Introduction
Prostate cancer (PC) is a common and extremely
variable disease (1). A major
challenge of PC management is the large number of patients,
approximately 40%, who relapse after radical treatment with surgery
or radiation therapy. Standard therapy for these patients is
castration leading to a temporary decrease in tumor growth.
Unfortunately, this effect is transient, and tumor growth
ultimately resumes (in a median of 18–24 months) during a
hormone-independent phase, i.e. castration-resistant PC (CRPC),
eventually resulting in death due to widespread disease (1). Until recently, few treatment options
have been available for these patients.
For many years, PC was believed to be a
chemoresistant disease (2,3). Clinical trials with mitoxantrone in
the late 90’s showed only palliative effects in patients with CRPC
(4). In 2004, two large randomized
phase III trials [the Southwest Oncology Group (SWOG) 99-16 and the
Tax 327 studies] showed that docetaxel-based therapy not only
improves quality of life and decreases pain but also prolongs
survival in patients with CRPC (5,6).
Thus, for the first time, chemotherapy was considered a treatment
option for patients with CRPC, and taxane-based therapy was
considered to be the gold standard. However, the drawbacks of
treatment with docetaxel are that the effect on survival is slight
(2–3 months), and the duration of the effect is limited to a
6-month period. Furthermore, it causes several major side effects
such as neutropenia, neuropathy and fatigue (7).
Patients with CRPC include a large heterogeneous
group of patients ranging from asymtomatic patients where the only
sign of progression is a rising prostate-specific antigen (PSA) to
patients with symptomatic metastases (7,8).
Furthermore, these patients are often elderly with a high degree of
comorbidity. The timing and best strategy for treating these
different groups of CRPC patients are not clear, but there is an
obvious need for clinical trials to test different chemotherapy
regimens at different stages of the disease so that the optimum
therapy with the most convenient routes of administration and least
amount of side effects can be defined (3).
In recent years, significant advances have been made
in our understanding of the tumor biology of CRPC where both tumor
cells as well as the surrounding stroma appear to play a pivotal
role in tumor progression (8).
Theoretically, this means that the use of a combination therapy
where both tumor and stromal cells are targeted may confer a
synergistic antitumor effect. There is a vast source of both
preclinical and clinical data supporting this concept (8). Therefore, in the present study we
investigated whether a combination of secondary hormonal
manipulation and chemotherapy, administered metronomically, has an
effect on patients with CRPC.
Metronomic chemotherapy is a regimen where low doses
of chemotherapy are administered on a frequent or continuous
schedule with no extended interruptions (9–11).
Preclinical studies have shown that, even though the total dose of
chemotherapy is lower, the tumor-inhibiting properties are just as
beneficial and in some cases even better than conventionally
administered chemotherapy administered at a maximum-tolerated dose
(MTD) (10,11). The main course of action for
metronomic chemotherapy is thought to be by attacking tumor stroma
through its anti-angiogenic properties thereby interrupting oxygen
and the nutrient supply in tumors (10,11).
In patients with lung, breast and ovarian cancer who stopped
responding to MTD-based therapy, therapeutic effects were noted
when chemotherapy was administered daily or weekly (12–14).
Furthermore, maintenance therapy in childhood tumors such as
leukemia is very similar to metronomic chemotherapy and has been
used for years (13).
Several phase II trials with combination
chemotherapy administered in a metronomic way in patients with CRPC
have shown beneficial therapeutic effects (15–18).
One of the most promising regimens consists of ketoconazole in
combination with doxorubicin alternating with vinblastine in
combination with estramustine (KA/VE) (15,17).
In a phase II trial with 45 patients an 8-week treatment cycle
produced the most satisfactory response rates observed to date in
this patient population: PSA reductions of >50% in 31 (69%)
patients and >80% in 26 (58%) patients, and a median overall
survival of 18.1 months; i.e. PSA responses, palliative and
survival effects compared favorably and sometimes even better than
early docetaxel studies (15,17).
The drawback, however, was toxicity. It was concluded that more
tolerable regimens should be a high priority prior to moving to
phase III trials.
Based on these findings, we developed a metronomic
peroral chemo-hormonal treatment schedule for patients with CRPC
consisting of ketoconazole in combination with cyclophosphamide or
etoposide in combination with estramustine administered on
alternate weeks (KEES) throughout the entire treatment period.
Prednisone was administered the entire period to counteract
potential adrenal insufficiency secondary to ketoconazole. All of
these drugs have previously been used in PC, and much attention has
been focused on possible positive and negative interactions and
toxicity when choosing dose levels.
The aim of this pilot study was to study the effect
(reduction in serum PSA) and toxicity of the KEES protocol in 17
patients with CRPC.
Patients and methods
Patients
Between 2003 and 2005, 17 patients with CRPC were
enrolled in this study at the Oncology Department, Sahlgrenska
Hospital, Gothenburg and the Department of Urology, Uddevalla
Hospital, Uddevalla, Sweden. No randomization was performed. The
enrolled patients were those who personally asked for further
treatment once they developed CRPC. Treatment was designed to be
administered in an outpatient setting. The median age of the
patients was 60 years (range 40–75). All patients had
histopathologically confirmed PC and had previously been treated
with medical or surgical castration for a median time of 46 months
(range 6–108) prior to the study. The medical castration included
androgen deprivation, estrogen, anti-androgen monotherapy or
combined androgen blockade (CAB). All patients had previously been
treated with CAB for at least one period and all but five patients
had experienced the effect of a therapeutic anti-androgen
withdrawal. Six patients had received previous first-line treatment
for CRPC. All patients except one were screened with bone
scintigraphy. The clinical characteristics of the patients are
presented in detail in Table
I.
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| Pt. no. | Age (years) | Time of castration
(months) | CAB | AA withdrawal | Previous
treatment | Pain (1–3) | Other symptoms | Bone metastasis | Other sites of
metastasis |
|---|
| 1 | 50 | 45 | Yes | Yes | Atrasentan | 1 | Difficulty
urinating | Yes | Lymph node |
| 2 | 75 | 7 | Yes | Yes | None | 1 | | Yes | Liver |
| 3 | 40 | 6 | Yes | No | None | 3 | Partial ileus,
recurrent infection, fracture | Yes | Lymph node, liver,
bladder |
| 4 | 74 | 68 | Yes | Yes | Prednisone | 1 | | Yes | 0 |
| 5 | 62 | 108 | Yes | Yes | Prednisone | 1 | | Yes | 0 |
| 6 | 46 | 46 | Yes | Yes | None | 1 | | Yes | 0 |
| 7 | 63 | 62 | Yes | Yes | Predisone,
bisphosphonates, Sendoxan | 2 | | No | Lymph node |
| 8 | 65 | 84 | Yes | Yes | None | 1 | Edema | NC | 0 |
| 9 | 62 | 36 | Yes | Yes | None | 1 | | Yes | 0 |
| 10 | 58 | 56 | Yes | Yes | None | 1 | | Yes | 0 |
| 11 | 59 | 104 | Yes | No | Prednisone | 1 | Spinal cord,
compression, weakness in arms and legs | Yes | 0 |
| 12 | 70 | 106 | Yes | No | None | 1 | Incontinence after
TUR-P, lymphostasis | Yes | Lymph node |
| 13 | 57 | 14 | Yes | No | None | 1 | Urine retention,
supra-pubic catheter | Yes | Lymph node |
| 14 | 63 | 35 | Yes | Yes | Prednisone | 1 | | NC | Lymph node |
| 15 | 62 | 79 | Yes | Yes | None | 1 | | Yes | Lymph node |
| 16 | 55 | 15 | Yes | No | None | 2 | Lymphostasis | Yes | Lymph node |
| 17 | 59 | 17 | Yes | Yes | None | 1 | | Yes | Lymph node |
Chemotherapy planning
Each course of peroral chemotherapy lasted for 6
consecutive weeks, with a 2-week rest between each course. Five
agents were included in the KEES schedule and administered as
follows: cyclophosphamide (Sendoxan®) (50 mg twice
daily) in combination with ketaconazole (200 mg three times daily)
were administered for seven consecutive days during weeks 1, 3 and
5. During weeks 2, 4 and 6, patients received seven consecutive
days of etoposide (50 mg twice daily) in combination with
estramustine (140 mg twice daily). One patient received idarubicin
(5 mg daily 4 days a week) instead of cyclophosphamide because of
hypersensitivity. A 2-week rest followed each 6-week course.
Prednisone (10 mg daily) was administered throughout the entire
treatment period to couteract potential ketoconazole-induced
adrenal complications. At the start of treatment each patient was
provided with a KEES schedule to aid in the correct administration
of the various drugs.
Treatment planning
The baseline visit was conducted at the outpatient
clinic at Sahlgrenska University Hospital and included a physical
examination and blood tests: serum PSA (prostate specific antigen),
Hemoglobin (Hb), white blood cell count (WBC), platelets, aspartate
transaminase (AST), alanine transaminase (ALT), bilirubin and
creatinine. Blood tests were repeated monthly and at the start of
each new course. Monthly contact with the doctor was maintained
through telephone or when needed by a clinical appointment. A
clinical staff nurse could easily be reached and was available to
the patients. Prior to the start of each new chemotherapy course, a
physical examination was performed.
PSA levels were used to follow the effect of
chemotherapy and were recorded at least every 8 weeks. Patients
with bone metastases that demonstrated a serum-PSA reduction by at
least 50% after 2–3 courses were evaluated with the intention to
administer strontium (Sr-89) when no contraindications were found.
For patients with a PSA reduction of <50%, a third course was
administered. Patients with no bone metastases received a third
KEES course, when there were no contraindications and good
tolerability was noted. No patient received more than 3 courses of
chemotherapy. PSA response was determined by using the baseline
value and the nadir value after 2–3 courses (i.e. 4–6 months) of
chemotherapy.
The choice of the consolidating regimen depended on
patient symptoms and tumor burden. Those with soft tissue
metastases received cyclophosphamide 50 mg/daily while those with
skeletal metastases and bone pain received zoledronic acid, 4 mg
monthly. In both groups treatment continued for 6 months.
Statistics
This study was a retrospective analysis, and
descriptive statistics were used to analyze the data.
Results
PSA response
Of the 17 patients with CRPC treated with the KEES
regime, 11 (65%) patients demonstrated a median reduction in their
serum PSA levels of 87% (range 26–99%). Of these 11 patients, 7
(41%) patients had a reduction in PSA level of >80%, 3 (18%)
patients had a 57–77% reduction and one patient had a minor
reduction of 26%. Ten (59%) patients responded with a decrease in
PSA >50%.
All patients responding to treatment apart from one
(pt. no. 11) received 2 or 3 courses of KEES over a period of 4–6
months. Although this patient had a 57% reduction in PSA one month
after therapy, his general condition deteriorated due to diarrhea
and he died one month after the initiation of KEES therapy. No
autopsy was performed, and the cause of death is unknown. Six
patients (nos. 3, 4, 9, 10, 13 and 16) did not respond to
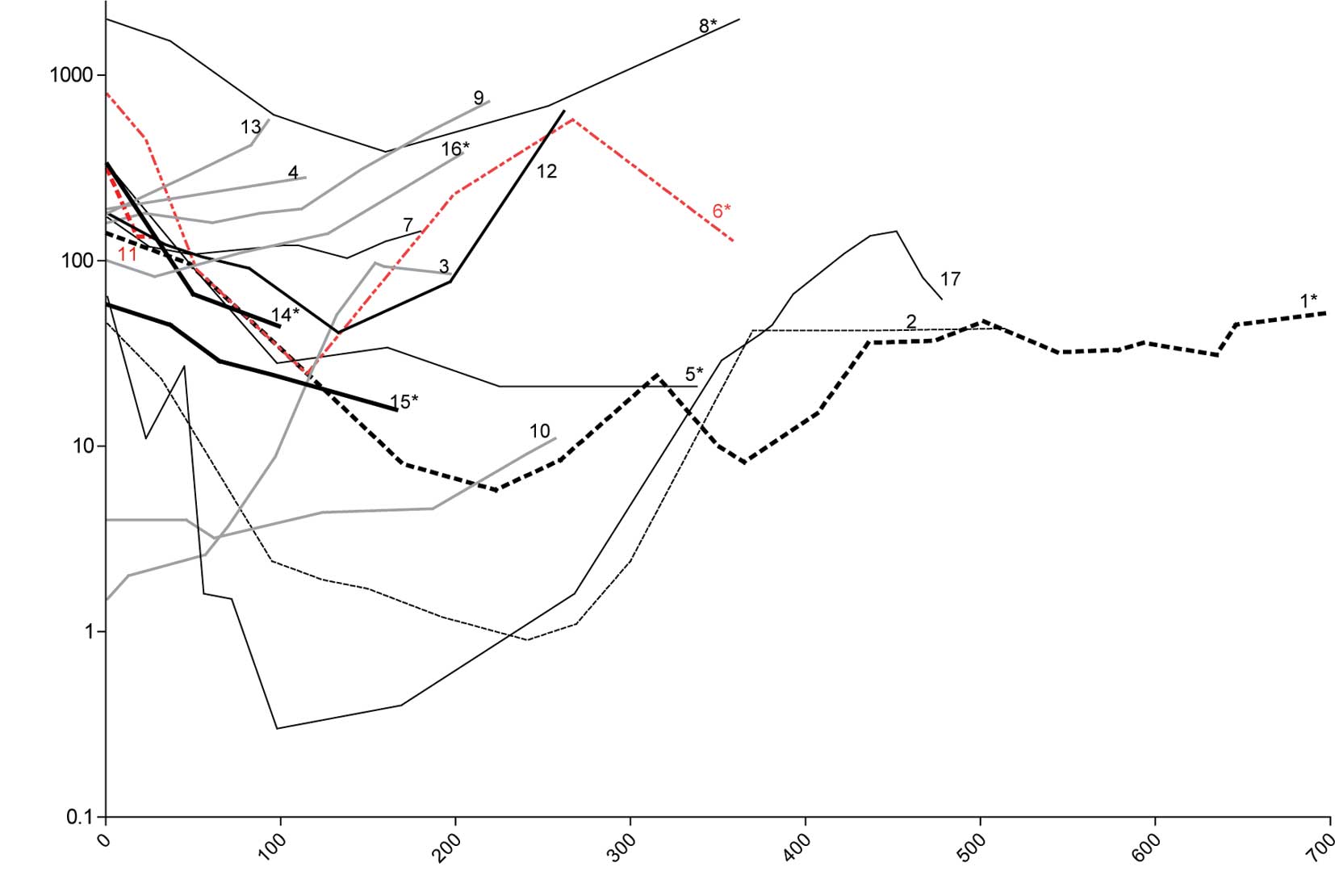
treatment, since no reduction in PSA was observed. Details of the
PSA level of each individual patient throughout the study are shown
in Fig. 1.
Treatment was discontinued in 4 patients. Three
patients (nos. 3, 4 and 13) were, in retrospect, in very poor
condition at baseline and did not tolerate chemotherapy. One
patient (no. 9) showed no response, and chemotherapy was
discontinued because he forgot his androgen deprivation. Eight
patients (nos. 1, 2, 5, 6, 8, 14, 15 and 17) were eligible for
Sr-89, 5 patients after 2 courses and 3 patients after 3 courses of
therapy. One of these patients (no. 2) could not be treated with
Sr-89 due to thrombocytopenia (Table
II).
 | Table II.Treatment planning and PSA levels
before and after treatment. |
Table II.
Treatment planning and PSA levels
before and after treatment.
| Pt. no. | Age (years) | Courses of KEES
administered | Reasons for
discontinued treatment | Sr-89
treatment | PSA at start of
treatment | PSA after
treatment | Change in PSA |
|---|
| 1 | 50 | 3 courses | | Yes | 140.0 | 8.0 | −94% |
| 2 | 75 | 2 1/2 courses |
Thrombocytopenia | No | 23.0 | 1.2 | −95% |
| 3 | 40 | <1/2 course | Infections,
compliance | No | 1.5 | 2.6 | - |
| 4 | 74 | 1/2 course | No response | No | 190.0 | 280.0 | +47% |
| 5 | 62 | 2 courses | | Yes | 330.0 | 28.0 | −91% |
| 6 | 46 | 2 courses | | Yes | 790.0 | 24.5 | −97% |
| 7 | 63 | 3 courses | | No | 171.0 | 144.0 | −16% |
| 8 | 65 | 3 courses | | Yes | 2400.0 | 386.0 | −84% |
| 9 | 62 | 1 1/2 course | No response | No | 160.0 | 190.0 | +19% |
| 10 | 58 | 3 courses | | No | 4.0 | 4.6 | +15% |
| 11 | 59 | 1/2 course | Death | No | 310.0 | 134.0 | −57% |
| 12 | 70 | 2 courses | | No | 180.0 | 41.0 | −77% |
| 13 | 57 | 1/2 course | Thrombocytopenia,
heart failure | No | 180.0 | 290.0 | +61% |
| 14 | 63 | 2 courses | | Yes | 330.0 | 44.4 | −87% |
| 15 | 62 | 3 courses | | Yes | 57.9 | 16.0 | −72% |
| 16 | 55 | 2 courses | | No | 100.0 | 110.0 | +10% |
| 17 | 59 | 2 courses | | Yes | 64.0 | 0.8 | −99% |
Side effects and morbidity
Thrombocytopenia was the most common side effect.
Four (24%) patients (nos. 2, 4, 13 and 16) demonstrated
thrombocytopenia. One patient (no. 2) needed repeated transfusions
over a period of 2 months. Two additional patients (nos. 4 and 16)
developed delayed thrombocytopenia. Anemia was the second most
frequently observed side effect. Three (18%) patients (nos. 2, 4
and 17) developed anemia that required erythrocyte transfusions.
One patient developed acute cholestasis (bilirubin 214 μmol/l,
reference value 5–25) and elevated serum chreatinine (208 μmol/l,
reference value 60–100) after his second KEES course. The signs and
symptoms as well as blood tests returned to normal in 3 weeks. This
same patient was retreated after one year with no observed side
effects, and a favorable secondary response was achieved.
Two patients (nos. 14 and 17) experienced vascular
complications in the form of thromboembolic events. One patient
(no. 14) was initially on anti-coagulant treatment. In spite of
this treatment, he developed a pulmonary embolus. Patient no. 17
developed a deep vein thrombosis. Other observed side effects were
mild gastrointestinal symptoms (29%), edema (12%), diarrhea (6%),
skin rash (6%) and elevation of liver enzymes (6%). Alopecia showed
a great degree of variability from mild to severe; only one patient
experienced complete alopecia. All side effects/morbidities are
presented in Table III.
 | Table III.Side effects/morbidity observed among
the patients treated with KEES. |
Table III.
Side effects/morbidity observed among
the patients treated with KEES.
| Pt. no. | Age (years) | Mild | Moderate | Severe |
|---|
| 1 | 50 | | Abdominal pain,
weight loss 5 kg | |
| 2 | 75 | | Thrombocytopenia
for >1 year, anemia (need of transfusions) | Thrombocytopenia
(need of transfusions for 2 months) |
| 3 | 40 | | Repeated
infections | |
| 4 | 74 | Hypotension | Anemia, delayed
thrombocytopenia | |
| 5 | 62 | Nausea | | |
| 6 | 46 | | | |
| 7 | 63 | Fatigue | | |
| 8 | 65 | Elevation of liver
enzymes | Heart failure | |
| 9 | 62 | Fatigue | | |
| 10 | 58 | Skin rash,
dyspepsia | | |
| 11 | 59 | Fatigue,
dyspepsia | Diarrhea | |
| 12 | 70 | | | |
| 13 | 57 | | Thrombocytopenia,
heart failure | |
| 14 | 63 |
Thrombophlebitis | | Pulmonary
embolism |
| 15 | 62 | Dyspepsia | | |
| 16 | 55 | | | Delayed
thrombocytopenia, one transfusion |
| 17 | 59 | 50% alopecia,
vomiting, abdominal pain, micturation problems | Anemia (need of
transfusion) | Deep vein
thrombosis, acute cholestasis |
One case of study fatality was reported. This
patient’s (no. 11) general condition deteriorated due to diarrhea,
and he died one month after initiation of KEES therapy.
Unfortunately no autopsy was performed, and the cause of death
remains unknown.
Discussion
Treatment of patients with castration-resistant PC
with a sequential metronomic chemo-hormonal therapy such as KEES
appears to be beneficial with favorable PSA responses that compare
with prior reports and with minor toxicity in contrast to what has
been previously described for composite chemotherapy regimens.
Even though the use of docetaxel in PC has shown a
life-prolonging effect, it only modestly alters the natural history
of castration resistant PC (17,19).
The timing and most effective strategy with which to treat the
heterogeneous population of patients with CRPC, who are often
elderly and with a great degree of comorbidity, remain largely
undefined although several clinical trials are underway. As always,
the goal is to define the optimum therapy with the most convenient
route of administration and the least amount of side effects
(7).
The KEES protocol was designed in an era when
docetaxel was not available, and our aim was to create an entirely
outpatient-based schedule with low toxicity using peroral
administration as an alternative to an in-house patient setting.
The KEES regimen described in this report was somewhat based upon
an MD Andersson (15,17,18)
protocol. It was modified and converted to a fully peroral regimen.
Therefore, doxorubicin and vinblastine were replaced with
cyclophosphamide and etoposide both of which are also active in PC
(3,16,20–24).
By combining secondary hormonal manipulation (ketoconazol,
estramustine) with chemotherapy (etoposide, Sendoxan and
estramustin) administered in a metronomic manner we sought to
achieve a synergistic antitumor effect as previously described in
experimental studies (23).
Here, we report PSA responses after 2–3 courses of
KEES (4–6 months of therapy) where 59% of patients responded with a
>50% decline in PSA levels. This equals the response rates noted
in docetaxel studies (TAX 327 and SWOG-9916) (5,6).
However, whether this reduction in PSA also corresponds to a
prolonged progression-free and overall survival and increased
quality of life remains to be shown, and a relevant study is
currently underway. Several patients receiving consolidating
therapy had long-lasting PSA responses (Fig. 1) (data not shown).
The toxicity observed in this study was less than
previously described for composite chemo-hormonal or
docetaxel-containing regimens (5,6,17,18).
A major concern was the risk of thromboembolic complications in
estramustine-containing protocols which in some trials has been as
high as 30% (25). We used a
smaller dose of estramustine (140 mg twice daily) which is less
than previous studies and may therefore explain the fewer incidents
of thromboembolic events (18%). This could, however, also be
explained by the small number of patients. A major complication in
docetaxel studies has been neutropenia which was not noted in this
trial. In contrast, thrombocytopenia was the most frequently
observed hematological side effect. Two of four patients required
transfusions, but no events of bleeding were observed. Importantly,
thrombocyte levels returned to normal levels for all four patients.
Ketoconazol treatment is associated with an increased risk of liver
toxicity, but in our study only one patient had elevated liver
enzymes which is less than previously described (26,27).
To further minimize the risk of liver toxicity we used low-dose
ketoconazole (200 mg twice daily) which has been shown to achieve
equal responses as high-dose ketoconazol (26,27).
Taken together, with the exception of an increased risk of
thrombocytopenia, the KEES protocol was well-tolerated.
It is not possible to analyze the contribution of
the various therapeutic components to the responses observed in
this small trial, yet it is likely that some drugs contributed to a
greater extent than others. As mentioned earlier, all drugs were
previously tested as single drugs or in combination for patients
with CRPC. One could argue that some of the treatment effect could
be contributed to prednisone which is probably true. However,
single drug studies with prednisone in androgen-independent PC have
shown PSA response rates of 16–24% which is far less than the rates
noted in the present study (28).
In this small preliminary study, we showed that
sequentially administered chemo-hormonal metronomic therapy appears
to be less toxic than conventional scheduling with an MTD dose of
docetaxel but with an equal effect on PSA levels. By yielding a
lower accumulated dose, a more prolonged treatment was possible
which is important considering the palliative situation of patients
with CRPC. A treatment with low toxicity with the prospect of being
used for an extended period of time with the added advantage of
being easy to administer is the most favorable at the present
time.
In conclusion, this small preliminary pilot study
demonstrated that sequentially administered metronomic
chemohormonal therapy is feasible for patients with
castration-resistant PC.
Acknowledgements
This study was supported by grants
from the Jubilee Clinic Cancer Research Foundation, Gothenburg,
Swedish Cancer Society, Jubilee Fund, Stockholm, Foundation for
Yngve Lands Memory, The Swedish Society of Medicine, The Health and
Medical Care Committee of the region Västra Götaland.
References
|
1.
|
Damber JE and Aus G: Prostate cancer.
Lancet. 371:1710–1721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Calabro F and Sternberg CN: Current
indications for chemotherapy in prostate cancer patients. Eur Urol.
51:17–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mike S, Harrison C, Coles B, Staffurth J,
Wilt TJ and Mason MD: Chemotherapy for hormone-refractory prostate
cancer. Cochrane Database Syst Rev. CD005247:2006.PubMed/NCBI
|
|
4.
|
Kantoff PW, Halabi S, Conaway M, et al:
Hydrocortisone with or without mitoxantrone in men with
hormone-refractory prostate cancer: results of the cancer and
leukemia group B 9182 study. J Clin Oncol. 17:2506–2513.
1999.PubMed/NCBI
|
|
5.
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gignac GA, Morris MJ and Hussain M:
Castration resistant, taxane-naive metastatic prostate cancer:
current clinical approaches and future directions. J Urol.
178:S30–S35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bradley DA and Hussain M: Promising novel
cytotoxic agents and combinations in metastatic prostate cancer.
Cancer J. 14:15–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hanahan D, Bergers G and Bergsland E: Less
is more, regularly: metronomic dosing of cytotoxic drugs can target
tumor angiogenesis in mice. J Clin Invest. 105:1045–1047. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kerbel RS, Klement G, Pritchard KI and
Kamen B: Continuous low-dose anti-angiogenic/metronomic
chemotherapy: from the research laboratory into the oncology
clinic. Ann Oncol. 13:12–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kikuchi Y, Kita T, Takano M, Kudoh K and
Yamamoto K: Treatment options in the management of ovarian cancer.
Expert Opin Pharmacother. 6:743–754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stempak D, Seely D and Baruchel S:
Metronomic dosing of chemotherapy: applications in pediatric
oncology. Cancer Invest. 24:432–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tonini G, Schiavon G, Silletta M, Vincenzi
B and Santini D: Antiangiogenic properties of metronomic
chemotherapy in breast cancer. Fut Oncol. 3:183–190. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ellerhorst JA, Tu SM, Amato RJ, et al:
Phase II trial of alternating weekly chemohormonal therapy for
patients with androgen-independent prostate cancer. Clin Cancer
Res. 3:2371–2376. 1997.PubMed/NCBI
|
|
16.
|
Lord R, Nair S, Schache A, et al: Low dose
metronomic oral cyclophosphamide for hormone-resistant prostate
cancer: a phase II study. J Urol. 177:2136–2140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Millikan R, Thall PF, Lee SJ, et al:
Randomized, multicenter, phase II trial of two multicomponent
regimens in androgen-independent prostate cancer. J Clin Oncol.
21:878–883. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tu SM, Millikan RE, Mengistu B, et al:
Bone-targeted therapy for advanced androgen-independent carcinoma
of the prostate: a randomised phase II trial. Lancet. 357:336–341.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Logothetis CJ and Millikan R: Chemotherapy
for advanced prostate cancer: 25 years later. J Clin Oncol.
26:2423–2424. 2008.PubMed/NCBI
|
|
20.
|
Berruti A, Fara E, Tucci M, et al: Oral
estramustine plus oral etoposide in the treatment of hormone
refractory prostate cancer patients: a phase II study with a 5-year
follow-up. Urol Oncol. 23:1–7. 2005.PubMed/NCBI
|
|
21.
|
Bracarda S, Tonato M, Rosi P, et al: Oral
estramustine and cyclophosphamide in patients with metastatic
hormone refractory prostate carcinoma: a phase II study. Cancer.
88:1438–1444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Dimopoulos MA, Panopoulos C, Bamia C, et
al: Oral estramustine and oral etoposide for hormone-refractory
prostate cancer. Urology. 50:754–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Eigl BJC, Eggener SE, Baybik J, et al:
Timing is everything: preclinical evidence supporting simultaneous
rather than sequential chemohormonal therapy for prostate cancer.
Clin Cancer Res. 11:4905–4911. 2005. View Article : Google Scholar
|
|
24.
|
Glode LM, Barqawi A, Crighton F, Crawford
ED and Kerbel R: Metronomic therapy with cyclophosphamide and
dexamethasone for prostate carcinoma. Cancer. 98:1643–1648. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fizazi K, Le Maitre A, Hudes G, et al:
Addition of estramustine to chemotherapy and survival of patients
with castration-refractory prostate cancer: a meta-analysis of
individual patient data. Lancet Oncol. 8:994–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wilkinson S and Chodak G: An evaluation of
intermediate-dose ketoconazole in hormone-refractory prostate
cancer. Eur Urol. 45:581–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Harris KA, Weinberg V, Bok RA, Kakefuda M
and Small EJ: Low dose ketoconazole with replacement doses of
hydrocortisone in patients with progressive androgen independent
prostate cancer. J Urol. 168:542–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Haines IE and Stanley RM: Perspective on
‘Chemotherapy for advanced prostate cancer: 25 years later’: Is it
a mirage or an oasis? J Clin Oncol. 26:4049–4051. 2008.
|