Introduction
Angiogenesis plays a pivotal role in tumorigenesis
and metastasis (1). Tumor
angiogenesis is a complex process and is based on the concept that
a tumor requires a vascular blood supply to grow beyond 1 or 2 mm
(2,3). Tumors that do not establish a
neovascular supply may remain dormant for a long time (4). Neovascularization has been thought to
result exclusively through proliferation, migration and remodeling
of fully differentiated endothelial cells derived from pre-existing
blood vessels. In addition, vascular endothelial growth factor
(VEGF) has been found to induce mobilization of bone marrow-derived
endothelial progenitor cells resulting in increased numbers of
differentiated endothelial progenitor cells and augmented
neovascularization (5,6).
Conventional cytotoxic chemotherapeutic drugs are
sensitive to endothelial cells in addition to directly sacrificing
or inhibiting the proliferation of rapidly dividing tumor cells
(7). However, conventional
chemotherapy, which is administered at the more toxic
maximum-tolerated dosage (MTD), requires 2- to 3-week rest periods
between successive cycles of therapy. The anti-angiogenic efficacy
of chemotherapy appears to be optimized by administering
comparatively low dosages of the drug on a frequent (daily, several
times a week or weekly) or continuous schedule, with no extended
interruptions. This concept is sometimes referred to as
‘metronomic’ chemotherapy (8). In
such a situation, mature circulating endothelial cells (CECs) and
endothelial progenitor cells (CEPs) have been used as a potentially
useful surrogate marker for anti-angiogenic activity (9).
Recently, various drugs have been shown to have
significant anti-angiogenic activity when administered at a low
dosage using a metronomic schedule (10,11).
Irinotecan (CPT-11), which has resulted in improved prognosis of
patients with metastatic colorectal cancer (12,13),
is always administered using a therapeutic MTD approach; thus, the
antitumor and anti-angiogenic efficacy of metronomic CPT-11
administration is unknown.
Humanized monoclonal antibody bevacizumab against
VEGF demonstrated an antitumor effect through its administration
combined with chemotherapy using CPT-11 and 5-FU/LV in a phase III
trial for advanced colorectal cancer (14,15).
Angiogenesis inhibitors also have effects on CECs and CEPs, and
these changes have emerged as a potentially useful surrogate marker
(16). However, the serial change
in the number of CECs/CEPs in chemotherapy, in particular in
metronomic chemotherapy is still unknown. In the present study, we
investigated the serial change of CECs/CEPs, and the relationship
between antitumor efficacy and CECs/CEPs, in metronomic
chemotherapy using CPT-11 combined with or without bevacizumab for
colon cancer.
Materials and methods
Drugs
Bevacizumab was a kind gift from Genentech (South
San Francisco, CA). CPT-11 was a gift from Yakult Honsha (Tokyo,
Japan). CPT-11 solution was freshly prepared in 0.9% saline at a
concentration of 1 mg/ml.
Cell culture
The human colon carcinoma cell line KM12SM, which
produces a high level of VEGF in monolayer culture (supernatant:
2822 pg/ml/106/48 h, unpublished data), was kindly
provided by Dr M. Nakajima (Johnson & Johnson KK, Tokyo,
Japan). The tumor cells were harvested from subconfluent cultures
by a 5-min treatment with trypsin-EDTA (Invitrogen, Tokyo, Japan).
The dislodged cells were first washed in RPMI-1640 (Invitrogen)
supplemented with 10% fetal bovine serum and re-suspended in
phosphate-buffered saline (PBS) for injection. Only single cells in
suspension with >90% viability were used for the injections.
Animals
Male BALB/c/nu/nu mice, aged 4 weeks, were purchased
from Clea Japan, Inc. (Tokyo, Japan). The mice were maintained in a
laminar-flow cabinet under specific pathogen-free conditions and
were used for experiments at the age of 5 weeks. The mice were
maintained in facilities according to the regulations and standards
of the Kurume University School of Medicine.
Tumor xenografts and assessment of
antitumor effects
A total of 1×106 KM12SM cells/PBS was
transplanted into the subcutis of the dorsal skin in each nude
mouse. The maximum tumor diameter was set at 5 mm and then CPT-11
combined with or without bevacizumab was administered
intraperitoneally at a dosage of 10–40 mg/kg of CPT-11 [up to
one-fourth and one-sixteenth the dosage of the LD50 of
177.5 mg/kg (17)] and 5 mg/kg of
bevacizumab for 28 days. After confirming that the implanted tumor
had grown 5 mm in size, mice were divided into 4 groups. Group A
received 40 mg/kg of CPT-11 every two weeks (Conv-40), and group B
received 10 mg/kg of CPT-11 twice weekly (Metro-10). Group C
received 10 mg/kg of CPT-11 twice weekly combined with 5 mg/kg of
bevacizumab twice weekly (Metro-10 + Beva). The control group
received 0.2 ml of PBS every week (Table I). We calculated the body weight of
each mouse from day 0 to 29, and these data were used as an
indicator of side effects. The tumor size was measured twice weekly
using calipers, and the tumor volume was calculated by the formula:
[(Maximum tumor diameter)2 × Minimum tumor diameter/2].
We then resected the tumors 29 days after the start of the drug
administration, and the tumors were fixed with 10% formalin for
histological examination.
 | Table I.Administration schedules of CPT-11 and
bevacizumab. |
Table I.
Administration schedules of CPT-11 and
bevacizumab.
| Treatment groups | Dose of CPT-11
(mg/kg) | Dose of bevacizumab
(mg/kg) | Application | Total dose of CPT-11
over 4 weeks (mg/kg) |
|---|
| Group A
(Conv-40) | 40 | | Day 1, 15 i.p. | 80 |
| Group B
(Metro-10) | 10 | | Twice weekly
i.p. | 80 |
| Group C (Metro-10 +
Beva) | 10 | 5 | Twice weekly
i.p. | 80 |
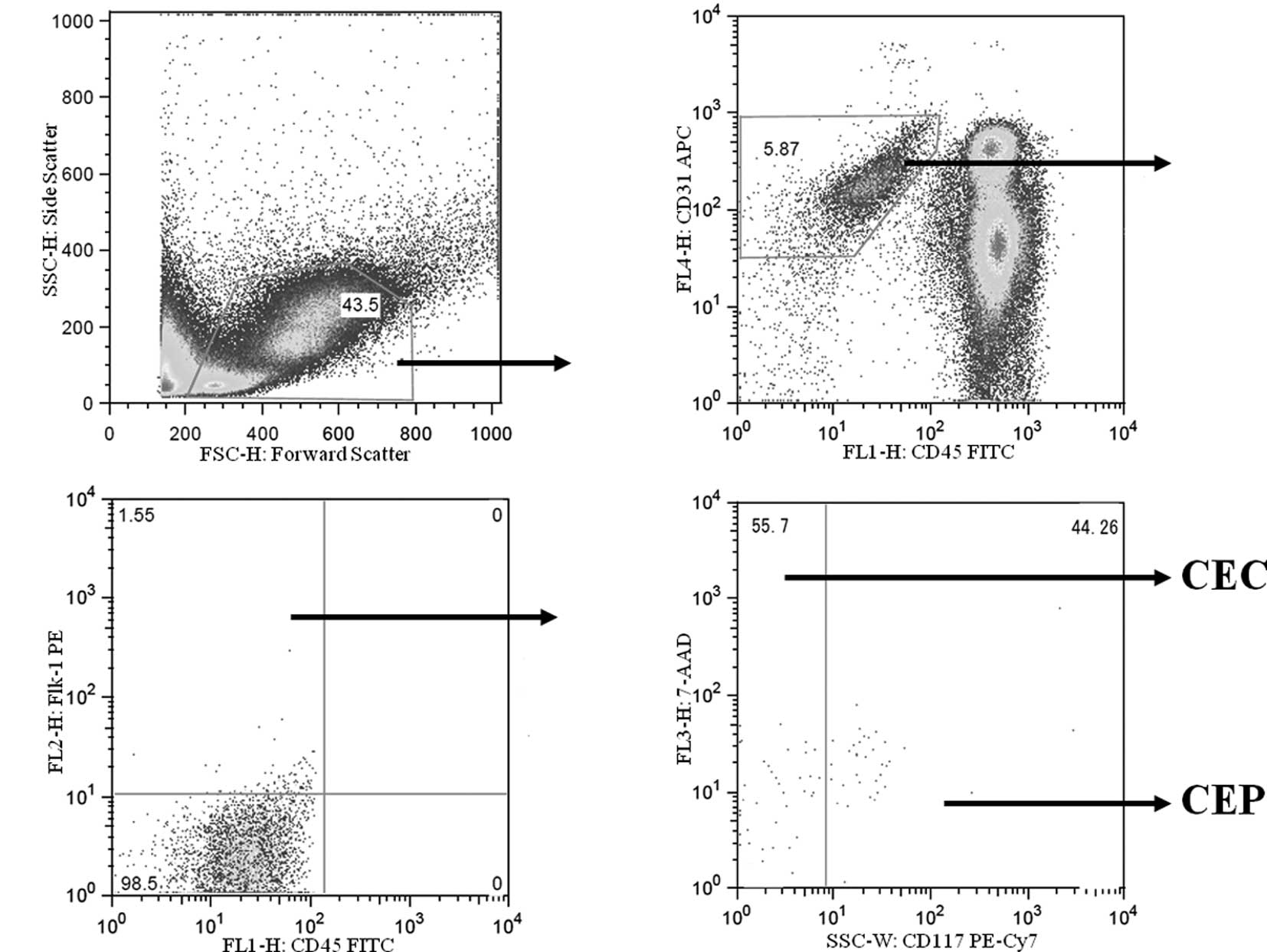
Measurement of CECs and CEPs by flow
cytometry
Mice were euthanized with diethyl ether on days 0,
4, 8 and 15, in each group, and heparinized blood was obtained from
the heart for CEC and CEP evaluation. CECs and CEPs were counted
using a FACSVantage SE flow cytometer (BD Biosciences, San Jose,
CA), and the acquired data were analyzed with FlowJo version 6.3.2
flow cytometry analysis software (Tree Star, Inc., Ashland, OR).
Heparinized whole blood was hemolyzed and stained with anti-mouse
CD45 monoclonal antibody, anti-mouse Flk-1 antibody, anti-mouse
CD31 monoclonal antibody and anti-mouse CD117 monoclonal antibody
(all from BD Bioscience, San Diego, CA). After red cell lysis, cell
suspensions were evaluated by a FACSVantage SE using analysis gates
designed to exclude dead cells, platelets and debris.
CD45+ cells were excluded by gating, and then
CD31+ and Flk-1+ cells were separated from
the CD45 cells. Among these cells, CD117− cells were
regarded as CECs, and CD117+ cells were regarded as CEPs
(Fig. 1). After acquisition of at
least 100,000 cells/sample, analyses were considered as informative
when adequate numbers of events (i.e., >50, typically 100–200)
were collected in the CEC and CEP enumeration gates (18,19).
Immunohistochemistry for microvessels and
assessment of microvessel density
The dorsal subcutaneous tumor was fixed by formalin
and embedded into paraffin. Serial sections 3 μm were cut from each
block. One section was stained using hematoxylin and eosin
(H&E), and a second was immunostained for CD31.
Immunoreactivities were determined using the avidin-biotin
peroxidase complex method (Vector Laboratories, Burlingame, CA)
using anti-mouse CD31 (Abcam, Cambridge, MA) at no dilution as the
primary antibody. Hematoxylin was used as the counter stain. The
negative controls used reagents except for the primary antibody.
Positive staining of a small tubular formation for CD31 was defined
as a macrovessel, and the microvessel density (MVD) was assessed as
the average number of vessels/mm2, over three areas, at
x200 magnification (20).
Statistical analysis
The deta were analyzed using the Student’s t-test.
The tumor volume was analyzed using two-way repeated ANOVA. A
P-value <0.05 was considered statistically significant. Analyses
were computed using the StatView v.5.0 software (SAS Institute
Inc., Cary, NC).
Results
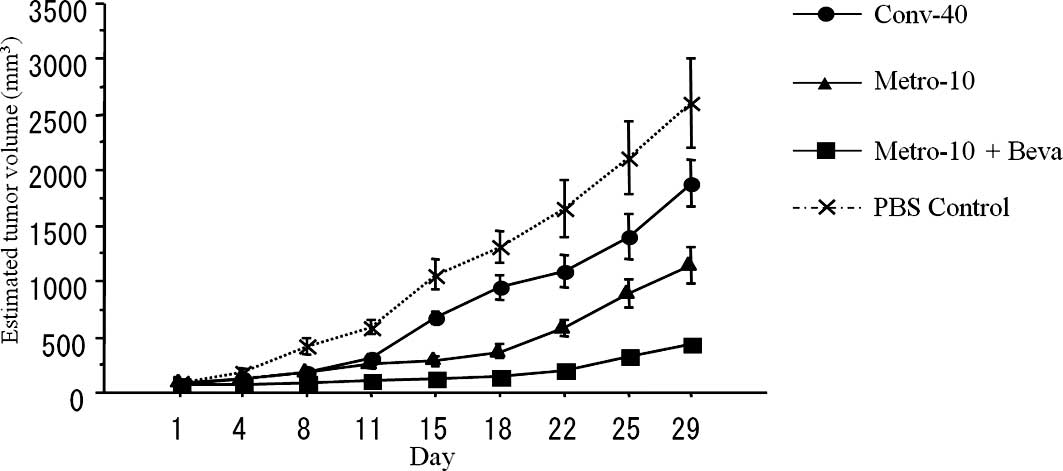
Growth inhibition of the tumors implanted
into the mouse subcutis
Conventional treatment of CPT-11 (Conv-40) showed
significantly higher antitumor activity compared with the PBS
control group (P=0.019). In addition, metronomic treatment using
CPT-11 (Metro-10) showed more effective antitumor activity compared
to the conventional (Conv-40) group (P<0.01). An additive
antitumor effect was found when bevacizumab was combined with
metronomic chemotherapy using CPT-11 (Metro-10 + Beva) (n=10 in
each group) (P<0.01) (Fig.
2).
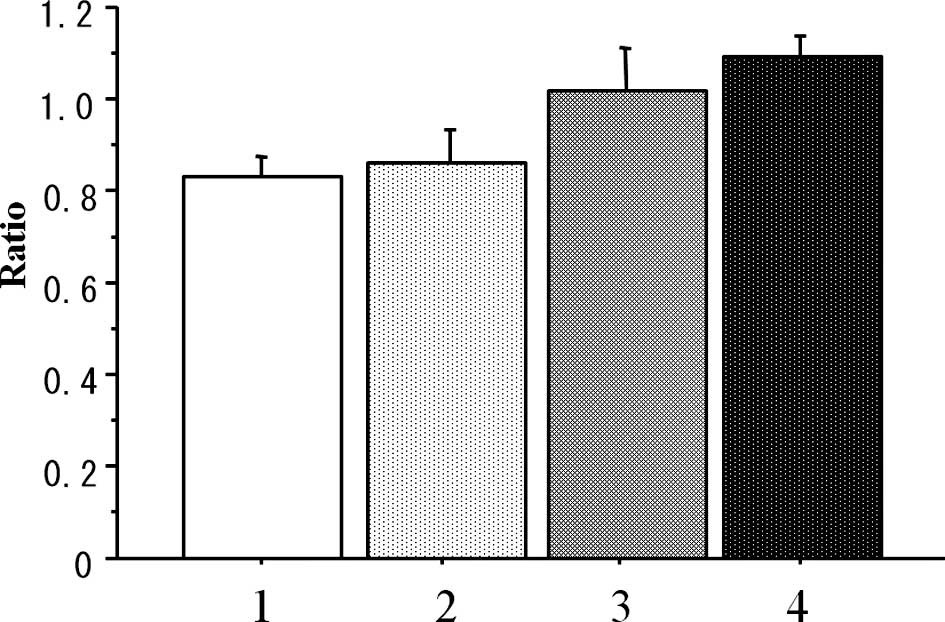
The weight-loss ratio (day 29/day 0) was
statistically lower in the conventional group (Conv-40) than that
in the metronomic-treated (Metro-10 ± Beva) groups (P=0.004),
although there was no significant difference in the weight loss
ratio between the conventional (Conv-40) group and that in the PBS
control group (n=7 in each group) (P=0.909) (Fig. 3).
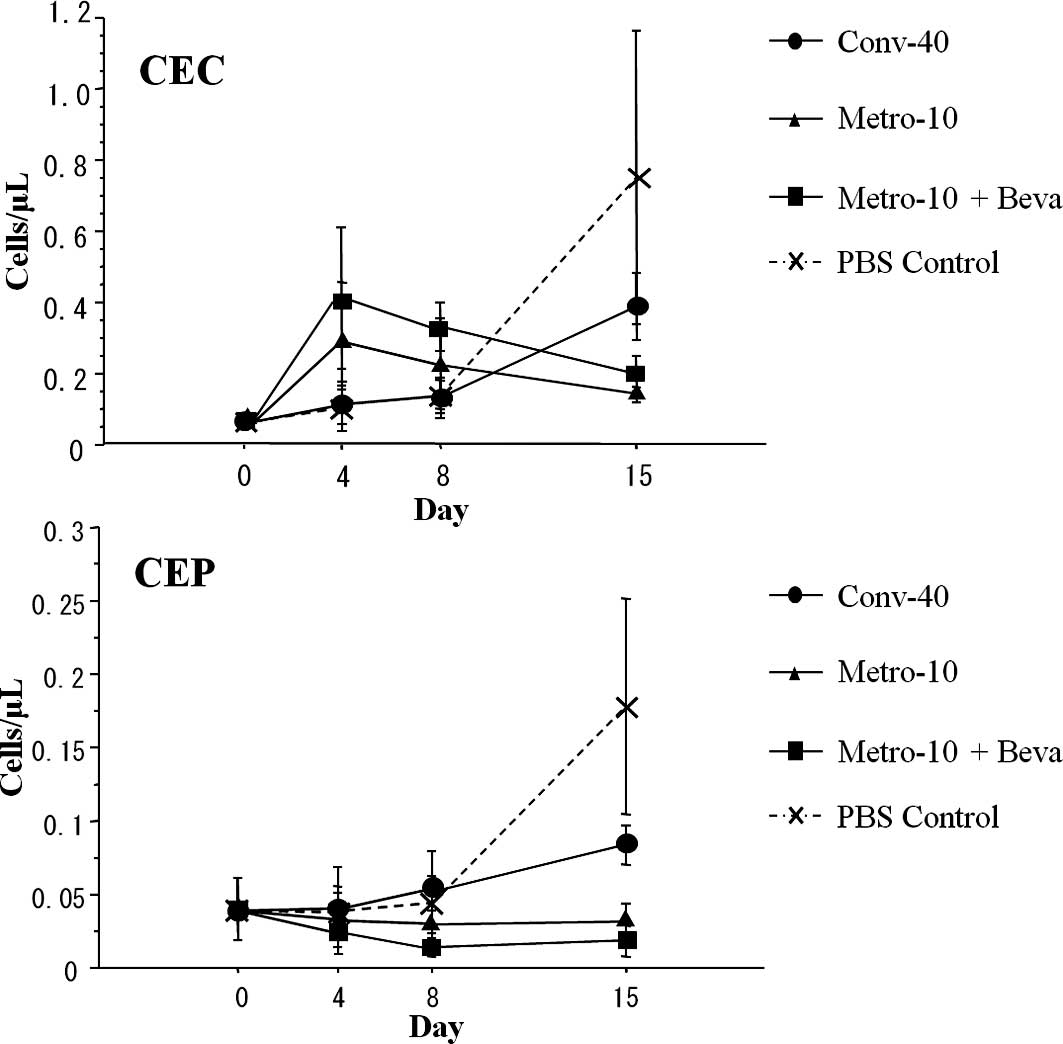
Serial changes of CECs and CEPs
CEC and CEP enumeration by flow cytometry is
depicted in Fig. 1. The numbers of
CECs in the control group and the conventional (Conv-40) group on
day 4 and 8 showed no difference compared to the numbers on day 0,
while the numbers of CECs on day 4 and 8 tended to increase in the
metronomic therapy (Metro-10 ± Beva) groups. While the numbers of
CECs increased in the control group and the conventional (Conv-40)
group on day 15, the numbers of CECs on day 15 in the metronomic
therapy groups tended to decrease compared to the numbers on day 4
and 8, but did not reach significance.
There was no significant difference in the number of
CEPs between each group on day 4 and 8. However, the numbers of
CEPs tended to decrease in the metronomic therapy groups on day 8
compared to those on day 0. Although a statistically significant
increase in the numbers of CEPs in the control and conventional
(Conv-40) group on day 15 was noted compared to those on day 0, 4
and 8 (P=0.028), there was no increase in numbers of CEPs in the
metronomic therapy groups (n=5 in each group) even on day 15
(Fig. 4).
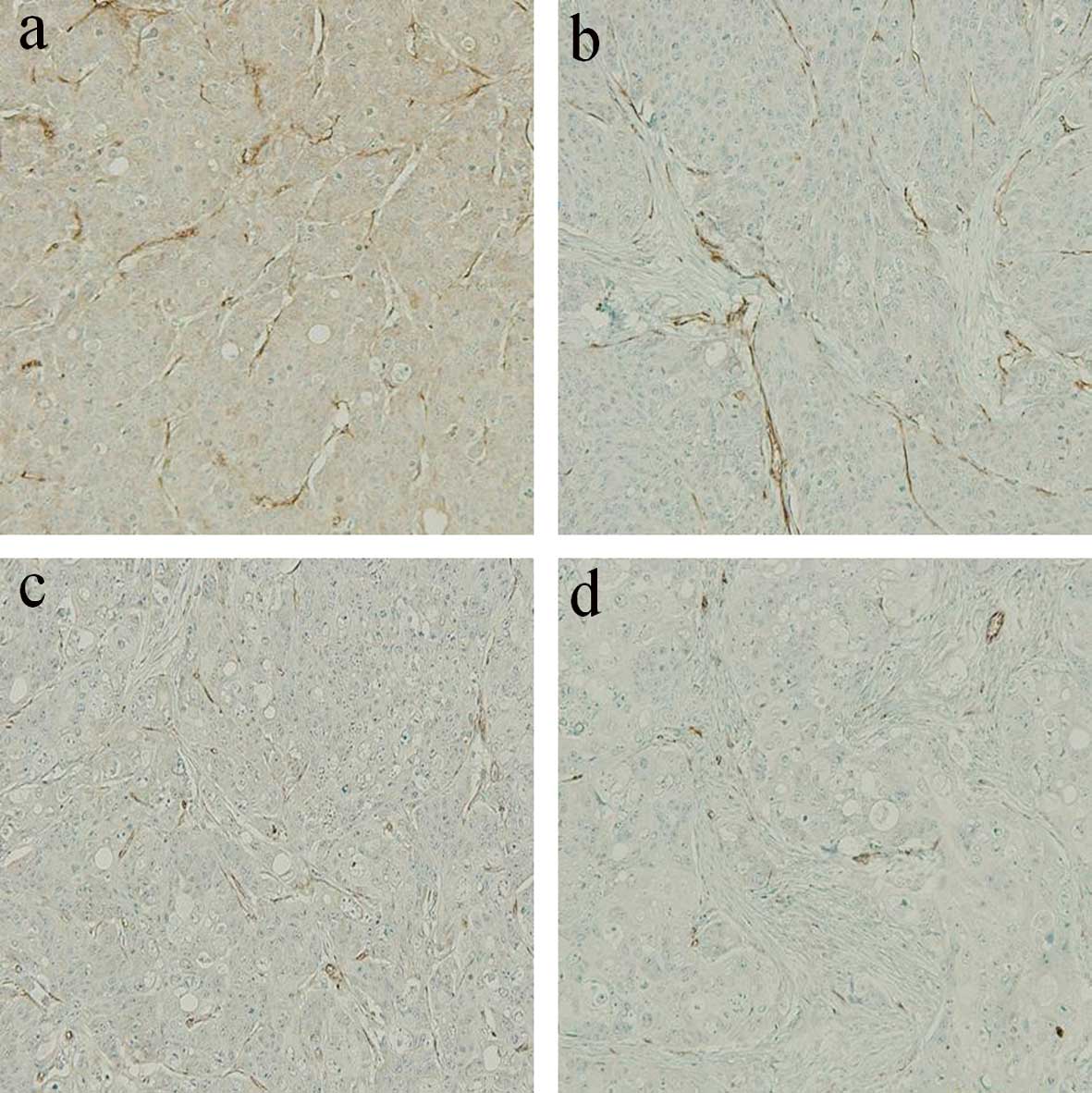
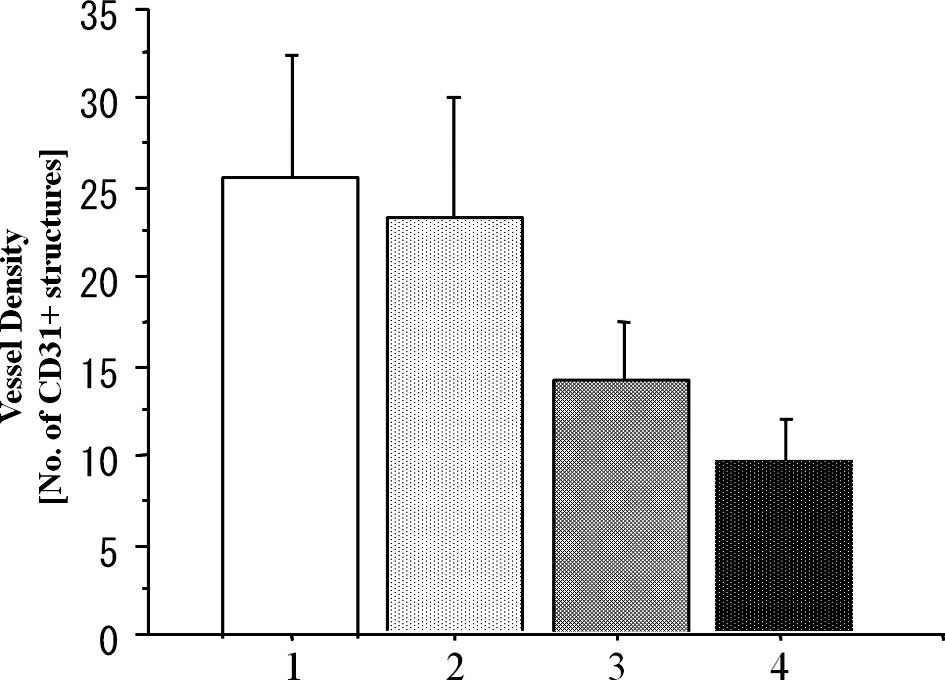
Analysis of MVD
To investigate the antitumor mechanism of the
metronomic CPT-11 treatment combined with or without bevacizumab,
we evaluated the MVD in the implanted colon cancer tumors in the
subcutis on day 15 after the beginning of drug administration
(Fig. 5). The MVD values on day 15
in the metronomic treatment (Metro-10 ± Beva) groups were
significantly lower than that in the conventional (Conv-40) group
(P<0.001), although there was no significant difference between
the conventional group and the PBS control group (P=0.173).
Additive effects of the inhibition of vascularization were found
when bevacizumab was combined with metronomic treatment of CPT-11
(Metro-10 + Beva vs. the Metro-10; P<0.001) (n=7 in each group)
(Fig. 6).
Discussion
The purpose of the present study was to clarify the
efficacy of metronomic chemotherapy using CPT-11 and its
combination with bevacizumab, a specific anti-angiogenic agent, and
the significance of CECs and CEPs as a surrogate marker for
efficacy in metronomic chemotherapy/anti-angiogenic therapy for
colon cancer. The concept of metronomic chemotherapy was summarized
by Kerbel and Kamen (8) and
Klement et al (21) as
follows. (i) Conventional cytotoxic anticancer drugs have
anti-angiogenic effects which could contribute to their efficacy.
(ii) The anti-angiogenic effects of chemotherapy appear to be
optimized by administering such drugs ‘metronomically’, in other
words in small dosages using a frequent schedule (daily, several
times a week or weekly) in an uninterrupted manner, over a
relatively long period. (iii) Conventional chemotherapy, which is
administered at a more toxic MTD, requires 2- to 3-week rest
periods between successive cycles of therapy (which counteracts the
potential for sustained therapeutically effective anti-angiogenic
effects). (iv) In preclinical models, metronomic chemotherapy can
be effective in treating tumors in which cancer cells have
developed resistance to the same chemotherapeutics in an MTD
administration (which also has the advantage of being less acutely
toxic, therefore making a more extended treatment possible). (v)
The efficacy of metronomic chemotherapy can be significantly
increased when administered in combination with anti-angiogenic
drugs, such as antibodies against VEGF or VEGF receptor 2. Finally,
(vi) some metronomic chemotherapy regimens induce sustained
suppression in CEPs and increase the levels of the endogenous
angiogenesis inhibitor thrombospondin-1, both of which can suppress
neovascularization.
In our experiment, the metronomic dispensing method
of CPT-11 showed a higher tumor proliferation-controlling effect
associated with reduced tumor MVD in nude mice transplanted with
KM12SM colon carcinoma cells when compared with the conventional
dispensing method. Moreover, the tumor proliferation-controlling
effect of metronomic administration of CPT-11 was significantly
increased when combined with bevacizumab, an anti-angiogenic agent.
In addition, in the metronomic administration groups weight loss as
an adverse effect was milder compared with that in the conventional
MTD administration group. These results from our colon cancer model
also support the concept of low-dosage metronomic chemotherapy
suggesting the ability of long-term administration and tumor
proliferation control.
It has been reported that, although the numbers of
CEPs markedly increase during rest periods between MTD
administrations of a chemotherapeutic agent to tumor-bearing mice,
CEPs are absent during the metronomic administration and the
development of tumors was not noted (18). We also investigated the serial
changes in CECs/CEPs and their relationship with tumor vasculature
(MVD) after treatment with CPT-11 and the vascular-targeting agent
bevacizumab. Our data provide evidence that metronomic
administration of CPT-11 and its combination with bevacizumab can
have opposing effects in the early phase on days 4 and 8 and then
similar effects in the late phase on day 15 on the number of CEPs
and mature CECs just prior to the next MTD administration. Namely,
the metronomic chemotherapy tended to increase the numbers of
mature CECs and to decrease the numbers of CEPs in the early phase
after the beginning of treatment (day 4 and 8), and tended to
decrease both CECs and CEPs in the late phase after the beginning
of treatment (day 15) in the KM12SM cell tumor-bearing mice. In
particular, the difference in the numbers of CEPs in the late phase
between the metronomic chemotherapy and conventional MTD
chemotherapy was statistical significant. The small numbers of CEPs
was associated with a concomitant inhibition in tumor vasculature
and in tumor growth, suggesting that continuous suppression of CEPs
may be a marker for anti-angiogenic activity, including metronomic
chemotherapy in a clinical situation.
Our results support the conclusion that the
antitumor effects of low dosage metronomic chemotherapy are
attributable, at least in part, to a mechanism involving inhibition
of tumor blood vessel formation. In addition to anti-angiogenic
mechanisms in which fully differentiated endothelial cells are
growth-inhibited and/or sacrificed by metronomic low-dosage
chemotherapy (6), an
anti-vasculogenic process may also be involved which is mediated by
reduced CEP mobilization and viability. It is also interesting to
consider whether MTD chemotherapy may sometimes accelerate tumor
(re)growth and drug resistance by increased mobilization of CEPs.
This may also help explain the robust reversal of the damage
inflicted by MTD chemotherapy on tumor blood vessel endothelial
cells as noted by Browder et al (22). An influx of mobilized CEPs during
the rest periods between cycles of MTD therapy may replace damaged
or sacrificed endothelial cells. In this regard, evaluating the
mobilization, viability, and levels of CEPs detected in cancer
patients treated with low-dosage metronomic chemotherapy regimens,
(e.g., daily low-dosage oral chemotherapy and twice weekly oral
methotrexate for breast cancer (23) or leukeran for lymphoma) (24) may be of considerable interest. Such
studies and our data may provide a surrogate marker with which to
monitor the anti-vasculogenic effects of metronomic chemotherapy
protocols. In murine studies, the anti-angiogenic agent endostatin
decreased the number of viable CEPs (25), whereas cyclophosphamide either
induced or inhibited CEPs depending on whether it was administered
in a conventional (every 21 days) or metronomic (every 6 days)
dosing schedule (18).
With regard to the increase in the number of CECs
early after the start of metronomic chemotherapy, it was found that
mature CECs increased after 3 days of treatment with ZD6474
targeting the tumor vasculature in tumor-bearing mice but not in
non-tumor-bearing mice (16),
suggesting that the increase in mature CECs was due, at least in
part, to the presence of the tumor and that ZD6474 or metronomic
chemotherapy has at least some degree of selectivity for tumor
endothelial cells rather than endothelial cells from normal
vasculature. On the other hand, metronomic chemotherapy or
anti-angiogenic therapy decreased the number of CECs on day 15 as
well as the number of CEPs, while the CECs increased on day 15 in
the control group and the MTD conventional chemotherapy group. The
changes in number of CECs were similar to the changes of CEPs on
day 15 after each treatment and in the control. These data suggest
that mature CECs may originate from differentiation of CEPs in
addition to the sloughing of tumor endothelial cells. Thus
metronomic chemotherapy can consistently inhibit an increase in
CEPs for a long time, while the number of CECs may be dependent on
various factors such as anti-angiogenic efficacy, tumor volume, the
status of tumor vasculature and time after chemotherapy, resulting
in large individual variations in the number of CECs.
Recently, oral daily fluoropyrimidines such as
capecitabine and UFT/LV have not been proven inferior to bolus
and/or infusion MTD chemotherapy using 5-FU in randomized control
studies for colon cancer (26,27).
Also combination therapies of oral fluoropyrimidine and
oxaliplatin/CPT-11 have been developed for colorectal cancer
(28,29). Oral fluoropyrimidine would be a
typical agent for metronomic chemotherapy (30). We previously reported the safety
and efficacy of metronomic chemotherapy using low-dosage weekly
CPT-11 and daily 5′-deoxy-5-fluorouridine, an intermediate
metabolite of capecitabine, for advanced colorectal cancer
(31). However, one of the major
problems is a definition of the optimal dosage based on the concept
of metronomic chemotherapy. This is a key reason why metronomic
chemotherapy has not been widely adopted in clinical trials. Our
data suggests that one possible means of determining the
recommended dosage for metronomic chemotherapy is to monitor the
serial change of CEPs rather than that of unstable CECs. The
optimal dosage for metronomic chemotherapy can be established as
the lowest level which is associated with no increase or decrease
in the number of CEPs for an individual patient.
We conclude that metronomic chemotherapy using
CPT-11 against colon cancer was more effective than conventional
therapy, via an anti-angiogenic effect. The combination with the
specific anti-angiogenic agent, bevacizumab, may realize the
advantage of metronomic chemotherapy. Measurement of CEPs may be a
consistent predictive factor for metronomic chemotherapy in colon
cancer. The assessment of serial changes in CEP values is
recommended in clinical trials of metronomic chemotherapy.
Acknowledgements
This study was supported by a
Grant-in-Aid for Scientific Research (C) (no. 20591597) from the
Ministry of Education, Culture, Sports, Science and Technology, of
Japan.
References
|
1.
|
Eskens FA: Angiogenesis inhibitors in
clinical development; where are we now and where are we going? Br J
Cancer. 90:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Warren RS, Yuan H, Matli MR, et al:
Regulation by vascular endothelial growth factor of human colon
cancer tumorigenesis in a mouse model of experimental liver
metastasis. J Clin Invest. 95:1789–1797. 1995. View Article : Google Scholar
|
|
4.
|
Takahashi Y, Ellis LM and Mai M: The
angiogenic switch of human colon cancer occurs simultaneous to
initiation of invasion. Oncol Rep. 10:9–13. 2003.PubMed/NCBI
|
|
5.
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Asahara T, Takahashi T, Masuda H, et al:
VEGF contributes to postnatal neovascularization by mobilizing bone
marrow-derived endothelial progenitor cells. EMBO J. 18:3964–3972.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bocci G, Francia G, Man S, et al:
Thrombospondin 1, a mediator of the anti-angiogenic effects of
low-dose metronomic chemotherapy. Proc Natl Acad Sci USA.
100:12917–12922. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Monestiroli S, Mancuso P, Burlini A, et
al: Kinetics and viability of circulating endothelial cells as
surrogate angiogenesis marker in an animal model of human lymphoma.
Cancer Res. 61:4341–4344. 2001.PubMed/NCBI
|
|
10.
|
Bocci G, Nicolaou KC and Kerbel RS:
Protracted low-dose effects on human endothelial cell proliferation
and survival in vitro reveal a selective anti-angiogenic window for
various chemotherapeutic drugs. Cancer Res. 62:6938–6943.
2002.PubMed/NCBI
|
|
11.
|
Shaked Y, Emmenegger U, Man S, et al:
Optimal biologic dose of metronomic chemotherapy regimens is
associated with maximum anti-angiogenic activity. Blood.
106:3058–3061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Shimada Y, Yoshino M, Wakui A, et al:
Phase II study of CPT-11, a new camptothecin derivative, in
metastatic colorectal cancer. CPT-11 Gastrointestinal Cancer Study
group. J Clin Oncol. 11:909–913. 1993.PubMed/NCBI
|
|
13.
|
Rothenberg ML, Cox JV, deVore RF, et al: A
multicenter, phase II trial of weekly irinotecan (CPT-11) in
patients with previously treated colorectal carcinoma. Cancer.
85:786–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kim KJ, Li B, Winer J, et al: Inhibition
of vascular endothelial growth factor-induced angiogenesis
suppresses tumor growth in vivo. Nature. 362:841–844. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gerber HP and Ferrara N: Pharmacology and
pharmacodynamics of bevacizumab as monotherapy or in combination
with cytotoxic therapy in preclinical studies. Cancer Res.
65:671–680. 2005.PubMed/NCBI
|
|
16.
|
Beaudry P, Force J, Naumov G, et al:
Differential effects of vascular endothelial growth factor
receptor-2 inhibitor ZD6474 on circulating endothelial progenitors
and mature circulating endothelial cells: implications for use as a
surrogate marker of anti-angiogenic activity. Clin Cancer Res.
11:3514–3522. 2005. View Article : Google Scholar
|
|
17.
|
Nitta K, Yokokura T, Sawada S, et al:
Antitumor activity of novel derivatives of camptothecin. Jpn J
Cancer Chemother. 14:850–857. 1987.
|
|
18.
|
Bertolini F, Paul S, Mancuso P, et al:
Maximum tolerable dose and low-dose metronomic chemotherapy have
opposite effects on the mobilization and viability of circulating
endothelial progenitor cells. Cancer Res. 63:4342–4346. 2003.
|
|
19.
|
Capillo M, Mancuso P, Gobbi A, et al:
Continuous infusion of endostatin inhibits differentiation,
mobilization, and clonogenic potential of endothelial cell
progenitors. Clin Cancer Res. 9:377–382. 2003.PubMed/NCBI
|
|
20.
|
Mizobe T, Ogata Y, Murakami H, et al:
Efficacy of the combined use of bevacizumab and irinotecan as a
postoperative adjuvant chemotherapy in colon carcinoma. Oncol Rep.
20:517–523. 2008.PubMed/NCBI
|
|
21.
|
Klement G, Baruchel S, Rak J, et al:
Continuous low-dose therapy with vinblastine and VEGF receptor-2
antibody induces sustained tumor regression without overt toxicity.
J Clin Invest. 105:R15–R24. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Browder T, Butterfield CE, Kraling BM, et
al: Anti-angiogenic scheduling of chemotherapy improves efficacy
against experimental drug-resistant cancer. Cancer Res.
60:1878–1886. 2000.PubMed/NCBI
|
|
23.
|
Colleoni M, Rocca A, Sandri MT, et al:
Low-dose oral methotrexate and cyclophosphamide in metastatic
breast cancer: antitumor activity and correlation with vascular
endothelial growth factor levels. Ann Oncol. 13:73–80. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
De Bont ES, Guikema JE, Scherpen F, et al:
Mobilized human CD34+ hematopoietic stem cells enhance
tumor growth in a nonobese diabetic/severe combined immunodeficient
mouse model of human non-Hodgkin’s lymphoma. Cancer Res.
61:7654–7659. 2001.
|
|
25.
|
Schuch G, Heymach JV, Nomi M, et al:
Endostatin inhibits the vascular endothelial growth factor-induced
mobilization of endothelial progenitor cells. Cancer Res.
63:8345–8350. 2003.PubMed/NCBI
|
|
26.
|
Twelves C; Xeloda Colorectal Cancer group:
Capecitabine as first-line treatment in colorectal cancer. Pooled
data from two large, phase III trials. Eur J Cancer. 38(Suppl. 2):
S15–S20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Douillard JY, Hoff PM, Skillings JR, et
al: Multicenter phase III study of uracil/tegafur and peroral
leucovorin versus fluorouracil and leucovorin in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
20:3605–3616. 2002. View Article : Google Scholar
|
|
28.
|
Santini D, Vincenzi B, Schiavon G, et al:
Chronomodulated administration of oxaliplatin plus capecitabine
(XELOX) as first line chemotherapy in advanced colorectal cancer
patients: phase II study. Cancer Chemother Pharmacol. 59:613–620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Goto A, Yamada Y, Yasui H, et al: Phase II
study of combination therapy with S-l and irinotecan in patients
with advanced colorectal cancer. Ann Oncol. 6:968–973. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kato H, Ichinose Y, Ohta M, et al: A
randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ogata Y, Sasatomi T, Mori S, et al:
Significance of thymidine phosphorylase in metronomic chemotherapy
using CPT-11 and doxifluridine for advanced colorectal carcinoma.
Anticancer Res. 27:2605–2612. 2007.PubMed/NCBI
|