MSI cancers. CIMP status was similar to that of the BRAF
mutation, with the SSAs and MSI cancers being much more frequently
CIMP-positive than the TAs or MSS cancers. The hMLH1 mutation was
only observed in MSI cancers.
Discussion
In the present study, we demonstrated the distinct
genetic and epigenetic features between proximal and distal SSA.
Regarding genetic features, the BRAF mutation dominantly occurred
in proximal SSAs rather than in distal SSAs. The KRAS mutation was
more likely to be detected in distal than in proximal SSA.
Epigenetic analysis exhibited no hMLH1 methylation in either
proximal or distal SSAs, whereas proximal SSA was likely to possess
CIMP (p=0.012). MSI, which is speculated to be caused by both
genetic and epigenetic alterations, was detected in neither
proximal nor distal SSA. Features, such as harboring the BRAF
mutation and CIMP, characterized proximal SSA. On the other hand,
TA did not show any difference in genetic and epigenetic features
for either location.
Since MSI cancer is dominantly located in the
proximal colon, we compared the features of proximal MSI cancers to
those of proximal MSS cancers. Proximal MSI cancers showed a high
frequency of BRAF mutations, a low frequency of KRAS mutations, and
hMLH1 methylation in 50 and 66.7% were positive for CIMP. On the
other hand, proximal MSS cancers exhibited less frequent BRAF
mutations and more frequent KRAS mutations compared to proximal MSI
cancers (p<0.001 and p=0.01, respectively). Epigenetic
alterations, such as hMLH1 methylation and CIMP, were much less
frequently observed in proximal MSS cancers.
SSA was characterized by a high frequency of BRAF
mutations, and MSI cancers were distinguished by BRAF status.
Therefore, the molecular features of MSI cancers with or without
the BRAF mutation were further examined. Proximal MSI cancers
harbored significantly more CIMP in the presence of the BRAF
mutation than in its absence. High correlation was observed between
positive CIMP and the BRAF mutation in proximal MSI cancers. These
molecular features were also observed in proximal SSAs, but not in
proximal TAs, suggesting that proximal SSA could be a precursor
lesion of proximal MSI cancers harboring the BRAF mutation.
Considering the distinct characteristics of MSI cancers of the
proximal and distal colon (16,17),
MSI cancers probably share their molecular features with SSA in a
location-dependent manner. By contrast, MSS cancers shared their
molecular features with TAs in a location-independent manner.
Several reports have indicated that SSAs are
microsatellite stable (9,13,25),
and our data showed the same result. Vaughn et al reported
that hMLH1 methylation in proximal hyperplastic polyps is rare and
most MSI cancers show methylation (26). Similarly, in our study, proximal
SSAs did not show any hMLH1 methylation, whereas a considerable
amount of MSI cancers exhibited hMLH1 methylation. Similar CIMP
frequency was observed in proximal SSAs and proximal MSI cancers.
These results suggest the possibility that DNA methylation
initially occurs in a limited range of the genome sparing critical
regions for cancer progression in proximal SSAs. Then the promoter
region of the DNA mismatch repair gene can be methylated and
silenced, resulting in MSI. This hypothesis is supported by
previous reports which demonstrated that limited methylation in the
hMLH1 promoter region did not inhibit hMLH1 protein expression
(27,28).
Notably, a considerable number of proximal MSI
cancers did not show the BRAF mutation; however, a small percentage
of proximal MSS cancers did show the BRAF mutation. There are
several reports with similar results for the frequency of the BRAF
mutation in MSI and MSS cancers (29–31).
Lubomierski et al (31)
suggested that there were two genetically distinct entities of MSI
cancer with and without the BRAF mutation, which is consistent with
our data. We observed that 8.3% (2/24) of MSI cancers showed the
KRAS mutation, one of which was CIMP-positive and another one
exhibited hMLH1 methylation (data not shown). On the other hand,
9.5% (4/42) of MSS cancers exhibited the BRAF mutation. Considering
the extremely high correlation between SSA and the BRAF mutation,
we hypothesized that MSS cancers with the BRAF mutation could be
derived from SSA followed by intricate genetic/epigenetic
alterations in the adenoma-carcinoma pathway.
Another interesting finding was that more than 40%
of MSI cancers did not show the BRAF mutations and were negative
for CIMP, a characteristic of SSA. We found that 8.3% of MSI
cancers showed the KRAS mutation, which was frequently found in
TAs. From these results, we postulate that a certain number of MSI
cancers emerge from TA. A minor population of proximal TAs may
acquire MSI, which results in MSI cancers harboring KRAS and not
the BRAF mutation. This putative mutual relationship for
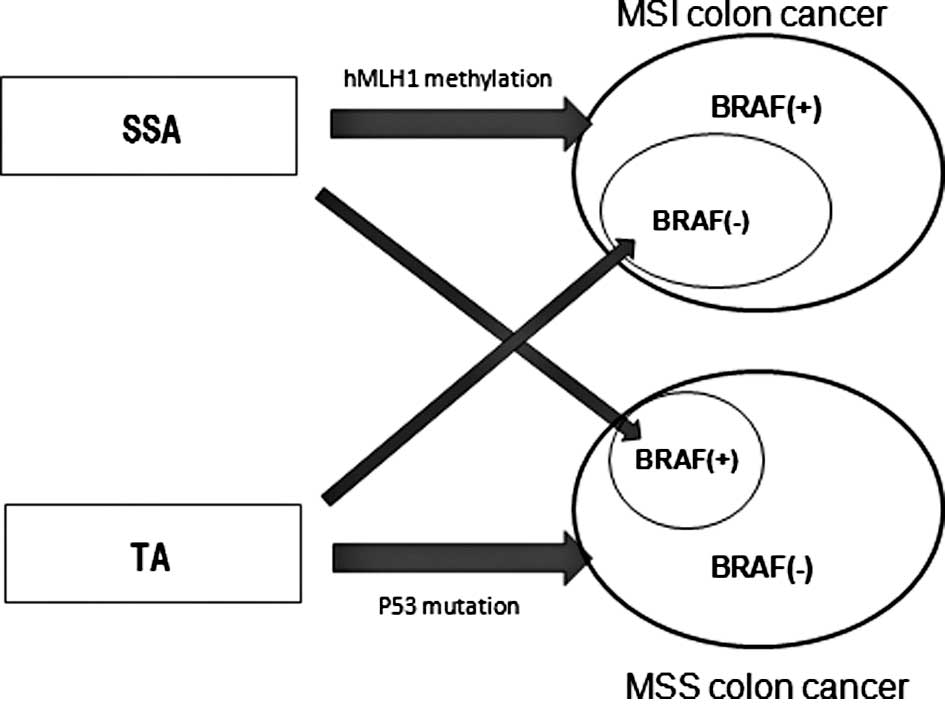
carcinogenesis of proximal colon cancer is shown in Fig. 2. We believe this concept is
consistent with the emerging fusion pathway proposed by Jass et
al (32) in which molecular
alterations that are characteristic of the serrated and
adenoma-carcinoma sequence co-occur in a minority of advanced
colorectal polyps.
In conclusion, similar genetic and epigenetic
features between proximal SSAs and MSI cancers with the BRAF
mutation suggest the strong possibility of proximal SSA as a
precursor lesion for proximal MSI cancers with the BRAF mutation.
The genetic and epigenetic features of proximal SSA and TA suggest
the possibility that both polyps develop into either proximal MSI
or MSS cancers.
Furthermore, the genetic and epigenetic features of
proximal SSAs and TAs suggest that in a small percentage of cases,
the former develop into proximal MSS cancers and the latter into
proximal MSI cancers.
References
|
1.
|
Longacre TA and Fenoglio-Preiser CM: Mixed
hyperplastic adenomatous polyps/serrated adenomas. A distinct form
of colorectal neoplasia. Am J Surg Pathol. 14:524–537. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Torlakovic E, Skovlund E, Snover DC,
Torlakovic G and Nesland JM: Morphologic reappraisal of serrated
colorectal polyps. Am J Surg Pathol. 27:65–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Torlakovic EE, Gomez JD, Driman DK, et al:
Sessile serrated adenoma (SSA) vs. traditional serrated adenoma
(TSA). Am J Surg Pathol. 32:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Torlakovic E and Snover DC: Serrated
adenomatous polyposis in humans. Gastroenterology. 110:748–755.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee SK, Chang HJ, Kim TI, et al:
Clinicopathologic findings of colorectal traditional and sessile
serrated adenomas in Korea: a multicenter study. Digestion.
77:178–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Leggett B and Whitehall V: Role of the
serrated pathway in colorectal cancer pathogenesis.
Gastroenterology. 138:2088–2100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Higuchi T and Jass JR: My approach to
serrated polyps of the colorectum. J Clin Pathol. 57:682–686. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yang AS, Estecio MR, Doshi K, Kondo Y,
Tajara EH and Issa JP: A simple method for estimating global DNA
methylation using bisulfite PCR of repetitive DNA elements. Nucleic
Acids Res. 32:e382004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sandmeier D, Benhattar J, Martin P and
Bouzourene H: Serrated polyps of the large intestine: a molecular
study comparing sessile serrated adenomas and hyperplastic polyps.
Histopathology. 55:206–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Carr NJ, Mahajan H, Tan KL, Hawkins NJ and
Ward RL: Serrated and non-serrated polyps of the colorectum: their
prevalence in an unselected case series and correlation of BRAF
mutation analysis with the diagnosis of sessile serrated adenoma. J
Clin Pathol. 62:516–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kambara T, Simms LA, Whitehall VL, et al:
BRAF mutation is associated with DNA methylation in serrated polyps
and cancers of the colorectum. Gut. 53:1137–1144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lash RH, Genta RM and Schuler CM: Sessile
serrated adenomas: prevalence of dysplasia and carcinoma in 2139
patients. J Clin Pathol. 63:681–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
O’Brien MJ, Yang S, Mack C, et al:
Comparison of microsatellite instability, CpG island methylation
phenotype, BRAF and KRAS status in serrated polyps and traditional
adenomas indicates separate pathways to distinct colorectal
carcinoma end points. Am J Surg Pathol. 30:1491–1501. 2006.
|
|
14.
|
Spring KJ, Zhao ZZ, Karamatic R, et al:
High prevalence of sessile serrated adenomas with BRAF mutations: a
prospective study of patients undergoing colonoscopy.
Gastroenterology. 131:1400–1407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Farina-Sarasqueta A, van Lijnschoten G,
Moerland E, et al: The BRAF V600E mutation is an independent
prognostic factor for survival in stage II and stage III colon
cancer patients. Ann Oncol. 21:2396–2402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Watanabe T, Kobunai T, Toda E, et al:
Distal colorectal cancers with microsatellite instability (MSI)
display distinct gene expression profiles that are different from
proximal MSI cancers. Cancer Res. 66:9804–9808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Cho YK, Kim HC, Kim SH, et al:
Location-related differences in sporadic microsatellite unstable
colorectal cancer. Dig Liver Dis. 42:611–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chan TL, Zhao W, Leung SY and Yuen ST:
BRAF and KRAS mutations in colorectal hyperplastic polyps and
serrated adenomas. Cancer Res. 63:4878–4881. 2003.PubMed/NCBI
|
|
20.
|
Takemoto N, Konishi F, Yamashita K, et al:
The correlation of microsatellite instability and
tumor-infiltrating lymphocytes in hereditary non-polyposis
colorectal cancer (HNPCC) and sporadic colorectal cancers: the
significance of different types of lymphocyte infiltration. Jpn J
Clin Oncol. 34:90–98. 2004. View Article : Google Scholar
|
|
21.
|
Xiong Z and Laird PW: COBRA: a sensitive
and quantitative DNA methylation assay. Nucleic Acids Res.
25:2532–2534. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Toyota M, Ho C, Ahuja N, et al:
Identification of differentially methylated sequences in colorectal
cancer by methylated CpG island amplification. Cancer Res.
59:2307–2312. 1999.PubMed/NCBI
|
|
23.
|
Toyota M, Ahuja N, Ohe-Toyota M, Herman
JG, Baylin SB and Issa JP: CpG island methylator phenotype in
colorectal cancer. Proc Natl Acad Sci USA. 96:8681–8686. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kusano M, Toyota M, Suzuki H, et al:
Genetic, epigenetic, and clinicopathologic features of gastric
carcinomas with the CpG island methylator phenotype and an
association with Epstein-Barr virus. Cancer. 106:1467–1479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kim YH, Kakar S, Cun L, Deng G and Kim YS:
Distinct CpG island methylation profiles and BRAF mutation status
in serrated and adenomatous colorectal polyps. Int J Cancer.
123:2587–2593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Vaughn CP, Wilson AR and Samowitz WS:
Quantitative evaluation of CpG island methylation in hyperplastic
polyps. Mod Pathol. 23:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Miyakura Y, Sugano K, Konishi F, et al:
Extensive methylation of hMLH1 promoter region predominates in
proximal colon cancer with microsatellite instability.
Gastroenterology. 121:1300–1309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Deng G, Chen A, Hong J, Chae HS and Kim
YS: Methylation of CpG in a small region of the hMLH1 promoter
invariably correlates with the absence of gene expression. Cancer
Res. 59:2029–2033. 1999.PubMed/NCBI
|
|
29.
|
Ogino S, Nosho K, Kirkner GJ, et al: CpG
island methylator phenotype, microsatellite instability, BRAF
mutation and clinical outcome in colon cancer. Gut. 58:90–96. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zlobec I, Kovac M, Erzberger P, et al:
Combined analysis of specific KRAS mutation, BRAF and
microsatellite instability identifies prognostic subgroups of
sporadic and hereditary colorectal cancer. Int J Cancer.
127:2569–2575. 2010. View Article : Google Scholar
|
|
31.
|
Lubomierski N, Plotz G, Wormek M, et al:
BRAF mutations in colorectal carcinoma suggest two entities of
microsatellite-unstable tumors. Cancer. 104:952–961. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Jass JR, Baker K, Zlobec I, et al:
Advanced colorectal polyps with the molecular and morphological
features of serrated polyps and adenomas: concept of a ‘fusion’
pathway to colorectal cancer. Histopathology. 49:121–131.
2006.PubMed/NCBI
|