Introduction
During the last decade, a number of important genes
responsible for the genesis of various types of cancers have been
discovered, at the same time their mutations have been precisely
established, and the pathway through which they act has been
characterized (1). The mismatch
repair (MMR) pathway, first described in bacteria, is involved in
the maintenance of genomic integrity by repairing DNA replication
errors (2), and MMR proteins
correct base substitution mismatches and small insertion-deletion
mismatches generated during DNA replication (3). The MMR system is well conserved from
Escherichia coli to mammals, and the E. coli MMR
system, where MutS, MutL and MutH complexes function, has been well
analyzed. In mammalian cells, heterodimers of MutS homologues
(MSH2-MSH6 and MSH2-MSH3) recognize replication
errors, and the heterodimer of the MutL homologue
(MLH1-PMS2) interacts with MutS homologues and recruits
further repair proteins (4).
Seven MMR genes exist in humans: MLH1, MLH3,
PMS1, PMS2, MSH2, MSH3 and MSH6 (5). The inactivation of these genes leads
to increased genetic instability, which in turn results in an
increased rate of mutation in ‘gatekeeper’ genes that regulate
human cell proliferation and death (6). A role for hMSH2 in cancer has
been firmly established in hereditary nonpolyposis colorectal
cancer (HNPCC) (7–9). Several single nucleotide
polymorphisms (SNPs), IVS12-6 T>C (rs2303428), G23A (rs4987188)
and IVS10+12 A>G (rs3732183) were identified in the hMSH2
gene and among them, only the IVS12-6 T>C polymorphism was
reported functional and therefore has been extensively studied in
recent years (3,10–21).
The IVS12-6 T>C is a common polymorphism located
at position −6 of the intronic splice acceptor site of exon 13 of
hMSH2. Although the genetic function of this polymorphism
has not been well determined, its variant type may result in
alternative splicing and deficiency of hMSH2 protein
(22,23).
To date, many studies have investigated the role of
this polymorphism in the etiology of cancers of various organs
including the lung, colon, rectum, ovary, and others (3,10–21).
However, the results of these studies remain conflicting rather
than conclusive. Considering the extensive role of hMSH2 in
the carcinogenic process, we performed a meta-analysis on all
eligible case-control studies to estimate the overall cancer risk
of this polymorphism and to quantify the potential between-study
heterogeneity.
Materials and methods
Identification and eligibility of
relevant studies
We searched the electronic literature PubMed for all
relevant reports (the last search update was March 22, 2011), using
the key words ‘MSH2’ or ‘hMSH2’, ‘cancer’ and
‘polymorphism’. The search was limited to English language
manuscripts. In addition, studies were identified by a manual
search of the reference lists of reviews and retrieved studies.
Studies were selected when there were available data for the
hMSH2 IVS12-6 T>C polymorphism with cancer risk in a
case-control design. Additional studies were identified by a hand
search of the references of the original studies. We also used the
PubMed option ‘Related Citations’ in each research article to
search potentially relevant articles. In our meta-analysis, the
studies had to meet the following criteria: i) was a study of the
hMSH2 IVS12-6 T>C polymorphism and cancer risk, ii) used
a case-control design and iii) contained available genotype
frequency.
Data extraction
Two of the authors independently extracted data and
reached a consensus on all of the items. For each study, the
following information was sought: the first author's last name,
year of publication, country of origin, ethnicity, source of
control groups (population- or hospital-based controls), numbers of
genotyped cases and controls, genotyping methods, and cancer type.
Different ethnic descents were categorized as Caucasian, Asian and
mixed (composed of an admixture of different ethnic groups). For
studies including subjects of different ethnic groups, data were
extracted separately for each ethnic group whenever possible.
Statistical analysis
For the control group of each study, the observed
genotype frequencies of hMSH2 IVS12-6 T>C were assessed
for Hardy-Weinberg equilibrium using the χ2 test. The
strength of the association between hMSH2 IVS12-6 T>C and
cancer risk was measured by odds ratios (ORs) with 95% confidence
intervals (CIs). We first estimated the risks of the CC and CT
genotypes on cancers, compared with the wild-type TT homozygote,
and then evaluated the risks of (CC/CT) vs. TT and CC vs. (CT/TT)
on cancers, assuming dominant and recessive effects of the variant
C allele, respectively. In order to evaluate the ethnicity-specific
effect, subgroup analyses were performed by ethnic group. In
consideration of the possibility of heterogeneity across the
studies, a statistical test for heterogeneity was performed based
on the Q-test. When the P-value was >0.10 in the Q-test which
indicates a lack of heterogeneity among studies, the summary OR
estimate of each study was calculated by the fixed-effects model of
Mantel-Haenszel (24). Otherwise,
the random-effects model of DerSimonian and Laird (25) was used. An estimate of potential
publication bias was carried out by the funnel plot, in which the
standard error of log (OR) of each study was plotted against its
log (OR). An asymmetric plot suggested a possible publication bias.
All statistical tests were performed with Stata software (version
10.0; Stata Corporation, College Station, TX, USA).
Results
Characteristics of the studies
Thirteen eligible publications were identified on
the association between the hMSH2 IVS12-6 T>C
polymorphism and cancer risk including 7,527 cancer cases and 8,762
controls (3,10–21).
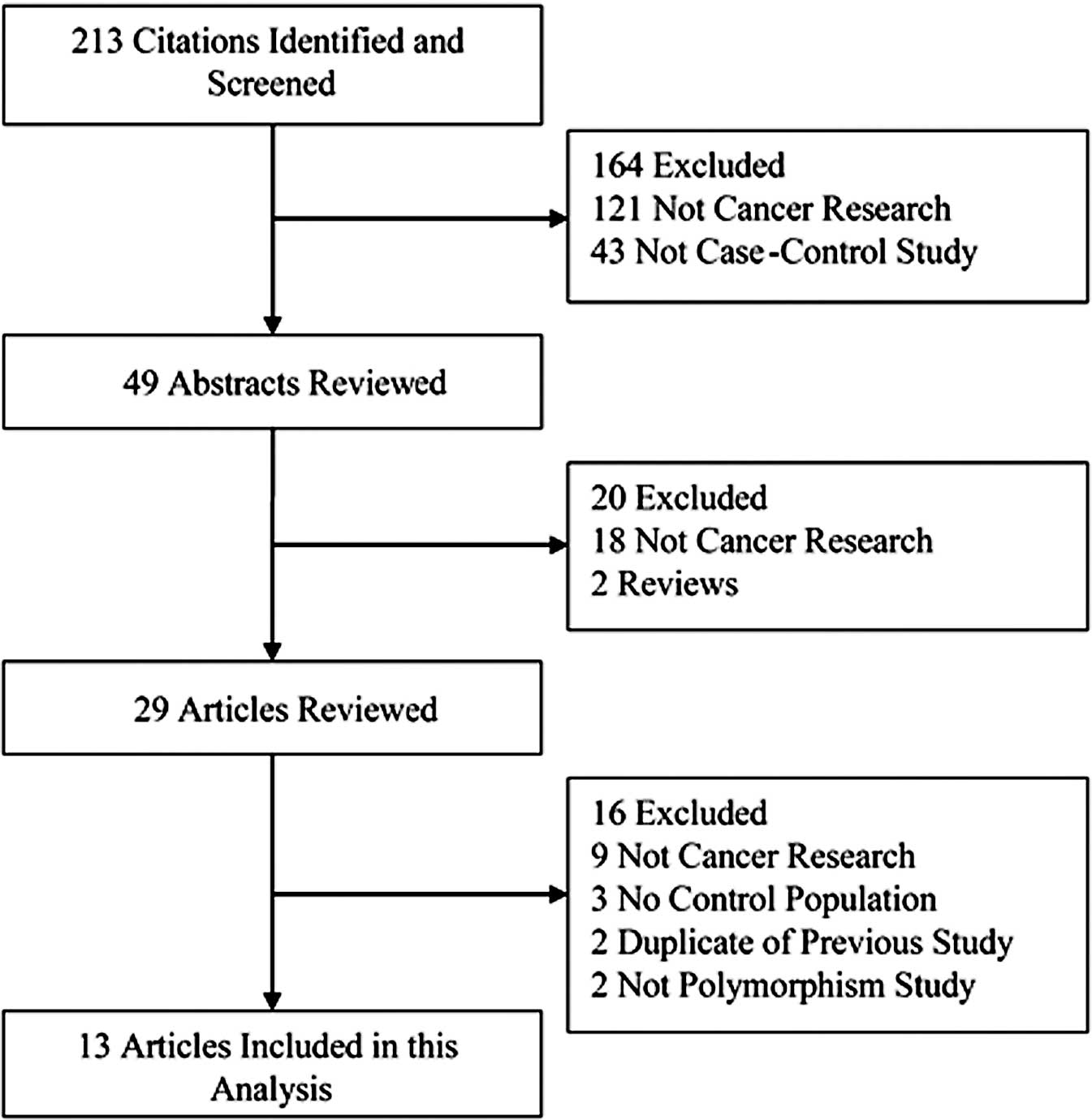
The selected study characteristics are listed in Table I and the criteria for inclusion and
exclusion are shown in Fig. 1. All
studies were case-control studies. There were six studies in
populations of Caucasian descent, four of Asian origin and three of
mixed race. A classic polymerase chain reaction-restriction
fragment length polymorphism (PCR-RFLP) assay was conducted in four
of the 13 studies. In addition, other methods were utilized to
detect genotypes. The genotype distributions among the controls of
all studies were in agreement with Hardy-Weinberg equilibrium for
all except three studies (16,17,21).
 | Table ICharacteristics of the eligible
studies included in the meta-analysis. |
Table I
Characteristics of the eligible
studies included in the meta-analysis.
| First author | Year | Country/Region | Ethnicity | Cancer type | Source of
controls | Genotyping
method | Cases | Controls | HWE |
|---|
| Goessl | 1997 | Germany | Caucasian | Colorectal
cancer | PB | FLUPD | 106 | 125 | 0.540 |
| Paz-y-mino | 2002 | Ecuador | Mixed | Non-Hodgkin's
lymphomas | HB | PCR-SSCP | 22 | 50 | 0.009 |
| Paz-y-mino | 2003 | Ecuador | Mixed | Lymphoma and
leukemia | HB | PCR-SSCP | 181 | 50 | 0.009 |
| Hishida | 2003 | Japan | Asian | Non-Hodgkin's
lymphomas | HB | PCR-RFLP | 103 | 487 | 0.942 |
| Kim | 2004 | Korea | Asian | Colorectal
cancer | PB | TaqMan | 107 | 330 | 0.091 |
| Jung | 2006 | Korea | Asian | Lung cancer | PB | PCR-RFLP | 432 | 432 | 0.613 |
| Beiner | 2006 | Canada | Caucasian | Endometrial
cancer | PB | MALDI-TOF | 665 | 654 | 0.000 |
| Song | 2006 | UK, Denmark,
USA | Caucasian | Ovarian cancer | PB | TaqMan | 1325 | 2044 | 0.411 |
| Hsu | 2007 | Taiwan | Asian | Lung cancer | PB | PCR-RFLP | 156 | 235 | 0.493 |
| Raptisa | 2007 | Canada | Caucasian | Colorectal
cancer | PB | TaqMan | 929 | 1098 | 0.444 |
| Raptisa | 2007 | Canada | Caucasian | Colorectal
cancer | PB | TaqMan | 430 | 275 | 0.195 |
| Koessler | 2008 | UK | Caucasian | Colorectal
cancer | PB | TaqMan | 2294 | 2279 | 0.752 |
| Tulupova | 2008 | Czech | Caucasian | Colorectal
cancer | HB | TaqMan | 611 | 608 | 0.689 |
| Demokan | 2010 | Turkey | Mixed | Head and neck
cancer | HB | PCR-RFLP | 166 | 95 | 0.054 |
Quantitative synthesis
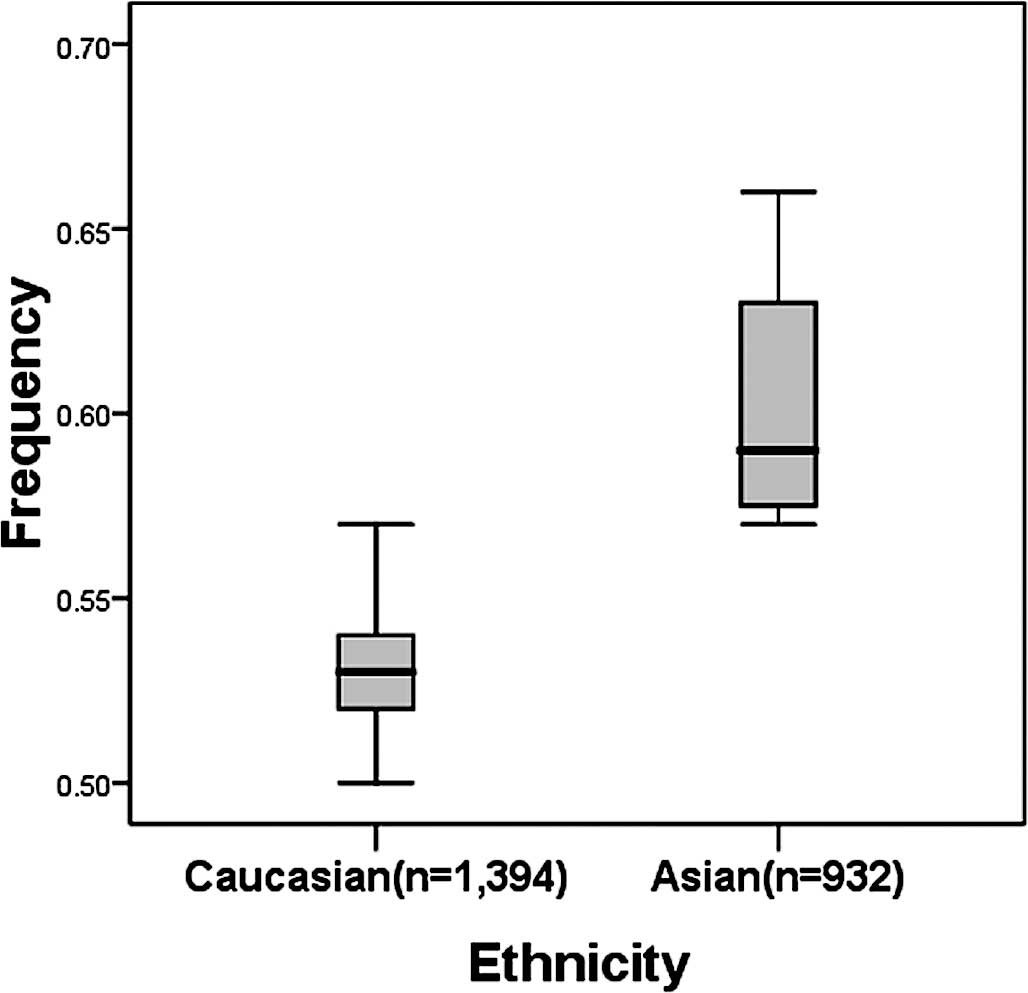
There was a wide variation in the C allele frequency
of the hMSH2 IVS12-6 T>C polymorphism among the controls
across different ethnicities. For Asian populations, the IVS12-6 C
allele frequency was 0.60 (95% CI 0.54–0.67), which was
significantly higher than that in Caucasian populations (0.53; 95%
CI 0.51–0.55; P=0.003) (Fig.
2).
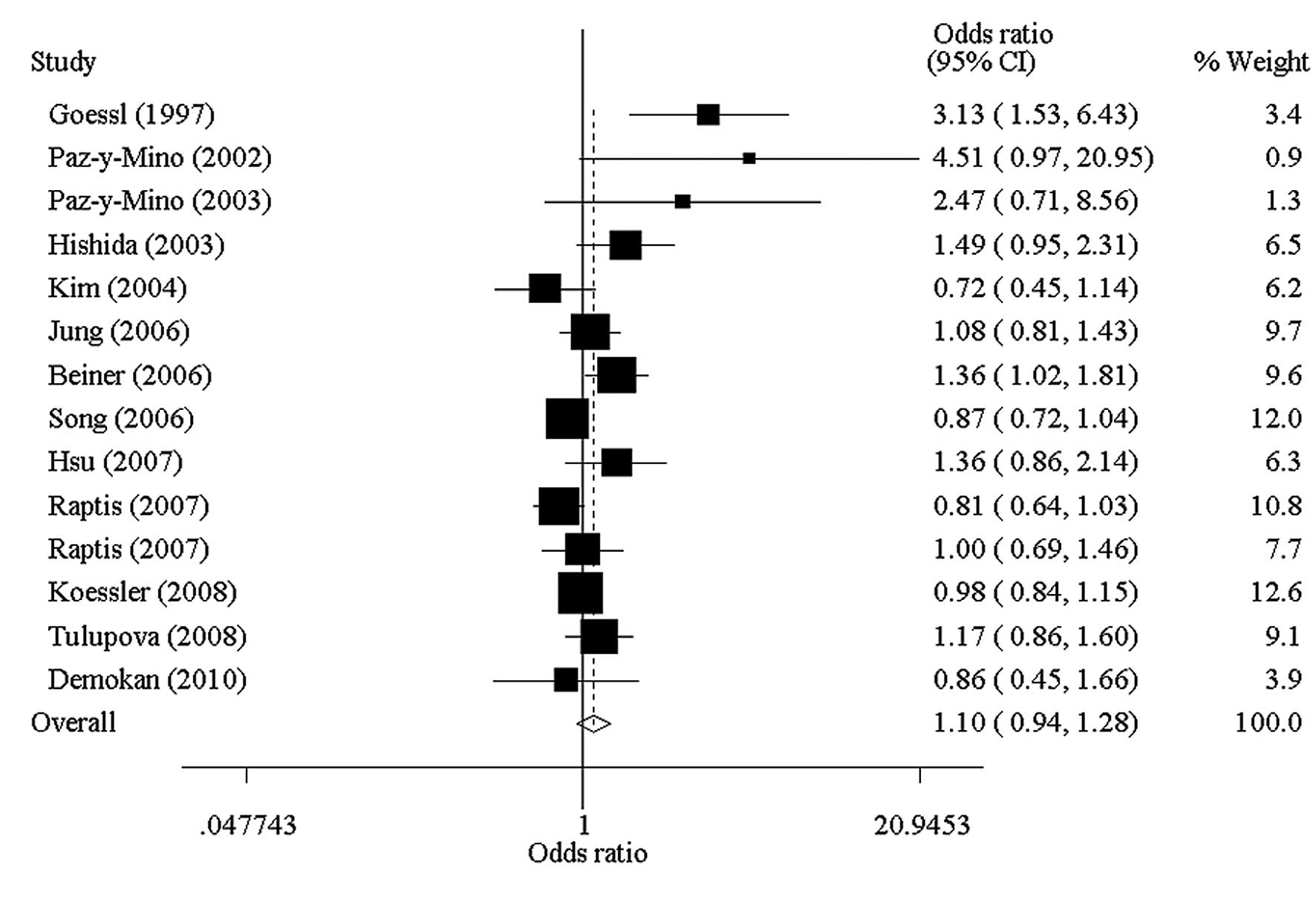
As shown in Table
II, no significant association was found in any genetic model
among studies of these cancers. Forest plot of heterozygote
comparison was given (Fig. 3). In
the stratified analysis by cancer type, significant increased risks
were observed for non-Hodgkin's lymphoma patients (heterozygote
comparison: OR=1.62; 95% CI 1.06–2.47) (Table II). According to the source of the
controls, a significant effect was observed in hospital-based
studies (heterozygote comparison: OR=1.28; 95% CI 1.02–1.61). Among
studies of lung cancer, colorectal cancer and other cancers, no
significant associations were found in any genetic model, in
neither Asian nor Caucasian individuals (Table II).
 | Table IIStratified analyses of the
hMSH2IVS12-6 T>C polymorphism on cancer risk. |
Table II
Stratified analyses of the
hMSH2IVS12-6 T>C polymorphism on cancer risk.
| Variables | n | Cases/controls | CT vs. TT
| CC vs. TT
| CC/CT vs. TT
(dominant)
| CC vs. CT/TT
(recessive)
|
|---|
| OR (95% CI) | P-valuea | OR (95% CI) | P-valuea | OR (95% CI) | P-valuea | OR (95% CI) | P-valuea |
|---|
| Total | 14 | 7527/8762 | 1.10
(0.94–1.28)b | 0.002 | 0.92
(0.74–1.15) | 0.881 | 1.07
(0.93–1.23)b | 0.003 | 0.89
(0.72–1.10) | 0.892 |
| Cancer types | | | | | | | | | | |
| Lung cancer | 2 | 588/667 | 1.15
(0.90–1.46) | 0.392 | 1.02
(0.70–1.46) | 0.818 | 1.11
(0.89–1.40) | 0.477 | 0.93
(0.66–1.30) | 0.878 |
| NHL | 2 | 125/537 | 1.62
(1.06–2.47) | 0.173 | 0.98
(0.43–2.27) | 0.948 | 1.50
(1.00–2.27) | 0.247 | 0.81
(0.36–1.81) | 0.951 |
| Colorectal
cancer | 6 | 4477/4715 | 1.02
(0.82–1.28)b | 0.008 | 1.00
(0.68–1.45) | 0.896 | 1.03
(0.83–1.28)b | 0.008 | 1.04
(0.71–1.51) | 0.867 |
| Other
cancers | 4 | 2337/2843 | 1.09
(0.77–1.55)b | 0.031 | 0.68
(0.42–1.09) | 0.261 | 1.03
(0.73–1.44)b | 0.027 | 0.67
(0.42–1.08) | 0.319 |
| Ethnicities | | | | | | | | | | |
| Asian | 4 | 798/1484 | 1.11
(0.92–1.34) | 0.114 | 1.04
(0.76–1.41) | 0.981 | 1.09
(0.91–1.31) | 0.243 | 0.96
(0.72–1.29) | 0.792 |
| Caucasian | 7 | 6360/7083 | 1.07
(0.88–1.28)b | 0.002 | 0.87
(0.63–1.20) | 0.809 | 1.06
(0.88–1.27)b | 0.002 | 0.87
(0.63–1.20) | 0.863 |
| Mixed | 3 | 369/195 | 1.78
(0.64–4.94)b | 0.080 | 0.25
(0.05–1.14) | 0.637 | 1.43
(0.57–3.64)b | 0.080 | 0.25
(0.05–1.12) | 0.694 |
| Source of
controls | | | | | | | | | | |
|
Hospital-based | 5 | 1083/1290 | 1.28
(1.02–1.61) | 0.214 | 0.78
(0.40–1.51) | 0.444 | 1.22
(0.98–1.52) | 0.223 | 0.70
(0.37–1.33) | 0.520 |
|
Population-based | 9 | 6444/7472 | 1.04
(0.88–1.23)b | 0.003 | 0.94
(0.74–1.19) | 0.899 | 1.03
(0.88–1.20)b | 0.004 | 0.92
(0.73–1.15) | 0.900 |
Test of heterogeneity
There was significant heterogeneity for the
heterozygote comparison (CT vs. TT, P=0.001) and dominant model
comparison (CC/CT vs. TT, P=0.002). We then assessed the source of
heterogeneity for the heterozygote comparison (CT vs. TT) by cancer
type, ethnicity and source of controls. As a result, the source of
controls (χ2=4.11, df=1, P=0.043), but not cancer type
(χ2=6.21, df=3, P=0.102) or ethnicity
(χ2=1.85, df=2, P=0.396) was found to contribute to the
substantial heterogeneity.
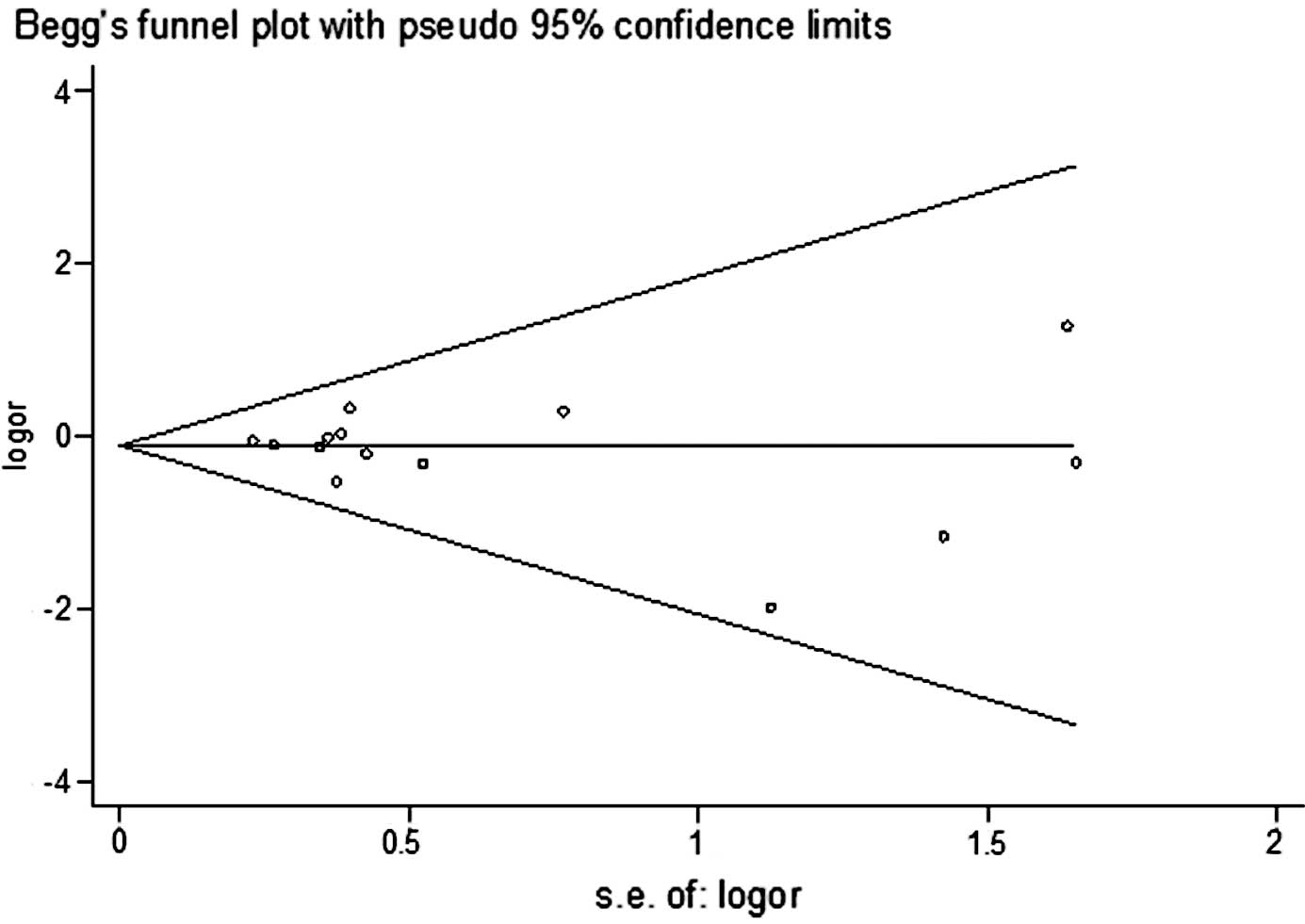
Publication bias
Begg's funnel plot and Egger's test were performed
to assess the publication bias of the literature studies. The
shapes of the funnel plots did not reveal any evidence of obvious
asymmetry. The Egger's test was subsequently used to provide
statistical evidence of funnel plot symmetry. The results did not
show any evidence of publication bias (t=0.83, P=0.423 for CC vs.
TT) (Fig. 4).
Discussion
The mismatch repair pathway plays an important role
in the maintenance of genome integrity and acts conservatively in
species. With its main function to repair mismatches during DNA
duplication, the MMR pathway ensures the integrity and stability of
the genome (3). Genome point
mutations, microsatellite instability (MSI) and loss of
heterozygosity (LOH) are all mutation phenotypes caused by
impairment in any component of the MMR pathway (3,26).
Studies have revealed the presence of MSI in more than 90% of
hereditary non-polyposis colorectal cancer (HNPCC) and in 15% of
non-family colorectal cancer cases (27), and mutations of MLH1, MSH2
and MSH6 germ cells in more than 95% of HNPCC cases with MSI
(28). The presence of MSI has
been confirmed in other primary cancers such as prostate (29), endometrial (30), pancreatic (31), gastric (32,33)
and others (34). Meanwhile, a few
SNPs were found to be involved in the formation and development of
solid tumors through the alteration of the biological activities of
DNA repairase (35–37); for example, MLH1 I219V and
breast cancer (38), PMS2
rs7797466 and ovarian cancer (19), and MLH1 −93 G>A,
MSH2 −118 T>C, MSH6 G39E and colorectal cancer
(18,39). hMSH2 is the first MMR gene
to be associated with HNPCC invasion. Its expression is varied not
only in gastric cancer, lung cancer, endometrial cancer and others,
but also is also associated with the prognosis of cancers. High
expression indicates better repair and low expression is indicative
of a worse repair ability (40).
Although the genetic function of the hMSH2 IVS12-6 T>C
polymorphism is not clear, its variant type may lead to alternative
splicing and deficiency of hMSH2 protein (22,23).
In the present study, we performed a meta-analysis
of published studies based on 13 case-control studies in order to
reveal the association between the hMSH2 IVS12-6 T>C
polymorphism and cancer risk. The results indicate that there is no
significant association between this polymorphism and cancer risk.
In the stratified analyses we found that the C allele was a risk
factor for developing non-Hodgkin's lymphoma (NHL). This may be due
to the limited number of studies analyzed and the small sample
size. The ethnically mixed population in our meta-analysis
consisted of only Ecuadorian and Trukese individuals from two
continents representing a huge discrimination of human race. Our
results also demonstrated that no significant associations were
found in any genetic model among studies of lung cancer, colorectal
cancer and others. A moderate association was observed in the
hospital-based controls, but not in the population-based controls
when stratifying the source of controls. This may have resulted
from a differential effect of selection criteria in different
cancers, which was dictated by the sample size in our
meta-analysis, as well as the weight of each study. Other factors
such as matched criteria may also have conferred an effect. The
above differences may contribute to the inconsistent results.
Therefore, it is very important to determine the unified selection
criteria and to choose larger sample population studies.
We would like to note the differences in the genetic
background and gene-environment interactions in the etiology. The
IVS12-6 C allele frequency among the controls in Asian populations
was significant higher than that in European populations,
suggesting a possible ethnic difference (Fig. 2). Moreover, there is no reported
study on African populations. Therefore, additional studies are
needed to further validate ethnic differences on the effect of this
SNP on cancer risk, particularly in Africans.
Identification of the source of heterogeneity is one
of the most important goals of meta-analysis. In this analysis, we
found that the source of heterogeneity was from the origin of the
controls, suggesting that certain effects of the genetic
polymorphism were population-specific.
Various limitations of this meta-analysis should be
mentioned. First, the lack of original data of the reviewed studies
limited our further evaluation of potential interactions, as
interactions between gene-gene or gene-environment may modulate
cancer risk. Second, our result was based on unadjusted estimates,
while more precise analyses were needed to be performed had
individual data been available, which would have allowed for an
adjusted estimate by age or gender. However, our present
meta-analysis also had advantages. First, a substantial number of
cases and controls was pooled from different studies, which greatly
increased the statistical power of the analysis. Second, the
quality of case-control studies included in this meta-analysis was
satisfactory according to our selection criteria.
In conclusion, our meta-analysis suggests that the
hMSH2 IVS12-6 T>C polymorphism is not associated with
cancer risk. A significant risk effect on cancer was observed in
NHL patients while no statistical results were found for the other
cancer types. Human race variation in the distribution of genotypes
may have also affected the analysis. The role of this variant in
other populations should be investigated by carrying out additional
studies including a wider spectrum of subjects particularly African
individuals. Further functional studies between the hMSH2
IVS12-6 T>C polymorphism and cancer risk should be conducted in
order to reveal its mechanism. Additional well-designed extensive
studies are warranted to validate the association between the
hMSH2 IVS12-6 T>C polymorphism and the susceptibility of
cancer.
References
|
1
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schofield MJ and Hsieh P: DNA mismatch
repair: molecular mechanisms and biological function. Annu Rev
Microbiol. 57:579–608. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koessler T, Oestergaard MZ, Song H, et al:
Common variants in mismatch repair genes and risk of colorectal
cancer. Gut. 57:1097–1101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi M, Shimodaira H, Andreutti-Zaugg
C, Iggo R, Kolodner RD and Ishioka C: Functional analysis of human
MLH1 variants using yeast and in vitro mismatch repair assays.
Cancer Res. 67:4595–4604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kunkel TA and Erie DA: DNA mismatch
repair. Annu Rev Biochem. 74:681–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinzler KW and Vogelstein B:
Cancer-susceptibility genes. Gatekeepers and caretakers. Nature.
386:7617631997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolodner RD: Mismatch repair: mechanisms
and relationship to cancer susceptibility. Trends Biochem Sci.
20:397–401. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Modrich P and Lahue R: Mismatch repair in
replication fidelity, genetic recombination, and cancer biology.
Annu Rev Biochem. 65:101–133. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demokan S, Suoglu Y, Ulusan M and Dalay N:
Analysis of the hMSH2 gene variants in head and neck cancer. DNA
Cell Biol. 29:449–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goessl C, Plaschke J, Pistorius S, et al:
An intronic germline transition in the HNPCC gene hMSH2 is
associated with sporadic colorectal cancer. Eur J Cancer.
33:1869–1874. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hishida A, Matsuo K, Hamajima N, et al:
Polymorphism in the hMSH2 gene (gIVS 12-6T-->C) and risk of
non-Hodgkin lymphoma in a Japanese population. Cancer Genet
Cytogenet. 147:71–74. 2003.PubMed/NCBI
|
|
13
|
Hsu HS, Lee IH, Hsu WH, Kao WT and Wang
YC: Polymorphism in the hMSH2 gene (gISV12-6T > C) is a
prognostic factor in non-small cell lung cancer. Lung Cancer.
58:123–130. 2007.PubMed/NCBI
|
|
14
|
Jung CY, Choi JE, Park JM, et al:
Polymorphisms in the hMSH2 gene and the risk of primary lung
cancer. Cancer Epidemiol Biomarkers Prev. 15:762–768. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JC, Roh SA, Koo KH, et al: Genotyping
possible polymorphic variants of human mismatch repair genes in
healthy Korean individuals and sporadic colorectal cancer patients.
Fam Cancer. 3:129–137. 2004. View Article : Google Scholar
|
|
16
|
Paz-y-Mino C, Fiallo BF, Morillo SA, et
al: Analysis of the polymorphism [gIVS12-6T > C] in the hMSH2
gene in lymphoma and leukemia. Leuk Lymphoma. 44:505–508. 2003.
|
|
17
|
Paz-y-Mino C, Perez JC, Fiallo BF and
Leone PE: A polymorphism in the hMSH2 gene (gIVS12-6T>C)
associated with non-Hodgkin lymphomas. Cancer Genet Cytogenet.
133:29–33. 2002.
|
|
18
|
Raptis S, Mrkonjic M, Green RC, et al:
MLH1 −93G>A promoter polymorphism and the risk of
microsatellite-unstable colorectal cancer. J Natl Cancer Inst.
99:463–474. 2007.
|
|
19
|
Song H, Ramus SJ, Quaye L, et al: Common
variants in mismatch repair genes and risk of invasive ovarian
cancer. Carcinogenesis. 27:2235–2242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tulupova E, Kumar R, Hanova M, et al: Do
polymorphisms and haplotypes of mismatch repair genes modulate risk
of sporadic colorectal cancer? Mutat Res. 648:40–45. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beiner ME, Rosen B, Fyles A, et al:
Endometrial cancer risk is associated with variants of the mismatch
repair genes MLH1 and MSH2. Cancer Epidemiol Biomarkers Prev.
15:1636–1640. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bubb VJ, Curtis LJ, Cunningham C, et al:
Microsatellite instability and the role of hMSH2 in sporadic
colorectal cancer. Oncogene. 12:2641–2649. 1996.PubMed/NCBI
|
|
23
|
Xia L, Shen W, Ritacca F, et al: A
truncated hMSH2 transcript occurs as a common variant in the
population: implications for genetic diagnosis. Cancer Res.
56:2289–2292. 1996.PubMed/NCBI
|
|
24
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
25
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung DC and Rustgi AK: The hereditary
nonpolyposis colorectal cancer syndrome: genetics and clinical
implications. Ann Intern Med. 138:560–570. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rowley PT: Inherited susceptibility to
colorectal cancer. Annu Rev Med. 56:539–554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lynch HT and de la Chapelle A: Hereditary
colorectal cancer. N Engl J Med. 348:919–932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Wang J, Fraig MM, et al: Defects
of DNA mismatch repair in human prostate cancer. Cancer Res.
61:4112–4121. 2001.PubMed/NCBI
|
|
30
|
Lax SF: Molecular genetic pathways in
various types of endometrial carcinoma: from a phenotypical to a
molecular-based classification. Virchows Arch. 444:213–223. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilentz RE, Goggins M, Redston M, et al:
Genetic, immunohistochemical, and clinical features of medullary
carcinoma of the pancreas: a newly described and characterized
entity. Am J Pathol. 156:1641–1651. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schneider BG, Bravo JC, Roa JC, et al:
Microsatellite instability, prognosis and metastasis in gastric
cancers from a low-risk population. Int J Cancer. 89:444–452. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beghelli S, de Manzoni G, Barbi S, et al:
Microsatellite instability in gastric cancer is associated with
better prognosis in only stage II cancers. Surgery. 139:347–356.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lawes DA, SenGupta S and Boulos PB: The
clinical importance and prognostic implications of microsatellite
instability in sporadic cancer. Eur J Surg Oncol. 29:201–212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trojan J, Zeuzem S, Randolph A, et al:
Functional analysis of hMLH1 variants and HNPCC-related mutations
using a human expression system. Gastroenterology. 122:211–219.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hutter P, Couturier A and Rey-Berthod C:
Two common forms of the human MLH1 gene may be associated with
functional differences. J Med Genet. 37:776–781. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hutter P, Wijnen J, Rey-Berthod C, et al:
An MLH1 haplotype is over-represented on chromosomes carrying an
HNPCC predisposing mutation in MLH1. J Med Genet. 39:323–327. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith TR, Levine EA, Freimanis RI, et al:
Polygenic model of DNA repair genetic polymorphisms in human breast
cancer risk. Carcinogenesis. 29:2132–2138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mrkonjic M, Raptis S, Green RC, et al:
MSH2 118T>C and MSH6 159C>T promoter polymorphisms and the
risk of colorectal cancer. Carcinogenesis. 28:2575–2580. 2007.
|
|
40
|
Lynch HT, Smyrk T and Lynch JF: Molecular
genetics and clinical-pathology features of hereditary nonpolyposis
colorectal carcinoma (Lynch syndrome): historical journey from
pedigree anecdote to molecular genetic confirmation. Oncology.
55:103–108. 1998. View Article : Google Scholar
|