Introduction
Acute gastric mucosal injury is a serious clinical
problem worldwide. The symptoms cause severe gastric ulceritis and,
with sustained exposure, eventually lead to gastric cancer.
Researchers have developed many in vivo gastric lesion
models: the water immersion restraint (WIRE) stress model, the
indomethacin-induced model and the ethanol-induced model using
animals (1). The WIRE model has
previously been used to observe various aspects affecting the
formation of and recovery from gastric mucosal lesions, including
sulphydryls, prostaglandins, growth factors and polyamines
(2). WIRE stress-induced gastric
lesions have also been used to study the roles of cell death and
gastric acid secretion in ulcerogenesis (3).
In general terms, gastric lesions are a disorder of
the gastric blockade, which typically protects against cavernous
problems caused by hydrogen ions and other toxic substances
generated in the lumen (4). One
main component of the gastric barrier is gastric microcirculation;
a disturbance in gastric mucosal perfusion results in the formation
of lesions and ulcers, such as those that present in animal models
of ischemic gastric lesions (5).
Gastric blood flow is classically controlled by signaling
molecules, such as prostaglandins and nitric oxide (NO), which play
key roles in sustaining mucosal tissue integrity (6). NO is produced by nitric oxide
synthase in particular, which is generally classified into two
categories: i) constitutive nitric oxide synthase (cNOS) and ii)
inducible nitric oxide synthase (iNOS). The cNOS isoforms include
endothelial nitric oxide synthase (eNOS), which controls vascular
homeostasis in endothelial cells, platelets and mesangial cells
(7), and neuronal nitric oxide
synthase (nNOS), which produces NO to act as a neurotransmitter in
central and peripheral nerve cells (8). The third isoform, iNOS, is a
calcium-independent enzyme which can be found in various human
tissues, including immune cells (9). The NOS isoforms have been
characterized in the gastrointestinal tract, and NO produced from
cNOS and iNOS has been shown to play a key role in the formation of
gastric lesions (10).
Many studies have investigated methods for curing
and/or preventing gastric lesions with chemically synthesized drugs
(11). Recently, it has also been
reported that many traditional Asian herbal remedies act on
gastrointestinal diseases (12).
Acer mono Max. sap (AmMs) has been used in East Asian
countries for centuries due to its anti-osteoporosis effects
(13); however, traditional East
Asian medical literature claims that the sap has potential
anti-oxidative and immunomodulatory efects (14,15).
Previous in vitro and in vivo studies have confirmed
that constituents from AmMs have anti-osteoporosis effects by
increasing calcium ion absortion (13), but no prior study has investigated
whether or not AmMs has gastroprotective effects.
Therefore, we investigated whether AmMs exhibits
protective effects against WIRE stress-induced gastric lesions in
mice as well as its effects activated through changes in iNOS/nNOS
and COX-2 mRNA expression, as their expression may be an indicator
controlling the status of WIRE-induced gastric ulceration.
Materials and methods
Chemicals
TRIzol® reagent was purchased from
Invitrogen (Carlsbad, CA, USA). L-arginine and omeprazole
{5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]
sulfinyl]-1H-benzimidazole; MW, 345.42} were obtained from
Sigma-Aldrich Co. (St. Louis, MO, USA) as positive controls.
QA-Agarose and the Maxime RT-PCR PreMix kit were purchased from
Qbiogene, Inc. (Irvine, CA, USA) and iNtRON Biotechnology (Sungnam,
Korea), respectively. All other chemicals were molecular biology
grade.
Preparation and treatment with AmMs
The sap (1,000 ml) of Acer mono Max. was
collected from a habitat of Acer mono on Mt. Myun Bong
(altitude 550 m), Juk Jang Myun, Pohang, Korea, between February 24
and 25, 2008. The collected AmMs was centrifuged and aseptically
filtered using a membrane filter (0.45 μm), and then stored at
−70°C until it was freeze-dried to obtain a powder. Freeze-drying
was carried out using a lyophilizer (Il-Shin BioBase, FMCD series,
Seoul, Korea) overnight, and 13.5 g of powder was finally obtained.
The color of the freeze-dried powder was pale white. The voucher
specimen was deposited in the Food Enzyme Biotechnology Laboratory,
Kyungpook National University, Daegu, Korea. The mineral content
(mg/l) showed that the sap contained high amounts of calcium
(36.5-fold), potassium (17-fold) and magnesium (4-fold) [(13) and data not shown].
Animal care and experiment
Six-week-old male Balb/c SPF mice, weighing 20–23 g,
were purchased from Samtaco Korea (Osan, Korea) and were fed a
commercial diet (Purina, Korea) and given water ad libitum.
They were housed in an air-conditioned animal room at a temperature
of 22±1°C and a humidity of 55±5%. All procedures were performed in
compliance with the rules and in-house guidelines for animal
experiments, including ethical care under the guidance of the
university committee (16), and
the guidelines of the International Association for the Study of
Pain Committee for Research and Ethical Issues (17). Animals were allowed to acclimate to
the laboratory atmosphere for at least 1 week prior to the
experiments. The number of each experimental group was limited to
six.
Induction of gastric mucosal lesions
The WIRE stress-induced gastric lesion model was
produced as previously described (18). The mice were randomly separated
into control (no treatment), L-arginine (300 mg/kg), omeprazole (3
mg/kg) and AmMs (30 or 150 mg/kg) groups (6 mice per group).
L-arginine was utilized as a positive control since it exhibits
potent protective effects against acute gastric mucosal induction
(18). Omeprazole is also well
known as an anti-ulcerative worldwide (19). Prior to treatment of the mice, food
was withdrawn for 24 h, while water was provided ad libitum.
The L-arginine-treated mice (n=6) received one intraperitoneal
(i.p.) injection of L-arginine or omeprazole (each 3 mg/kg). After
administration of AmMs (30 or 150 mg/kg, p.o.) to the AmMs-treated
mice (n=6), the animals were maintained in their cages for 1 h to
allow metabolization. Individual cages were vertically immersed in
a water bath (temperature exposure 21°C) to the level of the
xyphoid process. The mice were denied access to food or drink
during the experiment. The mice were exposed for 6 h to WIRE stress
and then euthanized with diethyl ether, and stomach tissues were
extracted. Each tissue was engraved along the curvature, and the
gastric mucosa was removed using a pair of anatomical scissors. The
mucosal tissues were carefully examined for lesions at the
glandular part of the surface. Blood was also gathered from the
inferior vena cava of each mouse for blood analysis.
Determination of the ulcer index
Mucosal lesions were counted as previously reported
(20). In brief, 1 point was
assigned for small round hemorrhagic corrosions; 2 points were
assigned when the length of the hemorrhagic corrosions was <1
mm; 3 points were assigned when the length was 1–2 mm; 4 points
were assigned when the length was 2–3 mm; and 5 points were
assigned when the length was >4 mm. This score was then added
and doubled when the width of the corrosions was >1 mm.
Reverse transcription-polymerase chain
reaction analysis of mRNA expression
Frozen gastric tissues (100 mg) were normalized in 1
ml TRIzol® reagent, and total RNA was arranged as
previously described (21). The
total RNA was measured using a spectrophotometer at 260 nm
(VICTOR3; Perkin Elmer, Wellesley, MA, USA), and the RNA was
quantified by 1% formaldehyde-agarose gel electrophoresis. The
samples were then stored at −80°C until use. Single-step RT-PCR was
carried out by specific primer sets using the Maxime RT-PCR PreMix.
These primers were constructed using the Primer3 software
(Whitehead Institute, MIT Center for Genome Research, Cambridge,
MA, USA) to evaluate sequences deposited in the NCBI GenBank
database. The primers were synthesized by Bioneer Co. (Daejeon,
Korea), as previously described (18). Briefly, first-strand cDNA was
reverse-transcribed from 200 ng of total RNA at 45°C for 30 min;
the samples were denatured for 5 min at 95°C; and PCR amplification
was executed with either 32 cycles (eNOS, nNOS and iNOS) or 27
cycles (18s RNA) of 45 sec at 95°C, 45 sec at 54°C for eNOS, 55°C
for nNOS, 45 sec at 53°C for iNOS and 60 sec at 72°C. Following the
amplification process, 20 μl of each RT-PCR product was determined
by 1.5% agarose gel electrophoresis and visualized by EtBr
staining. The position of the predicted product was verified by
comparison to a 1-kb DNA ladder (Solgent, Daejeon, Korea). The
relative amounts of cDNA in each matched set were normalized with
regard to 18s RNA expression using the Molecular Imager Gel Doc XR
System and Quantity One 1-D analysis software (Bio-Rad,
Philadelphia, PA, USA).
Statistical analysis
Results were presented as the means ± standard
deviation (SD) of three independent values. Statistical
significance was determined by the Student-Newman-Keuls method for
independent means, using the Sigma Plot program (22). The critical level for significance
was set at p<0.05; p<0.05 was considered statistically
significant.
Results
Effect of AmMs on WIRE stress-induced
gastric mucosal damage
One hour prior to the initiation of WIRE stress,
negative control mice received a single saline administration p.o.,
whereas the positive control mice received a single i.p. injection
of L-arginine (300 mg/kg) or p.o. administration of omeprazole (3
mg/kg). AmMs-treated mice received a single p.o. administration of
AmMs (30 or 150 mg/kg). After 6 h of WIRE-induced stress, all mice
were euthanasized and their stomach tissues were examined for
gastric injury, which was visualized as either disrupted or intact
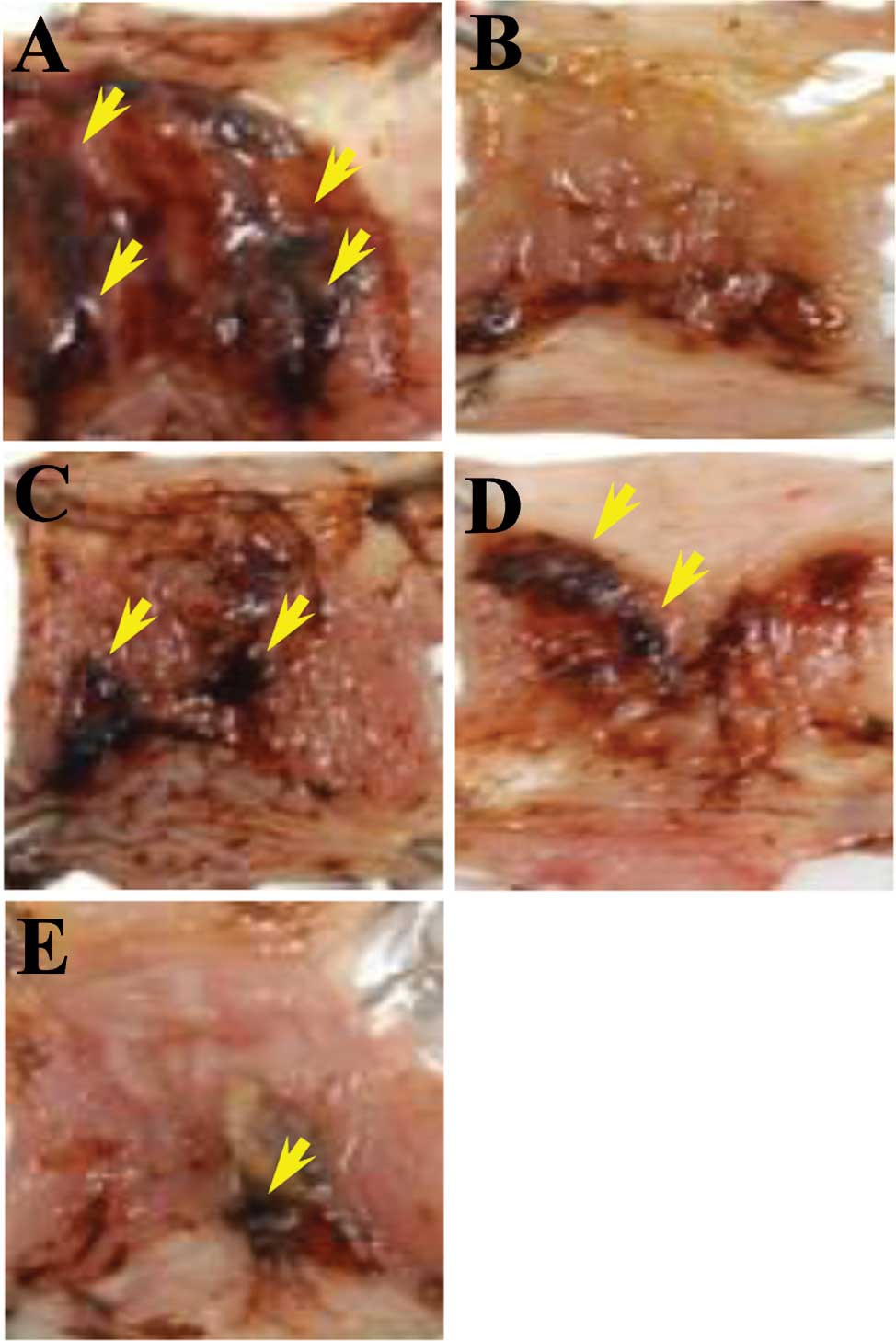
forms. In tissue samples from the stress-induced control, abundant
lesions were observed, most often 1–2 mm in size (Fig. 1A). By contrast, tissue samples from
the L-arginine- or omeprazole-treated mice presented very few
lesions (Fig. 1B and C) while the
stress-induced control group exhibited large-sized
blood-coagulating bunches (Fig.
1A). The positive control exhibited only small-sized bunches
with minor lesions (Fig. 1B and
C), which were not significant when compared to the
stress-induced control. Regardless of the dose, AmMs-treated mice
did not present any small- or large-sized corrosions (Fig. 1D and E; and data not shown). By
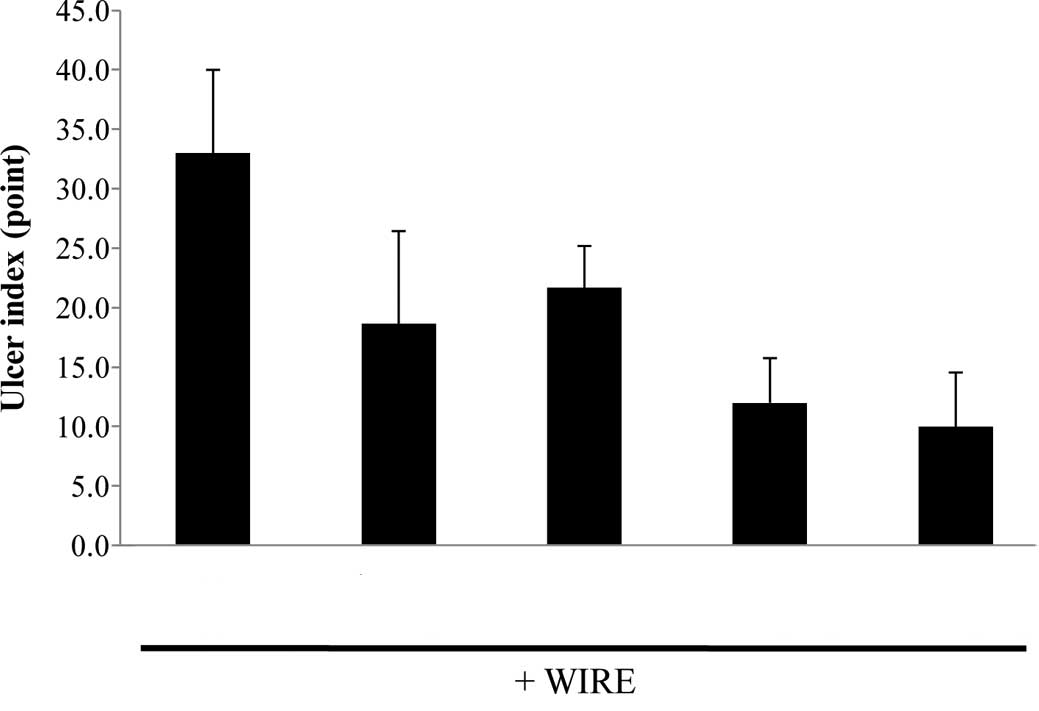
counting mucosal lesions according to a previously described manner
with a slight modification (20),
the ulcer index was calculated as shown in Fig. 2. The ulcer index of the
stress-induced control group was 23.0±4.1, whereas the indices of
the positive controls (L-arginine or omeprazole) were 8.0±5.5 and
8.0±5.5, respectively. Collectively, these findings reveal that
AmMs protects against WIRE stress-induced gastric mucosal lesions
in mice.
We next investigated how AmMs affects stress-induced
ulcers in mice. Classically, most anti-ulcer effects involve the
blockage of acid production on stomach epithelial cells during
stress onset. Therefore, we determined whether the sap reduces the
secretion of gastric acid. Treatment with sap slightly decreased
the volume of 0.1 N NaOH, which is required to neutralize the
gastric juice in the stomach, in a concentration-dependent manner
(data not shown), while the total volume was reduced, but not
dramatically, to 5.3%. This revealed that the sap partially
inhibits gastric secretion, indicating that other beneficial
effects of AmMs exist.
Expression profiles of mRNA of
anti-inflammatory enzymes
We investigated whether the detected changes in
serum and gastric tissue-derived enzyme levels were attributable to
changes in the expression levels of inflammation-related enzymes.
RT-PCR was used to scrutinize the mRNA expression levels of the
genes encoding these anti-inflammatory enzymes, such as NOS and
matrix metalloproteinases (MMPs). As shown in Fig. 3, the expression levels of CSE, BTC
and EGF were not altered in the various mouse groups. Fold
expression of cyclooxygenase-1 and -2 and MMP-2, -3 and -9 were
also not altered at the mRNA level, when compared to 18s RNA used
as a control. Fig. 4A shows that
the 305-bp gene product matching to iNOS cDNA was obviously reduced
in ulcerative tissues from the WIRE-induced tissues, whereas the
levels of the 307-bp eNOS band were similar to those of 18s RNA,
regardless of AmMs treatment. This fact was assessed by the
relative band intensity of mRNA. As a result, we found that the
iNOS gene product in the AmMs-treated (150 mg/kg) tissues was
5-fold lower than that of the tissues in the WIRE stress-induced
group, while the iNOS gene product in the omeprazole-treated mice
was decreased by 2-fold (Fig. 4B).
Notably, nNOS expression was dramatically decreased by treatment
with 150 mg/kg AmMs when compared to the positive controls
(Fig. 4A, third panel). This
decrease in the nNOS mRNA expression was ∼30% when compared with
the control, whereas the expression in the positive controls did
not change (Fig. 4C; columns 1, 2
and 3 vs. 5). These results indicate that AmMs ameliorates gastric
ulcers caused by the stress-induced WIRE model via a decrease in
nNOS as well as iNOS mRNA expression.
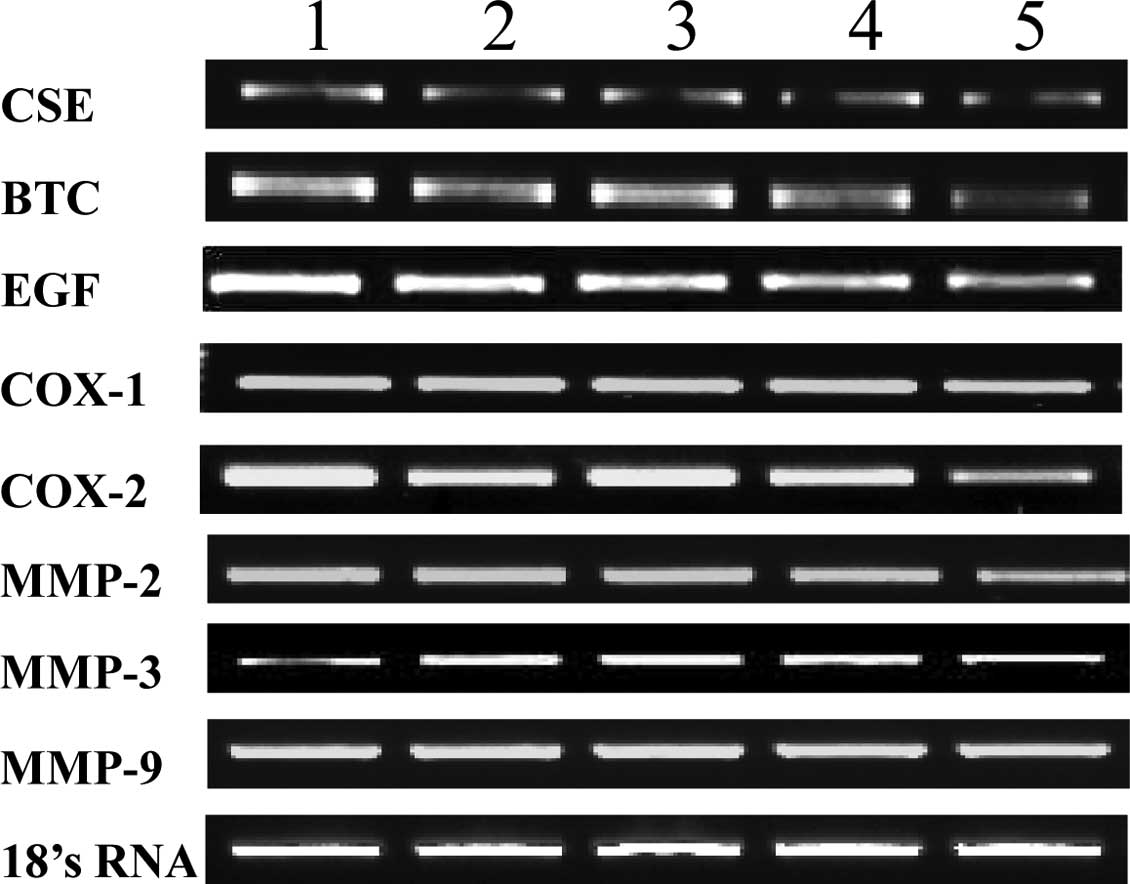 | Figure 3.mRNA expression levels of various
target proteins in AmMs-treated ulcerative tissues. mRNA expression
levels of CSE, BTC, EGF, COX-1, COX-2, MMP-2, MMP-3, MMP-9 and 18s
rRNA are shown. Lane 1, control group; lane 2, L-arginine-treated
group (300 mg/kg, i.p.); lane 3, omeprazole-treated group (3 mg/kg,
i.p.); lanes 4 and 5, AmMs-treated experimental groups (30 and 150
mg/kg, p.o., respectively). |
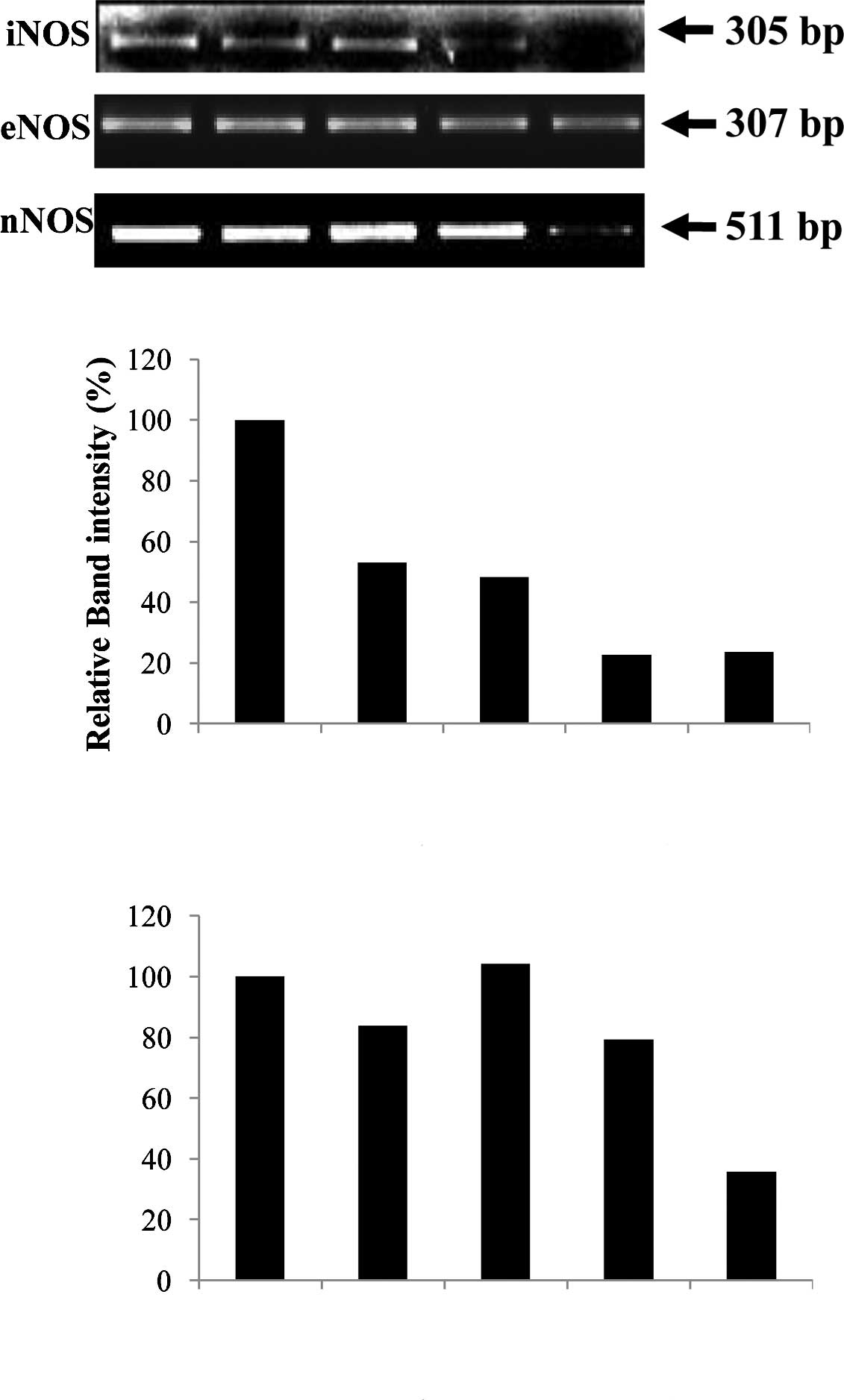 | Figure 4.Comparison of the mRNA expression
levels of NOSs. (A) The expression levels of iNOS, eNOS and nNOS
mRNA were normalized with that of 18s rRNA, using Quantity One
Version 4.6.1. One hour prior to the start of WIRE-induced stress,
control mice received a single saline administration p.o.; positive
control mice received a single i.p. injection of L-arginine (300
mg/kg) or omeprazole (300 mg/kg); and experimental mice received a
single p.o. administration of AmMs (30 and 150 mg/kg). Lane 1,
control group; lane 2, omeprazole-treated group (3 mg/kg, i.p.);
lane 3, L-arginine-treated group (300 mg/kg, i.p.); lanes 4 and 5,
AmMs-treated experimental groups (30 and 150 mg/kg, p.o.,
respectively). Relative band intensity (%) of (B) iNOS and (C) nNOS
comparing the expression levels in the various treatment groups.
The data are the representative results of three independent
experiments. |
Discussion
In the present study, we report for the first time
that treatment with AmMs safeguards against WIRE stress-induced
gastric mucosal injury in mice (Figs.
1 and 2). This finding
suggests that AmMs can be used as an ulcer remedy or for other
preventive and nutraceutical purposes.
Previous studies have revealed that WIRE
stress-induced gastric mucosal damage involves increased gastric
juice secretion, gastric peristalsis, gastrocirculation
disturbances in mucosal tissues, reduced prostaglandin levels, low
turnover of gastric mucosa and NO-induced changes in gastric mucus
cells (23–25). Among these patterns, NO-mediated
regulation is particularly important as it is associated with host
defense and inflammatory responses (26) and also plays a pivotal role in
gastric mucosal protection against damage induced by ethanol,
stress, endotoxins and drugs (27). NOS activity is particularly high in
gastric tissues (28), where NO
helps maintain gastric tissue integrity (29), controls gastromucosal-derived blood
flow (30) and increases gastric
mucus synthesis and secretion (28). L-arginine, a NOS substrate, has
been shown to facilitate gastric ulcer healing by increasing cell
regeneration in stomach tissues (31). In addition, inhibition of NOS
activity has been shown to intensify the stimulatory effects of NO
on gastric mucus synthesis and secretion, to inhibit angiogenesis
and to suppress ulcer healing (10,32).
In the present study, we demonstrated several
gastoprotective mechanisms against gastric ulceration as induced by
WIRE-stress mucosal damage. We initially determined whether the sap
inhibits gastric acid secretion. The results revealed that the
gastric acid was neutralized only to 5.3% with AmMs treatment at a
concentration of 20 μg/ml (data not shown). AmMs-treated mice
exposed to WIRE-induced stress had decreased serum and NO contents
in their stomach tissues when compared to the control stressed
mice. Similar results were obtained in the L-arginine-treated mice,
which were used as a positive control based on a previous report
that L-arginine treatment prohibits the development of
stress-induced gastric lesions (31). To analyze a feasible mechanism
causing a decrease in NO content, we examined the expression levels
of different NOS mRNAs. Our results showed that the gastric mucosal
expression of iNOS and nNOS mRNA was significantly lower in the
AmMs-treated mice vs. the control mice, whereas no such difference
was observed in eNOS mRNA expression levels (Figs. 3 and 4). Together these findings indicate that
the protective effects of NOS against WIRE stress-induced gastric
lesions in vivo may originate, at least in part, from the
suppression of NO signaling via decreased iNOS and nNOS mRNA
expression by AmMs.
Previous studies suggest that the significance of
constitutive NOSs in sustaining healthy gastric tissues is
important as these enzymes are prevalent in all types of tissues.
Decreased cNOS activity has been involved in gastric lesions, while
accelerated cNOS mRNA expression, activity and immunoreactivity has
been observed in the healing of gastric mucosa, particularly in
newly formed vessels and neurons (33). Consistent with the knowledge that
eNOS and nNOS are critical factors during the gastric tissue repair
process, a cigarette smoking-mediated decrease in gastromucosal NOS
activity was found to be related to reduced gastric blood flow and
inhibition of cell regeneration in ulcerative tissues (10); however, no changes were observed in
eNOS mRNA expression in the AmMs-treated mice exposed to
WIRE-induced stress (data not shown). Instead, changes were
detected in the mRNA expression levels of iNOS, suggesting that
this isoform of NOS may be involved in the mechanism of
AmMs-induced protection in ulcer tissues.
Large amounts of iNOS-induced NO have been
identified in gastric tissue damage during inflammatory processes
(34), and high levels of iNOS
expression and activity have been related to severe inflammation in
ulcerative mucosal cells (35).
Studies have revealed that iNOS is usually induced under oxidative
reactions, wherein high levels of NO reacts with the superoxide
anion, thus causing peroxynitrite generation, tyrosine-mediated
nitration, hydroxyl radical production, cellular toxicity and
tissue lesions (35,36). In the present study, we found high
levels of iNOS mRNA expression in gastric mucosal lesions in the
control mice, but significantly lower levels in the AmMs-treated
mice, indicating that nutritional factors of AmMs may diminish NO
production by preventing iNOS expression in WIRE-induced stress
conditions. This may then inhibit the abundant release of attacking
NO, leading to beneficial anti-ulcer effects.
In summary, we prove here for the first time that
exogenously administered AmMs protects against the development of
WIRE stress-induced gastric mucosal lesions in mice, and that this
protective effect may be attributable to decreased iNOS/nNOS mRNA
expression and subsequent decreases in damaging NO levels. Although
future studies are required to elucidate the specific mechanisms of
this protective effect, these novel findings suggest a new mode of
action for AmMs, and may imply that the sap of this traditional
tree can be developed for future use in ulcer prevention.
Abbreviations:
|
AmMs,
|
sap of Acer mono Max.;
|
|
WIRE,
|
water immersion restraint test;
|
|
RT-PCR,
|
reverse transcription-polymerase chain
reaction;
|
|
iNOS,
|
inducible nitric oxide synthase;
|
|
eNOS,
|
endothelial nitric oxide synthase;
|
|
nNOS,
|
neuronal nitric oxide synthase;
|
|
cNOS,
|
constitutive nitric oxide
synthase;
|
|
p.o.,
|
per oral;
|
|
i.p.,
|
intraperitoneal
|
Acknowledgements
This study was supported, in part, by
the Kyungpook National University Research Fund. The authors would
like to thank Mr. Sung-Gu Ji and Mr. Hae-Hee Yoon (JukJang Acer
Agricultural Cooperative) for the technical assistance of the AmMs
collection.
References
|
1.
|
MacLaren R, Jarvis CL and Fish DN: Use of
enteral nutrition for stress ulcer prophylaxis. Ann Pharmacother.
35:1614–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mózsik G, Bódis B, Figler M, Király A,
Karádi O, Pár A, Rumi G, Sütõ G, Tóth G and Vincze A: Mechanisms of
action of retinoids in gastrointestinal mucosal protection in
animals, human healthy subjects and patients. Life Sci.
69:3103–3112. 2001.
|
|
3.
|
Alarcón de la Lastra C, Barranco MD,
Motilva V and Herrerías JM: Mediterranean diet and health:
biological importance of olive oil. Curr Pharm Des. 7:933–950.
2001.PubMed/NCBI
|
|
4.
|
Konturek SJ, Konturek PC and Brzozowski T:
Melatonin in gastroprotection against stress-induced acute gastric
lesions and in healing of chronic gastric ulcers. J Physiol
Pharmacol. 57(Suppl 5): 51–66. 2006.PubMed/NCBI
|
|
5.
|
Pawlik M, Ptak A, Pajdo R, Konturek PC,
Brzozowski T and Konturek SJ: Sensory nerves and calcitonin
gene-related peptide in the effect of ischemic preconditioning on
acute and chronic gastric lesions induced by ischemia-reperfusion.
J Physiol Pharmacol. 52:569–581. 2001.
|
|
6.
|
Kwiecieñ S, Brzozowski T, Konturek PC,
Konturek SJ, Pawlik M, Pajdo R, Drozdowicz D, Ptak A and Hahn EG:
Effect of central and peripheral actions of histamine and its
metabolite N-alpha methyl histamine on gastric secretion and acute
gastric lesions. J Physiol Pharmacol. 52:625–638. 2001.PubMed/NCBI
|
|
7.
|
Nishida K, Harrison DG, Navas JP, Fisher
AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW and Murphy TJ:
Molecular cloning and characterization of the constitutive bovine
aortic endothelial cell nitric oxide synthase. J Clin Invest.
90:2092–2096. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Moncada S, Higgs EA and Furchgott R:
International union of pharmacology nomenclature in nitric oxide
research. Pharmacol Rev. 49:137–142. 1997.PubMed/NCBI
|
|
9.
|
Anggard E: Nitric oxide: mediator,
murderer, and medicine. Lancet. 343:1199–1206. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ma L, Chow JY and Cho CH: Cigarette
smoking delays ulcer healing: role of constitutive nitric oxide
synthase in rat stomach. Am J Physiol. 276:238–248. 1999.PubMed/NCBI
|
|
11.
|
La Vecchia C and Tavani A: A review of
epidemiological studies on cancer in relation to the use of
anti-ulcer drugs. Eur J Cancer Prev. 11:117–123. 2002.PubMed/NCBI
|
|
12.
|
Schmeda-Hirschmann G and Yesilada E:
Traditional medicine and gastroprotective crude drugs. J
Ethnopharmacol. 100:61–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee GS, Byun HS, Kim MH, Lee BM, Ko SH,
Jung EM, Gwak KS, Choi IG, Kang HY, Jo HJ, Lee HJ and Jeung EB: The
beneficial effect of the sap of Acer mono in an animal with
low-calcium diet-induced osteoporosis-like symptoms. Br J Nutr.
100:1011–1018. 2008.PubMed/NCBI
|
|
14.
|
Yang H, Lee MK and Kim YC: Protective
activities of stilbene glycosides from Acer mono leaves against
H2O2-induced oxidative damage in primary cultured rat hepatocytes.
J Agric Food Chem. 53:4182–4186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang H, Sung SH and Kim YC: Two new
hepatoprotective stilbene glycosides from Acer mono leaves.
J Nat Prod. 68:101–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Heo JC, Rho JR, Kim TH, Kim SY and Lee SH:
An aqueous extract of green tea Camellia sinensis increases
expression of Th1 cell-specific anti-asthmatic markers. Int J Mol
Med. 22:763–767. 2008.PubMed/NCBI
|
|
17.
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
An SM, Park CH, Heo JC, Park JY, Woo SW,
Seo JH, Lee MS, Cho KJ, Cho HS, Shin HM and Lee S-H: Gastrodia
elata Blume protects against stress-induced gastric mucosal
lesions in mice. Int J Mol Med. 20:209–215. 2007.
|
|
19.
|
Naesdal J, Bodemar G and Walan A: Effect
of omeprazole, a substituted benzimidazole, on 24-h intragastric
acidity in patients with peptic ulcer disease. Scand J
Gastroenterol. 19:916–922. 1984.PubMed/NCBI
|
|
20.
|
Nie SN, Qian XM, Wu XH, Yang SY, Tang WJ,
Xu BH, Huang F, Lin X, Sun DY, Sun HC and Li ZS: Role of TFF in
healing of stress-induced gastric lesions. World J Gastroenterol.
9:1772–1776. 2003.
|
|
21.
|
Roos-van Groningen MC, Eikmans M, Baelde
HJ, de Heer E and Bruijn JA: Improvement of extraction and
processing of RNA from renal biopsies. Kidney Int. 65:97–105.
2004.
|
|
22.
|
Falkeholm L, Grant CA, Magnusson A and
Moller E: Xylene-free method for histological preparation: a
multicentre evaluation. Lab Invest. 81:1213–1221. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kitagawa H, Fujiwara M and Osumi Y:
Effects of water-immersion stress on gastric secretion and mucosal
blood flow in rats. Gastroenterology. 77:298–302. 1979.PubMed/NCBI
|
|
24.
|
Garrick T, Leung FW, Buack S, Hirabayashi
K and Guth PH: Gastric motility is stimulated but overall blood
flow is unaffected during cold restraint in the rat.
Gastroenterology. 91:141–148. 1968.PubMed/NCBI
|
|
25.
|
Lamarque D and Whittle BJ: Involvement of
superoxide and xanthine oxidase in neutrophil-independent rat
gastric damage induced by NO donors. Br J Pharmacol. 116:1843–1848.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Calatayud S, Barrachina D and Esplugues
JV: Nitric oxide: relation to integrity, injury, and healing of the
gastric mucosa. Microsc Res Tech. 53:325–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Nishida K, Ohta Y and Ishiguro I: Role of
gastric mucosal constitutive and inducible nitric oxide synthases
in the development of stress-induced gastric mucosal lesions in
rats. Biochem Biophys Res Commun. 236:275–279. 1997. View Article : Google Scholar
|
|
28.
|
Brown JF, Hanson PJ and Whittle BJ: Nitric
oxide donors increase mucus gel thickness in rat stomach. Eur J
Pharmacol. 223:103–104. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Whittle BJ, Lopez-Belmonte J and Moncada
S: Regulation of gastric mucosal integrity by endogenous nitric
oxide: interactions with prostanoids and sensory neuropeptides in
the rat. Br J Pharmacol. 99:607–611. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Pique JM, Esplugues JV and Whittle BJ:
Endogenous nitric oxide as a mediator of gastric mucosal
vasodilatation during acid secretion. Gastroenterology.
102:168–174. 1992.PubMed/NCBI
|
|
31.
|
Brzozowski T, Konturek SJ, Sliwowski Z,
Drozdowicz D, Zaczek M and Kedra D: Role of L-arginine, a substrate
for nitric oxide-synthase, in gastroprotection and ulcer healing. J
Gastroenterol. 32:442–452. 1997. View Article : Google Scholar
|
|
32.
|
Konturek SJ, Brzozowski T, Majka J,
Pytko-Polonczyk J and Stachura J: Inhibition of nitric oxide
synthase delays healing of chronic gastric ulcers. Eur J Pharmacol.
239:215–217. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Brzozowski T, Konturek PC, Konturek SJ,
Pajdo R, Drozdowicz D, Kwiecien S and Hahn EG: Acceleration of
ulcer healing by cholecystokinin (CCK): role of CCK-A receptors,
somatostatin, nitric oxide and sensory nerves. Regul Pept.
82:19–33. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Barrachina MD, Panes J and Esplugues JV:
Role of nitric oxide in gastrointestinal inflammatory and
ulcerative diseases: Perspective for drug development. Curr Pharm
Des. 7:31–48. 2001. View Article : Google Scholar
|
|
35.
|
Kankuri E, Vaali K, Knowles RG, Lahde M,
Korpela R, Vapaatalo H and Moilanen E: Suppression of acute
experimental colitis by a highly selective inducible nitric-oxide
synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. J
Pharmacol Exp Ther. 298:1128–1132. 2001.
|
|
36.
|
Ding HL, Zhu HF, Dong JW, Zhu WZ, Yang WW,
Yang HT and Zhou ZN: Inducible nitric oxide synthase contributes to
intermittent hypoxia against ischemia/reperfusion injury. Acta
Pharmacol Sin. 26:315–322. 2005. View Article : Google Scholar : PubMed/NCBI
|