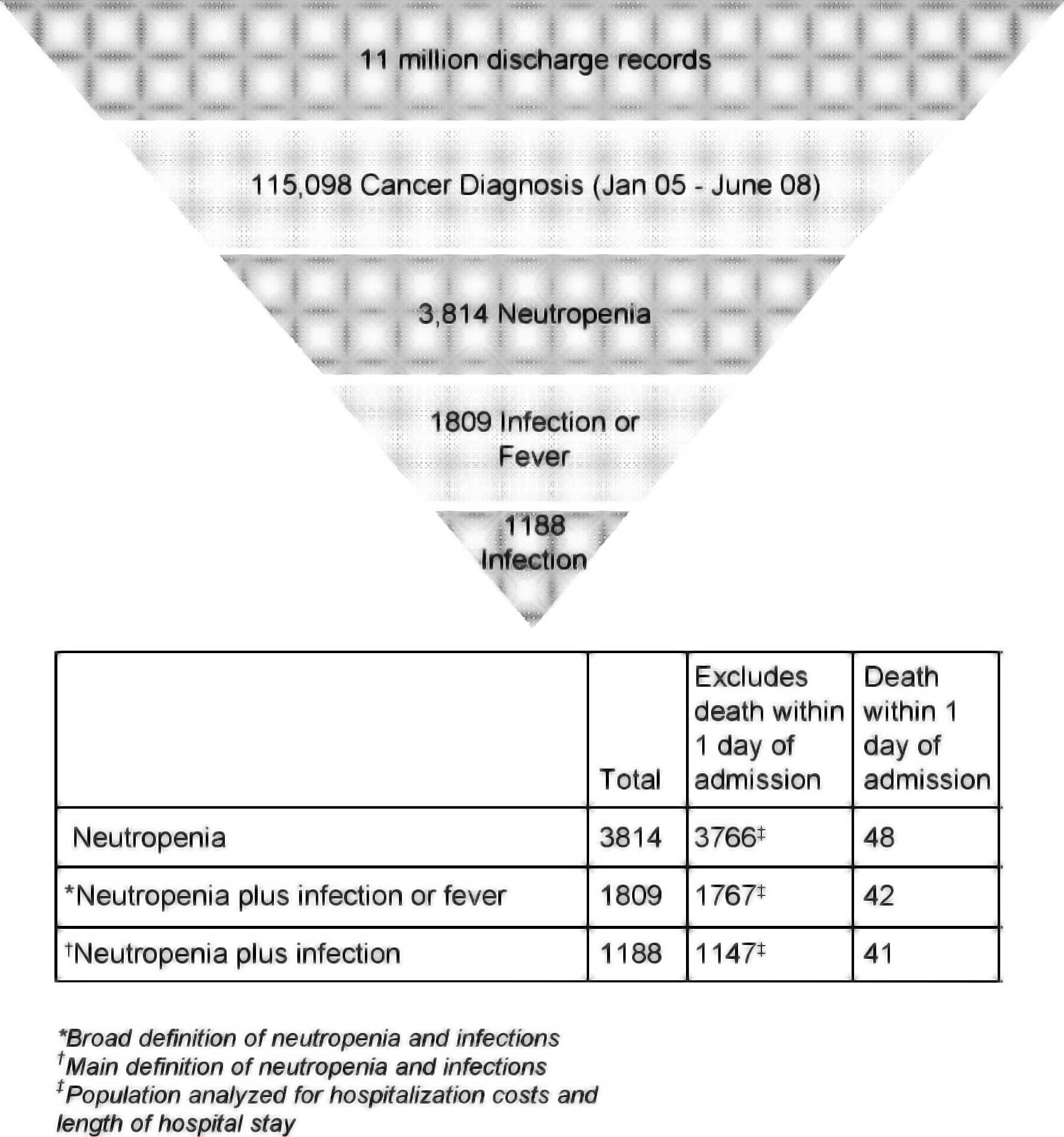
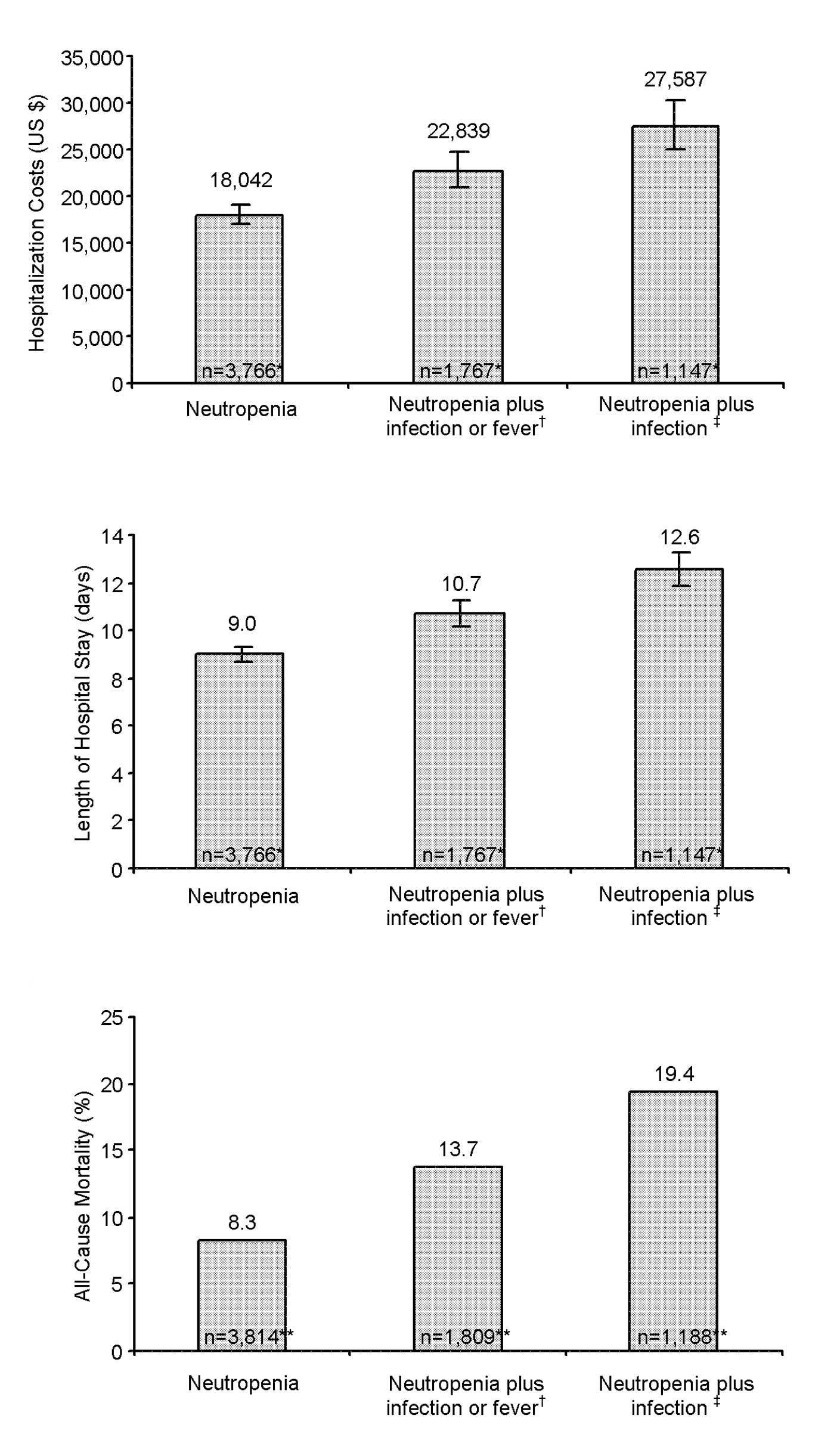
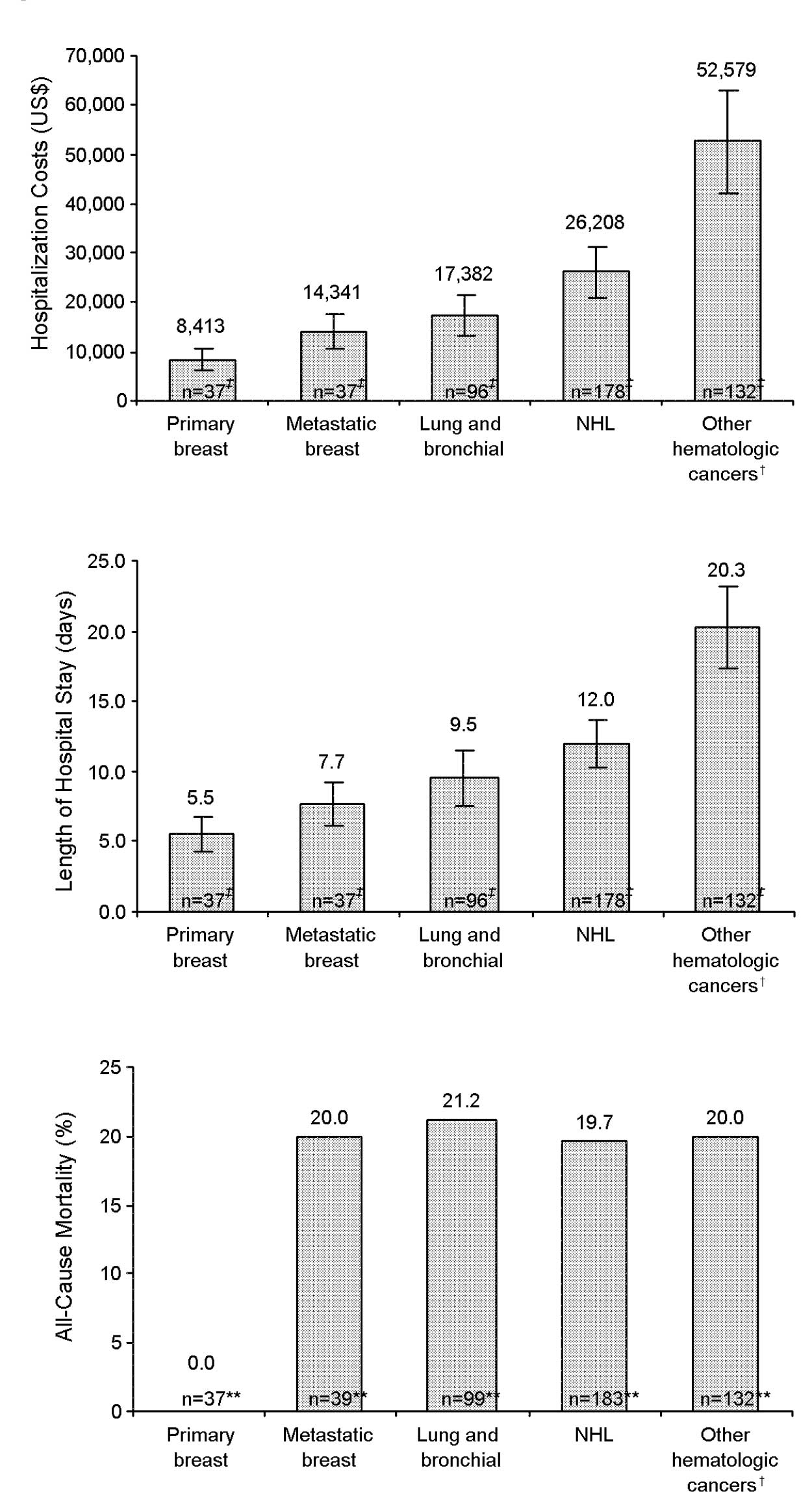
|
1.
|
Kuderer NM, Dale DC, Crawford J, et al:
Mortality, morbidity, and cost associated with febrile neutropenia
in adult cancer patients. Cancer. 106:2258–2266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Link BK, Budd GT, Scott S, et al:
Delivering adjuvant chemotherapy to women with early-stage breast
carcinoma: current patterns of care. Cancer. 92:1354–1367. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Adida C, Haioun C, Gaulard P, et al:
Prognostic significance of surviving expression in diffuse large
B-cell lymphomas. Blood. 96:1921–1925. 2000.
|
|
4.
|
Lyman GH, Dale DC and Crawford J:
Incidence and predictors of low dose-intensity in adjuvant breast
cancer chemotherapy: a nationwide study of community practices. J
Clin Oncol. 21:4524–4531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hryniuk WM: Average relative dose
intensity and the impact on design of clinical trials. Semin Oncol.
14:65–74. 1987.PubMed/NCBI
|
|
6.
|
Klastersky J, Paesmans M, Rubenstein EB,
et al: The Multinational Association for Supportive Care in Cancer
risk index: a multinational scoring system for identifying low-risk
febrile neutropenic cancer patients. J Clin Oncol. 18:3038–3045.
2000.PubMed/NCBI
|
|
7.
|
Talcott JA, Siegel RD, Finberg R and
Goldman L: Risk assessment in cancer patients with fever and
neutropenia: a prospective, two-center validation of a prediction
rule. J Clin Oncol. 110:316–322. 1992.PubMed/NCBI
|
|
8.
|
Smith TJ, Khatcheressian J, Lyman GH, et
al: 2006 update of recommendations for the use of white blood cell
growth factors: an evidence-based clinical practice guideline. J
Clin Oncol. 24:3187–3205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bonadonna G, Moliterni A, Zambetti M, et
al: 30 years’ follow up of randomized studies of adjuvant CMF in
operable breast cancer: cohort study. BMJ. 330:2172005.
|
|
10.
|
Epelbaum R, Faraggi D, Ben-Arie Y, et al:
Survival of diffuse large cell lymphoma. A multivariate analysis
including dose intensity variables. Cancer. 66:1124–1129. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kwak LW, Halpern J, Olshen RA and Horning
SJ: Prognostic significance of actual dose intensity in diffuse
large-cell lymphoma: results of a tree-structured survival
analysis. J Clin Oncol. 8:963–977. 1990.PubMed/NCBI
|
|
12.
|
Lepage E, Gisselbrecht C, Haioun C, et al:
Prognostic significance of received relative dose intensity in
non-Hodgkin’s lymphoma patients: application to LNH-87 protocol.
The GELA (Groupe d’Etude des Lymphomes de l’Adulte). Ann Oncol.
4:651–656. 1993.PubMed/NCBI
|
|
13.
|
Weycker D, Malin J, Edelsberg J, Glass A,
Gokhale M and Oster G: Cost of neutropenic complications of
chemotherapy. Ann Oncol. 19:454–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Caggiano V, Weiss RV, Rickert TS and
Linde-Zwirble WT: Incidence, cost, and mortality of neutropenia
hospitalization associated with chemotherapy. Cancer.
103:1916–1924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Health Insurance Portability and
Accountability Act of 1996, 42 USC. pp. 1320–1322. 1996
|
|
16.
|
US Dept of Health and Human Services:
Public Welfare-Protection of Human Subjects, 45 CFR 46. https://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm.
Accessed Aug 12, 2009.
|
|
17.
|
Weycker D, Malin J, Kim J, et al: Risk of
hospitalization for neutropenic complications of chemotherapy in
patients with primary solid tumors receiving pegfilgrastim or
filgrastim prophylaxis: a retrospective cohort study. Clin Ther.
31:1069–1081. 2009. View Article : Google Scholar
|
|
18.
|
Gold M, Siegel JE, Russell LB and
Weinstein MC: Cost-Effectiveness in Health and Medicine. Oxford
University Press; New York: pp. 91–92. 1996
|
|
19.
|
Center for Medicare and Medicaid Services:
Eliminating serious, preventable, and costly medical errors-never
events, CMS Fact Sheet, May, 18, 2006. https://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=1863&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=false&cboOrder=datehttps://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=1863&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=false&cboOrder=date.
Accessed Oct 5, 2009.
|
|
20.
|
National Comprehensive Cancer Network
clinical practice guidelines in oncology: Myeloid growth factors in
cancer treatment, version 2.2005[online]. www.nccn.org/professionals/physician_gls/PDF/myeloid_growth.pdf.2005.
Accessed Mar 12, 2009.
|
|
21.
|
Glaspy J, Hackett J, Flyer P, et al:
Febrile neutropenia is associated with an increase in the
incidence, duration, and severity of chemotherapy toxicities.
Blood. 98:432b2001.
|
|
22.
|
Brown RE, Hutton J and Burrell A: Cost
effectiveness of treatment options in advanced breast cancer in the
UK. Pharmacoeconomics. 19:1091–1102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Fortner BV, Stolshek B, Tauer KW, et al:
Final analysis: chemotherapy-induced neutropenia (CIN) is
associated with lower quality of life (QoL) in patients (pts) with
cancer. Ann Oncol. 13(Suppl 15): 1742002.
|
|
24.
|
Okon TA, Fortner BV, Schwartzberg L, et
al: Quality of life (QOL) in patients with grade IV
chemotherapy-induced neutropenia (CIN). Proc Am Soc Clin Oncol.
21:275b2002.
|