Introduction
Congenital heart diseases (CHDs) are common
congenital malformations. They are caused by abnormalities in the
embryonic heart and vasculature. The reported incidence of CHDs
varies between 12 and 14 per 1,000 live births (1). In China, approximately 100,000
neonates are born with CHDs every year. Only one-fifth of these
neonates obtain treatment in the approximately 300 units that
perform cardiac surgery (2).
Ventricular septal defect (VSD) is the most common form of CHD.
Currently, even though many genes related to cardiac development
have been identified and correction by surgery has yielded
favorable results as the main treatment option, the etiology and
pathological mechanisms of the disease are unknown.
The NADPH oxidase (NOX) family consists of the
homologs of gp91phox (glycosylated subunit of phagocyte
NADPH oxidase flavocytochrome); this family includes the
NOX1, NOX2, NOX3, NOX4 and NOX5
genes. ROS are derived by the NOX family as secondary messenger
molecules that participate in cell proliferation, transformation,
differentiation and apoptosis (3–5). The
embryonic development of the ventricular septum involves a balance
between various cell proliferation, differentiation and apoptosis
processes. NOX5 is a highly expressed embryonic gene, which
may be involved in this process.
Epigenetic mechanisms, such as miRNA expression and
histone modification, are crucially responsible for dysregulated
gene expression in CHD (6). By
contrast, the role of DNA methylation remains unknown. DNA
methylation is catalyzed by DNA methyltransferase and involves the
addition of a methyl group to the carbon-5 position of the cytosine
ring converting it to methyl cytosine (7), which is another well-characterized
epigenetic mark. DNA methylation plays an important role during
embryogenesis, normal mammalian development, cellular
differentiation and chromosome integrity. NOX5, a promoter
hypermethylation gene, was found in our laboratory by promoter
methylation microarrays (8). The
aim of this study was to verify the results of promoter methylation
microarrays and determine whether there is concordance between
NOX5 methylation and a decline in its mRNA expression in VSD
and normal fetuses.
Materials and methods
Tissues and DNA
Fetal myocardial tissue samples were obtained from
the Nanjing Maternal and Child Health Hospital. The representative
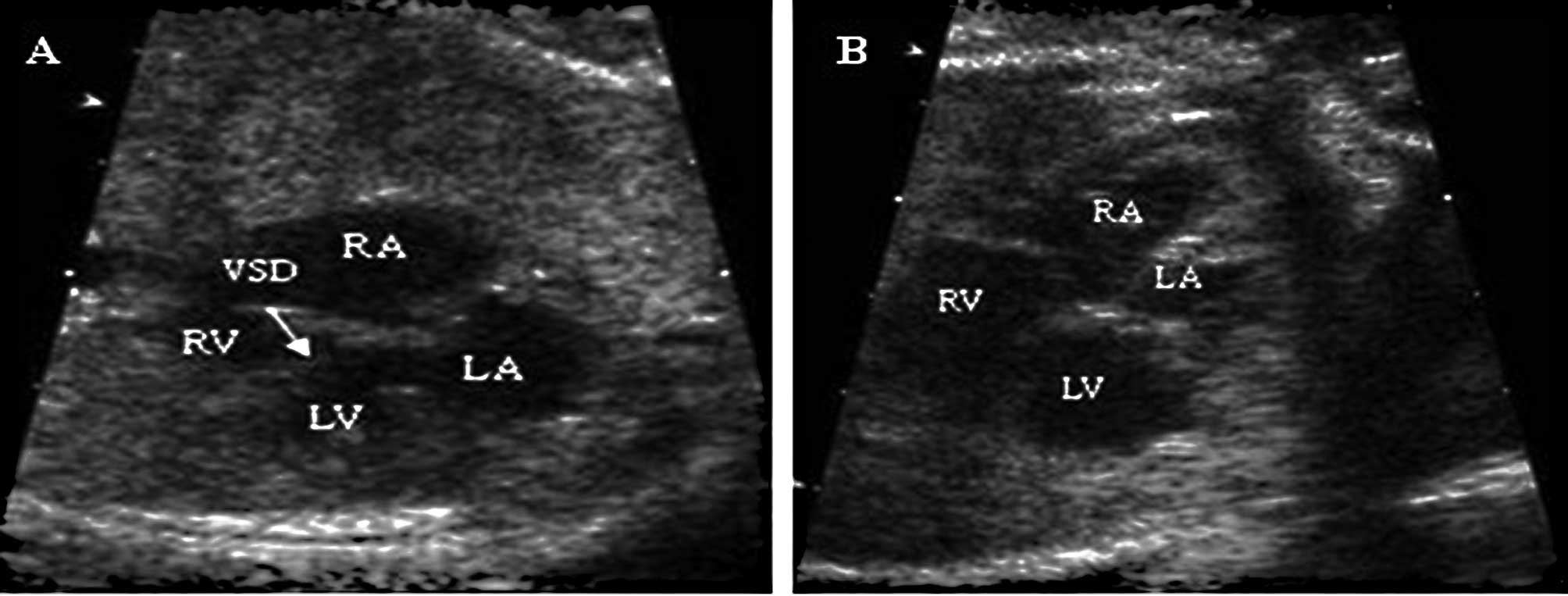
four-chamber view of VSD is shown in Fig. 1. Thirty-six fetuses at 26 weeks of
gestation were obtained during surgery for termination of pregnancy
owing to trauma of the pregnant women. All samples were collected
with the approval of the ethics committee of the appropriate
institute, and written consent was provided by each pregnant woman
and her family. The specimens were snap frozen in liquid nitrogen
immediately and then stored at −80°C until analysis. Genomic DNA
was purified from tissue specimens using the conventional
proteinase K digestion and phenol/chloroform extraction method.
After purification, genomic DNA was treated with sodium bisulfite.
The treatment converts unmethylated cytosines to uracils, while
leaving the methylated cytosines unaffected (9).
Methylation-specific PCR
The NOX5 gene sequence was obtained from
GenBank (Gene ID 79400). Methyl Primer Express® Software
was used to design the primer of methylation-specific PCR. The
primer designed for the unmethylated sequences does not amplify the
methylated sequence and vice versa. Bisulphite-modified DNA was
amplified by nested PCR using the primer sets described in Table I. Primers were purchased from
Invitrogen (Carlsbad, CA, USA). Myocardial tissue DNA samples,
untreated or methylated in vitro by excess CpG (Sss.I)
methyltransferase (NEB, USA), were used as positive controls for
unmethylated and methylated DNA, respectively. Distilled water was
used as a negative control. The first cycle of nested PCR was
carried out in a final volume of 25 μl, containing 2.5 μl 10X
buffer, 2.5 μl 10 mM dNTP mixture, 2.0 μl 50 mM MgCl2,
1.0 μl of each of the primers, 0.25 μl (5 U/μl) Taq DNA
polymerase and 2.0 μl DNA sample containing 10 ng DNA. The
amplification conditions were as follows: an initial incubation at
94°C for 2 min, followed by 36 cycles at 94°C for 30 sec, 57°C for
30 sec and 72°C for 45 sec; and a final extension at 72°C for 7
min. A 20-fold dilution of the product from the first cycle was the
template of the second cycle, while the other components were
similar. The amplification conditions for the second cycle were as
follows: an initial incubation at 94°C for 2 min, followed by 36
cycles at 94°C for 30 sec, 55°C for 30 sec and 72°C for 45 sec; and
a final extension at 72°C for 7 min. PCR products were separated in
a 1.5% agarose gel, stained with ethidium bromide and visualized
under UV illumination. Each experiment was repeated at least three
times.
 | Table I.Primer sequences for the methylated
(M) and unmethylated (U) NOX5 gene. |
Table I.
Primer sequences for the methylated
(M) and unmethylated (U) NOX5 gene.
| Primers | | Sequences | Temperature (°C) |
|---|
| First cycle | M forward |
5′-TATAGGGATCGCGTTTAAATTAC-3′ | 57 |
| M reverse |
5′-ACTAAAAACTTCATAAACGTCGTC-3′ | |
| U forward |
5′-TTTTATAGGGATTGTGTTTAAATTAT-3′ | |
| U reverse |
5′-AAACTAAAAACTTCATAAACATCATCC-3′ | |
| Second cycle | M forward |
5′-TATAGGGATCGCGTTTAAATTAC-3′ | 55 |
| M reverse |
5′-TATCAACGAAATACCGTCCTAC-3′ | |
| U forward |
5′-TTTTATAGGGATTGTGTTTAAATTAT-3′ | |
| U reverse |
5′-TATCAACAAAATACCATCCTACCTC-3′ | |
RT-PCR
Total RNA from the myocardial tissue samples was
extracted using the TRIzol method (Invitrogen). cDNA was
synthesized from 1 μg of total RNA using an AMV Reverse
Transcriptase kit (Promega A3500; Promega, Madison, WI, USA). An
aliquot (10%) of the resulting cDNA was amplified for PCR with the
primers listed in Table II. The
number of cycles and reaction temperatures used in the PCR assay
were optimized to provide a linear relationship between the amount
of input template and the amount of PCR product.
 | Table II.Primer sequences for RT-PCR. |
Table II.
Primer sequences for RT-PCR.
| Gene | | Primers | Temperature (°C) |
|---|
| NOX5 | Forward |
5′-AAGACTCCATCACGGGGCTGCA-3′ | 65 |
| Reverse |
5′-CCCTTCAGCACCTTGGCCAGAG-3′ | |
| GAPDH | Forward |
5′-CCATGTTCGTCATGGGTGTGAACCA-3′ | 60 |
| Reverse |
5′-GCCAGTAGAGGCAGGGATGATGTTC-3′ | |
Statistical analysis
Each experiment was performed at least three times.
For the analysis, data were classified using Fisher’s exact test
and Student’s t-test. A P-value <0.05 (2-sided) was regarded as
statistically significant. All data were analyzed with SPSS 13.0
for Windows (SPSS Inc., Chicago, IL, USA).
Results
Hypermethylation of the NOX5 promoter in
VSD fetuses
The results of the methylation status analysis of
the CpG islands in the NOX5 promoter region in the
myocardial tissue of normal and VSD fetuses are shown in Fig. 2. The NOX5 promoter was
hypermethylated in 66.67% of the VSD fetuses and in only 20% of the
normal fetuses (Table III).
Statistical analysis revealed that promoter hypermethylation of the
NOX5 gene was strongly correlated with the study groups
(P=0.008).
 | Table III.Hypermethylation status in the two
groups. |
Table III.
Hypermethylation status in the two
groups.
| Study group | No. | Hypermethylated
cases | Hypermethylation yes
(%) |
|---|
| VSD | 21 | 14 | 66.67 |
| Controls | 15 | 3 | 20.00 |
Expression of NOX5 gene mRNA in fetal
myocardial tissue
The expression levels of NOX5 in fetal
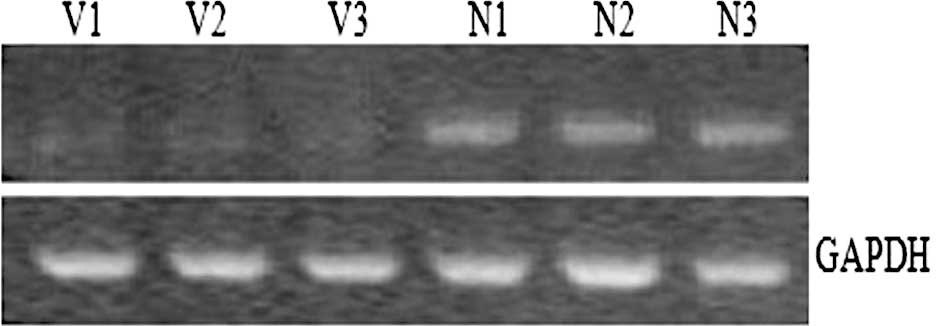
myocardial tissue samples were measured by RT-PCR. Fig. 3 shows that the expression levels of
NOX5 were much higher in the controls than in the VSD
fetuses. The result indicates that there is a significant
association between low expression of NOX5 with
hypermethylation of the gene in VSD.
Discussion
DNA methylation is the main epigenetic modification
in mammals and particularly in humans. The most striking feature of
vertebrate DNA methylation patterns is the presence of CpG islands.
Earlier studies estimated that ∼60% of human genes are associated
with CpG islands (10). Many
studies of DNA methylation have been carried out in mammalian
systems, in which genomic DNA methylation is found throughout the
genome (11). Thus, DNA
methylation provides information as to where and when the gene
should be expressed, but does not alter the structure or function
of a gene (12). DNA methylation
patterns are required for normal embryonic development. The DNA
methyltransferases DNMT1, DNMT3A and DNMT3B
cooperatively regulate cytosine methylation in CpG dinucleotides in
mammalian genomes (13); both
Dnmt3a and Dnmt3b function as de novo methyltransferases
that play important roles in normal development (14). Furthermore, the DNA methylation
levels vary throughout the mammalian developmental process
(15).
It has been recognized that environmental and
genetic factors play important roles in VSD, as in other CHDs.
However, recent studies have shown that CHD caused by single gene
or single locus defects is more common than expected (16). The interaction between histone
de-acetylation and DNA methylation existing between human end-stage
cardiomyopathic and heart failure has already been established
(17). Most recent studies have
focused on the crucial role of the NOX family in cardiac
pathophysiology. In this respect, it has been shown that there is
increased myocardial NOX family activity in the failing heart
(18). Expression of NOX2
has been demonstrated in human cardiomyocytes and was shown to be
up-regulated during myocardial infarction (19).
NOX5 was the last discovered gene in the NOX
family and it is highly divergent from other members of the family.
Evolutionary tree analysis has revealed that NOX5 may
represent the gene which is closest to primordial NOX (20), and the gene is unique as it
contains EF hand domains in the N-terminal region that bind calcium
and permit activation of the enzyme by an increase in intracellular
calcium (21). In prior studies,
NOX5 expression has been detected within blood vessels of
the spleen and lung and also in coronary blood vessels (22,23).
In blood vessels from individuals without coronary
artery disease, NOX5 expression is very low, but it is
substantially increased in blood vessels of individuals with the
disease (24). In this study, we
found that NOX5 promoter hypermethylation occurred more
often in VSD myocardial tissue by methylation-specific PCR. There
was a significant concordance between NOX5 methylation and a
decline in its mRNA expression. Thus, the low expression of
NOX5 in individuals without coronary artery disease may
account for the promoter hypomethylation of the gene. NOX5
promoter hypermethylation in VSD myocardial tissue contributes to
transcriptional silencing of the gene. The gene silencing leads to
abnormal reduction in ROS production. ROS, as a signaling
substance, stimulates the proliferation of mammalian cells.
However, the formation of VSD is an extremely complex pathological
process, which involves cell proliferation and differentiation
during embryogenesis as well as apoptosis (25). NOX5 silencing, which leads
to reduction in ROS, may be involved in the pathological process of
fetal VSD. On the other hand, in rodents, NOX1 and
NOX2 are the primary isoforms expressed in the spleen
(26), whereas in humans
NOX5 was initially characterized as a gene that is highly
expressed in the testis, spleen and lymph nodes; in lymphocytes,
this gene may participate in calcium signaling, proliferation,
differentiation and apoptosis (21). The immunological profile in CHD
children, including levels of IgG and IgA and complement components
C3 and C4, was found to be significantly impaired in all children
with CHD; T-helper cells were decreased and T-suppressor cells were
increased in all groups with CHD as compared to controls (27). The B-cell percentage was increased
in cyanotic children, but was not affected in acyanotic children
(27). Thus, another possibility
is that NOX5 gene silencing in VSD fetuses may result in
immune dysfunction and immunoregulatory disorders.
In summary, hypermethylation of the NOX5
promoter was detected in 66.67% of the VSD fetuses using
methylation-specific PCR. Our findings indicate that a high
frequency of methylation of the NOX5 gene promoter is an
important mechanism for NOX5 inactivation in VSD and normal
fetuses.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (nos.
30973213, 81070500 and 81070138).
References
|
1.
|
Hoffman JI and Kaplan S: The incidence of
congenital heart disease. J Am Coll Cardiol. 39:1890–1900. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wenxiang D: Status of paediatric cardiac
surgery in China. Heart Lung Circ. 10:16–19. 2001. View Article : Google Scholar
|
|
3.
|
Suh YA, Arnold RS, Lassegue B, Shi J, Xu
X, Sorescu D, Chung AB, Griendling KK and Lambeth JD: Cell
transformation by the superoxide-generating oxidase Mox1. Nature.
401:79–82. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ,
Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J and Kim SS: NOX2
stimulates muscle differentiation via NF-κB/iNOS pathway. Free
Radic Biol Med. 38:989–1001. 2005.PubMed/NCBI
|
|
5.
|
Pedruzzi E, Guichard C, Ollivier V, et al:
NAD(P)H oxidase NOX-4 mediates 7-ketocholesterol-induced
endoplasmic reticulum stress and apoptosis in human aortic smooth
muscle cells. Mol Cell Biol. 24:10703–10717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Van Rooij E and Olson EN: MicroRNAs:
powerful new regulators of heart disease and provocative
therapeutic targets. J Clin Invest. 117:2369–2376. 2007.PubMed/NCBI
|
|
7.
|
Bird A: DNA methylation pattern and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar
|
|
8.
|
Zhu C, Yu ZB, Chen XH, Pan Y, Dong XY,
Qian LM and Han SP: Screening for differential methylation status
in fetal myocardial tissue samples with ventricular septal defects
by promoter methylation microarrays. Mol Med Rep. 4:137–143.
2011.
|
|
9.
|
Herman JG, Graff JR, Myohanen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: a novel PCR assay for
methylation status of CpG islands. Proc Natl Acad Sci USA.
93:9821–9826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Antequera F and Bird A: Number of CpG
islands and genes in human and mouse. Proc Natl Acad Sci USA.
90:11995–11999. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Suzuki MM and Bird A: DNA methylation
landscapes: provocative insights from epigenomics. Nat Rev Genet.
9:465–476. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dal C and Guldberyg P: DNA methylation
analysis techniques. Biogerontology. 4:233–250. 2003. View Article : Google Scholar
|
|
13.
|
Tsumura A, Hayakawa T, Kumaki Y,
Takebayashi S, Sakaue M, Matsuoka C, Shimotohno K, Ishikawa F, Li
E, Ueda HR, Nakayama J and Okano M: Maintenance of self-renewal
ability of mouse embryonic stem cells in the absence of DNA
methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells.
11:805–814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Okano M, Bell DW and Haber DA: DNA
methyltransferases Dnmt3a and Dnmt3b are essential for de novo
methylation and mammalian development. Cell. 99:247–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Mayer W, Niveleau A, Walter J, Fundele R
and Haaf T: Embryogenesis-demethylation of the zygotic paternal
genome. Nature. 403:501–502. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Burn J, Brennan P, Little J, et al:
Recurrence risks in offspring of adults with major heart defects:
results from first cohort of British collaborative study. Lancet.
351:311–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mehregan M, Choy MK, Goddard M, Bennett
MR, Down TA and Foo RS: Differential DNA methylation correlates
with differential expression of angiogenic factors in human heart
failure. Plos One. 5:e85642010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Heymes C, Bendall JK, Ratajczak P, Cave
AC, Samuel JL, Hasenfuss G and Shah AM: Increased myocardial NADPH
oxidase activity in human heart failure. J Am Coll Cardiol.
41:2164–2171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Krijnen PA, Meischl C, Hack CE, Meijer CJ,
Visser CA, Roos D and Niessen HW: Increased Nox2 expression in
human cardiomyocytes after acute myocardial infarction. J Clin
Pathol. 56:194–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cheng G, Cao Z and Xu X: Homologs of
gp91phox: cloning and tissue expression of NOX3, NOX4, and NOX5.
Gene. 269:131–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bánfi B, Molnár G, Maturana A, Steger K,
Hegedûs B, Demaurex N and Krause KH: A Ca(2+)-activated NADPH
oxidase in testis, spleen, and lymph nodes. J Biol Chem.
276:37594–37601. 2001.
|
|
22.
|
BelAiba RS, Djordjevic T, Petry A, Diemer
K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C and Görlach
A: NOX5 variants are functionally active in endothelial cells. Free
Radic Biol Med. 42:446–459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Guzik TJ, Chen W, Gongora MC, Guzik B, Lob
HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski
J and Harrison DG: Calcium-dependent NOX5 nicotinamide adenine
dinucleotide phosphate oxidase contributes to vascular oxidative
stress in human coronary artery disease. J Am Coll Cardiol.
52:1803–1809. 2008. View Article : Google Scholar
|
|
24.
|
Fulton DJ: Nox5 and the regulation of
cellular function. Antioxid Redox Signal. 11:2443–2452. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kaynak B, von Heydebreck A, Mebus S,
Seelow D, Hennig S, Vogel J, Sperling HP, Pregla R,
Alexi-Meskishvili V, Hetzer R, Lange PE, Vingron M, Lehrach H and
Sperling S: Genome-wide array analysis of normal and malformed
human hearts. Circulation. 107:2467–2474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maru Y, Nishino T and Kakinuma K:
Expression of Nox genes in rat organs, mouse oocytes, and sea
urchin eggs. DNA Seq. 16:83–88. 2005.PubMed/NCBI
|
|
27.
|
Khalil A, Trehan R and Tiwari A:
Immunological profile in congenital heart disease. Indian Pediatr.
31:295–300. 1994.
|