Introduction
Hepatitis C virus (HCV) is the leading cause of
chronic liver disease. It accounts for 70% of all chronic hepatitis
cases, 40% of all cases of liver cirrhosis and 60% of
hepatocellular carcinomas (HCC) (1,2).
Since the successful eradication of HCV is associated with a
reduced risk of liver cirrhosis and HCC (3,4),
antiviral therapy plays a crucial role in the management of
HCV-infected patients. Currently, pegylated interferon (PEG-IFN)
plus ribavirin (RBV) is considered to be the most effective
standard of care for chronic hepatitis C (5–7), but
the rate of sustained virological response (SVR; HCV RNA-negative
for 24 weeks after the cessation of therapy) is approximately 50%
in patients with HCV genotype 1, the most common genotype in many
countries (5,6). Furthermore, 20–30% of HCV genotype 1
patients are non-responders to PEG-IFN/RBV therapy (3).
Prediction of the efficacy of PEG-IFN/RBV therapy is
important for determining its applications, since PEG-IFN/RBV
carries potential serious side effects and is costly when used over
a long period of time to achieve SVR. The outcome of interferon
therapy has been reported to be associated with both viral and host
factors. Representative viral factors are the HCV genotype, high
HCV RNA titers (8), amino acid
substitutions in the NS5A (9) and
core region (10). The host
factors include age, body mass index, insulin resistance and stage
of liver fibrosis (4,11). Furthermore, it has recently been
reported that a single nucleotide polymorphism (SNP) of the host
gene interleukin (IL)-28B is significantly associated with the
response to PEG-IFN/RBV therapy (12,13).
A genome-wide association study of the genetic determinants of a
therapeutic response in HCV-1 patients treated with PEG-IFN/RBV
identified a SNP upstream of the IL-28B gene on chromosome 19,
which was associated with an approximately 2-fold difference in SVR
rates in patients of European, African-American or Hispanic
ancestry (13). Non-responders
were required to have been more than 80% compliant to both
PEG-IFN/RBV dosing, and the ethnicity was defined by their genetic
ancestry (13).
This study investigated the relationship between the
early response of HCV to PEG-IFN/RBV therapy and the IL-28B genetic
polymorphism in Japanese patients with HCV infection.
Patients and methods
Study populations
A total of 144 patients with chronic HCV infection
(HCV serotype 1, n=105 and serotype 2, n=39), who were treated at
the Hospital of the Shiga University of Medical Science, the
Notogawa Hospital and the Social Insurance Shiga Hospital, were
included in the study. Table I
lists the demographic features of the subjects. Among these
patients, 59 were treated with PEG-IFN/RBV therapy. The patients
received weekly injections of PEG-IFN at 1.5 μg/kg body weight, and
the oral administration of ribavirin for 48 weeks. The amount of
ribavirin was adjusted based on the patient’s body weight (600 mg
for <60 kg, 800 mg for 60–80 kg and 1,000 mg for >80 kg).
Only patients with >75% compliance with the prescribed doses of
PEG-IFN/RBV were included in this study. Informed consent was
obtained from all patients. The ethics committee of each
participating medical center approved this study.
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
| Gender
(male/female) | 88/67 | |
| Age (years) | 59.8±11.2 | (28–92) |
| ALT (IU/l) | 83.3±115.4 | (17–1,199) |
| γ-GTP (IU/l) | 56.6±68.8 | (7.8–638) |
| Platelet
(106/μl) | 15.6±5.40 | (3.5–9.0) |
| HCV-RNA level
(log10/ml) | 6.1±0.64 | (3.5–9.0) |
| HCV serotype 1/2 | 105/39 | |
Serotyping
Serotyping was performed by an enzyme
immunoassay-based Murex assay (Murex Diagnostics Inc., Norcross,
GA, USA).
Genotyping
The samples were genotyped for IL-28B
rs8099917 using the TaqMan® SNP Assay (Applied
Biosystems Inc., Foster City, CA, USA) as previously described
(14). Homozygosity (GG) or
heterozygosity (TG) of the minor sequence was defined as having the
IL-28B minor allele, whereas homozygosity for the major sequence
(TT) was defined as having the IL-28B major allele. The HCV RNA
levels were analyzed using the TaqMan RT-PCR test. The measurement
ranges of these assays were 1.2–7.8 log IU.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) analysis was
performed in these subjects by comparing the detected distribution
of allele frequencies with the theoretical distribution estimated
from the SNP allelic frequencies. P>0.05 (Chi-square statistics)
was considered to indicate equilibrium. The categorical variables
were presented as frequencies and percentages when required. The
continuous variables were reported as the means ± SD (range). The
viral kinetics were assessed using the Chi-square test.
Multivariate logistic regression analysis with stepwise forward
selection was performed with a criterion of P<0.05 for the
inclusion or removal of variables. All statistical analyses used
the Ekuseru-toukei 2008 (Social Survey Research Information Co.,
Ltd., Tokyo, Japan) software with P<0.05 considered to be
statistically significant.
Results
The genotype frequencies of the IL-28B polymorphisms
in patients with HCV serotypes 1 and 2 are shown in Table II. The frequency of the IL-28B
major allele, defined as homozygosity for the major sequence (TT),
in HCV serotype 1 patients (75.2%) was lower than that in serotype
2 patients (84.6%) (not significant). The frequency of the
rs8099917 T allele was 87.1% in patients with serotype 1, and 92.3%
in patients with serotype 2. These data corroborate those of a
recent report of the Japanese population described by Ochi et
al (5).
 | Table II.Genotype distribution of IL-28B SNP
rs8099917. |
Table II.
Genotype distribution of IL-28B SNP
rs8099917.
| rs8099917
|
|---|
| TT | TG | GG | T (%) | G (%) |
|---|
| HCV serotype 1
(n) | 79 | 25 | 1 | | |
| (75.2%) | (23.8%) | (0.95%) | 87.1 | 12.9 |
| HCV serotype 2
(n) | 33 | 6 | 0 | | |
| (84.6%) | (15.4%) | (0.0%) | 92.3 | 7.7 |
| Total (n) | 112 | 31 | 1 | | |
| (77.8%) | (21.5%) | (0.69%) | 89.7 | 10.3 |
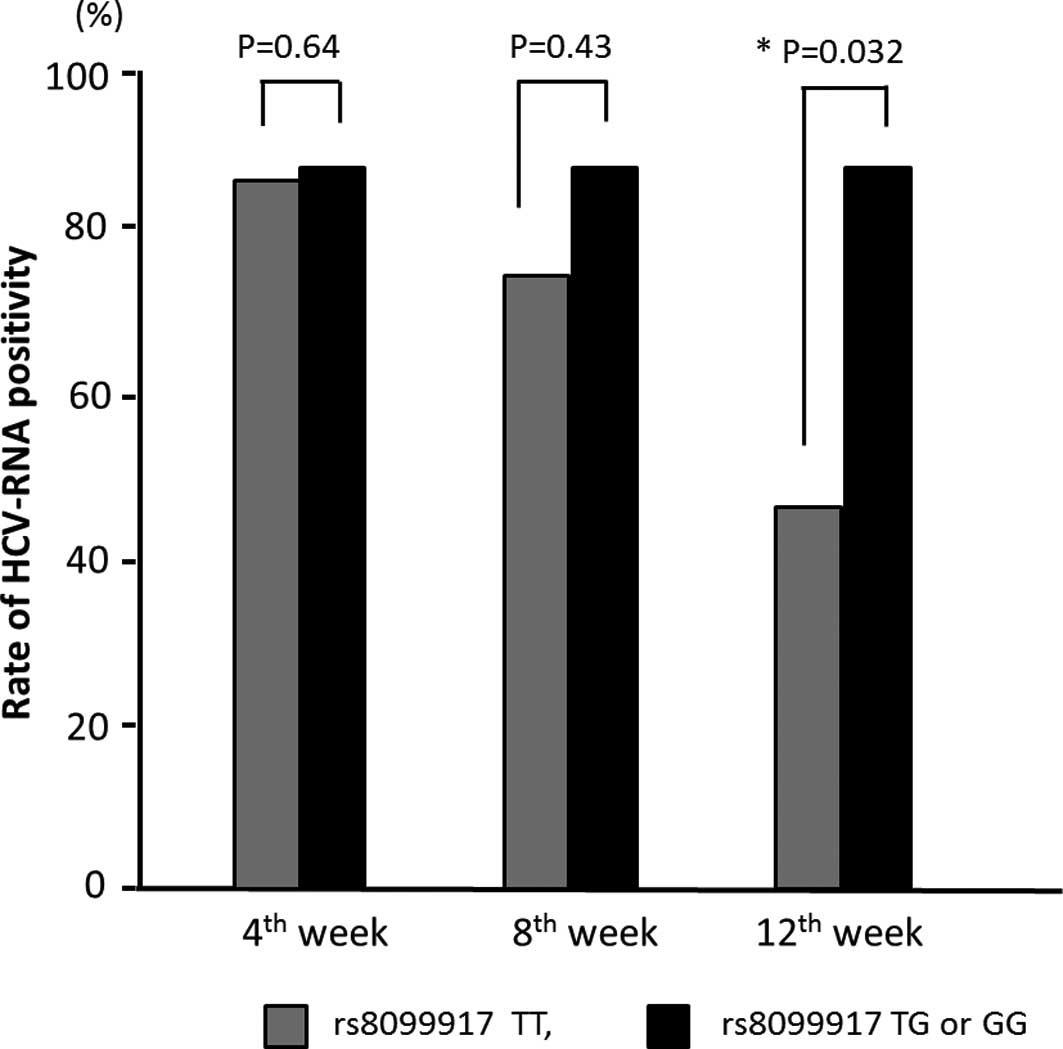
The patients with HCV genotype 1 were stratified
according to their IL-28B allele type, and the early responses of
HCV-RNA at 4, 8 and 12 weeks after the start of PEG-IFN/RBV therapy
were analyzed (Fig. 1). The rate
of HCV-RNA positivity was significantly lower at 12 weeks in
patients with the IL-28B major allele than in patients with the
IL-28B minor allele. This finding suggests that IL-28B has a great
impact on the early virological response to therapy. Using
multivariate analysis, the major allele of IL-28B (P=0.014) and the
number of platelets (P=0.030) were associated with negative HCV-RNA
at 12 weeks (Table III). No
significant difference was detected between the effects of
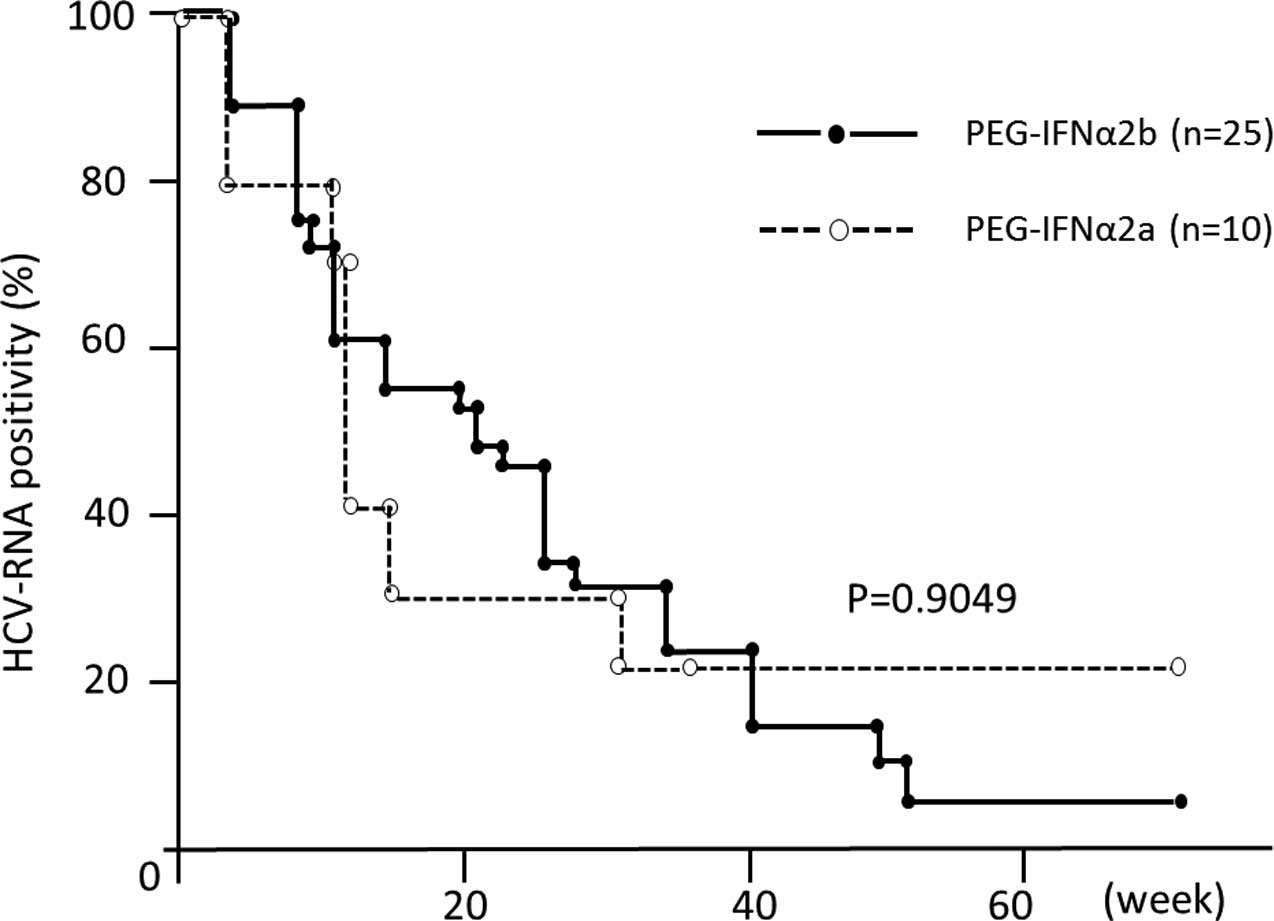
PEG-IFNα-2a and 2b (Fig. 2).
 | Table III.Multivariate analyses of factors
associated with HCV-RNA-negativity at week 12. |
Table III.
Multivariate analyses of factors
associated with HCV-RNA-negativity at week 12.
| Odds ratio | 95% CI | P-value |
|---|
| rs8099917 TT | 66.2 | 2.320-999.9 | 0.014a |
| Gender | 0.36 | 0.043–3.21 | 0.220 |
| Age | 1.07 | 0.990–1.18 | 0.220 |
| BMI | 0.72 | 0.430–1.21 | 0.210 |
| γ-GTP | 1.00 | 0.970–1.03 | 0.910 |
| Platelets | 1.33 | 1.030–2.12 | 0.030a |
Discussion
Twenty to thirty percent of patients infected with
HCV genotype 1 are non-responders to PEG-IFN/RBV therapy.
Furthermore, PEG-IFN/RBV therapy may be associated with
considerable toxicity. Therefore, a pre-treatment prediction of the
patient response to therapy is clinically valuable. However, there
have been no reliable baseline predictors for the response to
anti-viral therapy. This study demonstrated that the IL-28B
polymorphism was an overwhelming predictor of an early viral
response to PEG-IFN/RBV therapy. IL-28B encodes a protein known as
IFNλ 3, which is thought to suppress the replication of various
viruses, including HCV (15). The
results of the present study may provide a rationale for developing
diagnostic testing and a therapeutic approach for targeting IL-28B
in chronic hepatitis C in the future.
A polymorphism upstream of the IL-28B gene has been
reported to be strongly associated with a favorable response in
HCV-1 patients (13). In this
study, we confirmed the clinical relevance of this genetic
discovery. The polymorphism was significantly associated with early
viral clearance at week 12. The key marker for an improved
treatment response was the IL-28B major allele (TT type). The major
allele type was associated with improved early viral suppression,
such that by week 12, the reduction in viral positivity was doubled
in patients with the TT allele as compared to the non-TT IL-28B
type HCV-1 patients. Genotyping of IL-28B prior to PEG-IFN/RBV
therapy will aid clinicians and patients in PEG-IFN/RBV therapy
management. Those patients who have the major allele (TT type)
IL-28B have a greater likelihood of achieving early viral
reduction, and should be considered ideal candidates. By contrast,
patients with the minor allele (TG or GG type) are unlikely to
achieve an early response to therapy, and need excellent compliance
to the doses of PEG-IFN/RBV.
It has been reported that 20% of individuals
infected with HCV are obese, and that the obesity in these
individuals is associated with steatosis and the progression of
fibrosis (4,11,16).
These factors are shown to be associated with a non-response to
treatment with PEG-IFN/RBV (16).
However, we did not identify the BMI as one of the significant
factors for a poor early viral response. On the other hand, we
detected a significant association of platelet number. Since
platelet number decreases in accordance with the progression of
liver fibrosis (11), this
suggests that PEG-IFN/RBV is more effective for patients with
better histological changes of the liver.
Recent direct comparative trials, retrospective and
meta-analysis studies have demonstrated that treatment with
PEG-IFNα-2a is a significant and independent contributor to SVR in
patients infected with genotype 1 (17), as compared to treatment with
PEG-IFNα-2b, although the largest head-to-head trial (IDEAL study)
failed to detect a significant difference in SVR rates between
these two peg-IFNα formulations (18). Our observations also demonstrated
that there are no differences in the early viral response between
these two PEG-IFNα subtypes.
In conclusion, an IL-28B genotype is the strongest
baseline predictor of an early viral response to PEG-IFN/RBV
therapy in HCV-1 patients. It is likely that IL-28B SNP genotyping
may become part of the clinical assessment before standard
antiviral therapy in individuals chronically infected with
HCV-1.
Acknowledgements
This study was supported, in part, by
MSD K.K. (Tokyo, Japan).
References
|
1.
|
Alavian SM, Tabatabaei SV, Keshvari M, et
al: Peginterferon alpha-2a and ribavirin treatment of patients with
haemophilia and hepatitis C virus infection: a single-centre study
of 367 cases. Liver Int. 30:1173–1180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Welzel TM, Morgan TR, Bonkovsky HL, et al:
Variants in interferon-alpha pathway genes and response to
pegylated interferon-alpha2a plus ribavirin for treatment of
chronic hepatitis C virus infection in the hepatitis C antiviral
long-term treatment against cirrhosis trial. Hepatology.
49:1847–1858. 2009. View Article : Google Scholar
|
|
3.
|
Kurosaki M, Tanaka Y, Nishida N, et al:
Pre-treatment prediction of response to pegylated-interferon plus
ribavirin for chronic hepatitis C using genetic polymorphism in
IL28B and viral factors. J Hepatol. 54:439–448. 2011. View Article : Google Scholar
|
|
4.
|
Yasui K, Harano Y, Mitsuyoshi H, et al:
Steatosis and hepatic expression of genes regulating lipid
metabolism in Japanese patients infected with hepatitis C virus. J
Gastroenterol. 45:95–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ochi H, Maekawa T, Abe H, et al: IL-28B
predicts response to chronic hepatitis C therapy-fine-mapping and
replication study in Asian populations. J Gen Virol. Jan. 12–2011,
(E-pub ahead of print).
|
|
6.
|
Hadziyannis SJ and Koskinas JS:
Differences in epidemiology, liver disease and treatment response
among HCV genotypes. Hepatol Res. 29:129–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hadziyannis SJ, Sette H Jr, Morgan TR, et
al: Peginterferon-alpha2a and ribavirin combination therapy in
chronic hepatitis C: a randomized study of treatment duration and
ribavirin dose. Ann Intern Med. 140:346–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Iino S, Ichida F, Sakuma A and Suzuki H: A
randomized clinical trial with natural interferon-alpha monotherapy
for 24 or 48 weeks in patients with chronic hepatitis C having
genotype 1b infection in high viral titers. Hepatol Res.
24:338–345. 2002. View Article : Google Scholar
|
|
9.
|
Enomoto N, Sakuma I, Asahina Y, et al:
Mutations in the nonstructural protein 5A gene and response to
interferon in patients with chronic hepatitis C virus 1b infection.
N Engl J Med. 334:77–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Akuta N, Suzuki F, Kawamura Y, et al:
Predictors of viral kinetics to peginterferon plus ribavirin
combination therapy in Japanese patients infected with hepatitis C
virus genotype 1b. J Med Virol. 79:1686–1695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Izumi N, Asahina Y and Kurosaki M:
Predictors of virological response to a combination therapy with
pegylated interferon plus ribavirin including virus and host
factors. Hepat Res Treat. 2010:7036022010.PubMed/NCBI
|
|
12.
|
Tanaka Y, Nishida N, Sugiyama M, et al:
Genome-wide association of IL28B with response to pegylated
interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat
Genet. 41:1105–1109. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ge D, Fellay J, Thompson AJ, et al:
Genetic variation in IL28B predicts hepatitis C treatment-induced
viral clearance. Nature. 461:399–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Livak KJ: Allelic discrimination using
fluorogenic probes and the 5′ nuclease assay. Genet Anal.
14:143–149. 1999.
|
|
15.
|
Marcello T, Grakoui A, Barba-Spaeth G, et
al: Interferons alpha and lambda inhibit hepatitis C virus
replication with distinct signal transduction and gene regulation
kinetics. Gastroenterology. 131:1887–1898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Charlton MR, Pockros PJ and Harrison SA:
Impact of obesity on treatment of chronic hepatitis C. Hepatology.
43:1177–1186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tsubota A, Fujise K, Namiki Y and Tada N:
Peginterferon and ribavirin treatment for hepatitis C virus
infection. World J Gastroenterol. 17:419–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
McHutchison JG, Lawitz EJ, Shiffman ML, et
al: Peginterferon alpha-2b or alpha-2a with ribavirin for treatment
of hepatitis C infection. N Engl J Med. 361:580–593. 2009.
View Article : Google Scholar : PubMed/NCBI
|