Introduction
Surgical resection is considered to be the most
effective treatment for stage I-IIIA non-small-cell lung cancer
(NSCLC). Despite curative surgery, however, numerous patients are
diagnosed with distant or local recurrence following surgery, and
succumb to the disease as a result of the recurrent lung cancer.
Since there is no standard treatment and there are no clearly
defined guidelines for the treatment of patients with postoperative
recurrence, chemotherapy and/or radiotherapy are usually
considered, depending on the patient’s condition, the same as in
cases of inoperable advanced NSCLC.
The orally active epidermal growth factor receptor
(EGFR) tyrosine kinase inhibitor gefitinib (Iressa, AstraZeneca,
Wilmington, DE, USA) has been demonstrated to have significant
antitumor efficacy and provide significant symptom relief in
patients with NSCLC previously treated by chemotherapy that
included cis-diamminedichtoroplatinum (CDDP) in a Phase II study
(IDEAL-1, 2) (1,2). Based on the results of the IDEAL
studies, in 2002, gefitinib was approved in Japan for the treatment
of inoperable and recurrent NSCLC. Recent clinical phase studies of
patients with advanced and recurrent NSCLC have shown a correlation
between gefitinib response and a number of clinical factors,
including adenocarcinoma, absence of smoking history, female gender
and Asian ethnicity. (3–7)
Gefitinib treatment has already become a treatment
option in cases of recurrent NSCLC following surgical resection as
well as in inoperable advanced cases of NSCLC in general clinical
practice in Japan. We believe that gefitinib treatment should also
be evaluated by means of population-based studies, since the
subjects of clinical trials tend to consist of highly selected
populations that are not representative of NSCLC patients in
general clinical practice. We previously reported the first
population-based study of advanced and recurrent NSCLC treated with
gefitinib in general clinical practice in Japan (8). The results obtained, particularly
with respect to response rate, median survival time (MST), 1-year
survival, and adverse effects, were comparable to those reported in
previously published clinical trials whose criteria restricted
eligibility.
Since reports on the benefits of gefitinib
specifically in patients with postoperative recurrent NSCLC have
been rare, and the impact of gefitinib on population-based outcomes
is difficult to measure, in the present study, we performed a
subgroup analysis of NSCLC patients with recurrence following
surgery who were selected from the subjects in our previous report,
who consisted of patients with inoperable advanced NSCLC and
patients with postoperative recurrent NSCLC. To assess the benefits
of gefitinib in patients with postoperative recurrent NSCLC, in
this population-based study we retrospectively reviewed the
characteristics, response to therapy, toxicity and survival of
patients with recurrent NSCLC treated with gefitinib.
Patients and methods
Ibaraki Prefecture, Japan, has an area of 6095
km2 and a population of 3 million. This retrospective
population-based study was conducted by reviewing the records of
patients with NSCLC whose treatment had included gefitinib at 14
institutions (18 divisions) in Ibaraki Prefecture between July 2002
and September 2007. A pathological or cytological diagnosis of
NSCLC was required for inclusion in this study. The pathological
diagnosis of lung cancer was made according to the WHO
classification. Recurrence following surgery was diagnosed by chest
computed tomography (CT), brain magnetic resonance imaging (MRI),
bone scintigraphy, and ultrasonography and/or CT of the abdomen
during follow-up examinations. Eligible cases were identified in
the clinical database of each hospital, and the following
information was extracted from the data: data at the time of the
gefitinib therapy (age, gender, smoking history, histological
diagnosis and pathological stage), and objective tumor response.
Tumor response was evaluated according to the Response Evaluation
Criteria in Solid Tumors as complete response (CR), partial
response (PR), stable disease (SD), progressive disease (PD), or
not evaluable (NE). Toxicity was investigated with regard to
interstitial lung damage (ILD). Overall survival time was defined
as the interval between the start of gefitinib treatment and the
date of mortality or the last follow-up examination. This study
plan was approved by each institution’s Institutional Review
Board.
EGFR mutation data were provided by two
institutions. Surgical specimens were examined for mutations by
direct sequencing of clones. Samples were digested with SDS and
proteinase K, and DNA was extracted by using a DNA extractor WB kit
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) according to
the manufacturer’s instructions. Exons 18, 19 and 21 of the EGFR
gene were amplified by using the following primers: exon
18-forward, 5′-CTG TGT TCT TGT CCC CCC CAG-3′ and reverse, 5′-GGC
CTG TGC CAG GGA CCT TAC-3′; exon 19-forward, 5′-TTC CTT CTC TCT CTG
TCA TAG-3′ and reverse, 5′-CAC AGC AAA GCA GAA ACT CAC-3′; and exon
21-forward, 5′-GCA GGG TCT TCT CTG TTT CAG-3′ and reverse, 5′-GAC
CTA AAG CCA CCT CCT TAC-3′, and the sizes of the PCR products were
165 bp (exon 18), 141 bp (exon 19), and 198 bp (exon 21). We also
evaluated the correlation between the presence of a mutation and
the antitumor efficacy of gefitinib therapy.
Statistical analysis
Differences in proportions between two independent
subgroups were compared by the Chi-square test. A value of
p<0.05 was considered to be statistically significant. In the
analysis of survival, time zero was defined as the date gefitinib
administration was initiated. Survival probability was estimated by
the Kaplan-Meier method and compared using the log-rank test.
Multivariate analysis of significant prognostic factors identified
by the univariate analysis was performed by using Cox’s
proportional hazard model.
Results
Patient characteristics
Complete data sets of the 141 patients with
recurrence following surgery were obtained from the records of a
total of 626 NSCLC patients who had received gefitinib. The other
485 patients had advanced inoperable NSCLC. The characteristics of
the patients with postoperative recurrence are shown in Table I. There were 48 patients with local
recurrence (34%) and 93 patients with distant metastasis (66%). The
median follow-up period after the initiation of gefitinib therapy
was 9 months (1–60 months). The median age was 65 years (range
33–86), and 54.6% of the patients were female. The histological
diagnosis in 85.8% of the patients was adenocarcinoma, 48.9% of the
patients were non-smokers, and 68.1% of the patients had an ECOG
performance status (PS) of 0–1.
 | Table IPatient profiles. |
Table I
Patient profiles.
| Total no. of
patients | 141 |
| Recurrent site | |
| Local | 48 |
| Distant | 93 |
| Follow-up
(months) | |
| Median | 9 |
| Range | 1–60 |
| Age (years) | |
| Median | 65 |
| Range | 33–86 |
| Histology | |
|
Adenocarcinoma | 121 |
|
Non-adenocarcinoma | 20 |
| Gender | |
| Female | 77 |
| Male | 64 |
| Smoking status | |
| Non-smoker | 69 |
| Smoker | 70 |
| Unknown | 2 |
| PS | |
| 0–1 | 96 |
| 2–4 | 45 |
| Gefinitib Tx
line | |
| First | 25 |
| Second | 57 |
| Third | 59 |
| Tumor response | |
| CR, PR | 53 |
| SD | 36 |
| PD | 25 |
| NE | 27 |
Response to treatment
The objective response rate (ORR) to gefitinib
therapy was 37.6% (53 CR+PR). The response was evaluated as SD in
25.5% of the patients, and the disease control rate was 63.1%. A
total of 27 of the 141 patients were not evaluated (NE) for tumor
response. Higher responses were documented in the females with
adenocarcinoma than in the males with adenocarcinoma among
non-smokers and smokers (non-smokers, 43.6 vs. 40%; smokers, 43.8
vs. 31.6%; Table II).
 | Table IIBenefits and risks of each group of
patients. |
Table II
Benefits and risks of each group of
patients.
| n | Benefit
| Risk
|
|---|
| CR/PR (%) | MST (m) | 95% CI | ILD (%)a |
|---|
| Overall | 141 | 53 (37.6) | 12 | 6.4–17.6 | 5 (3.5%) |
| Adeno,
non-smoker | | | | | |
| Female | 55 | 24 (43.6) | 22 | 15.8–28.2 | 1 (1.8%) |
| Male | 10 | 4 (40.0) | 15 | 11.0–19.0 | 0 (0.0%) |
| Adeno, smoker | | | | | |
| Female | 16 | 7 (43.8) | 24 | 14.4–33.6 | 1 (6.3%) |
| Male | 38 | 12 (31. 6) | 7 | 4.8–9.3 | 2 (5.3%) |
| Non-adeno | | | | | |
| Non-smoker | 4 | 1 (25.0) | 4 | - | 0 (0%) |
| Smoker | 16 | 4 (25.0) | 6 | 2.9–9.1 | 1 (6.3%) |
Survival analysis
The MST was 12 months [95% confidence interval (CI),
6.4–17.6 months], and the 1-year and 2-year survival rates were
48.9% and 28.9%, respectively (Fig.
1A). The MST of the patients with advanced inoperable NSCLC was
7 months (95% CI, 5.9–8.1 months), and their 1-year and 2-year
survival rates were 32% and 13%, respectively (Fig. 1B). Survival after the initiation of
gefitinib therapy was improved in the group with postoperative
recurrence as opposed to the group with advanced inoperable NSCLC.
The subgroup analysis of postoperative recurrence according to
histological diagnosis, gender and smoking history (Table II) showed a higher MST in the
female subgroup with adenocarcinoma among the non-smokers (22
months; 95% CI, 15.8–28.2) and smokers (24 months; 95% CI,
14.3–33.6) than in the male subgroup with adenocarcinoma among the
non-smokers (15 months; 95% CI, 11.0–19.0) and smokers (7 months;
95% CI, 4.8–9.3).
To identify factors that affected survival, a
univariate analysis was performed with gender, PS, smoking history
and histological diagnosis as covariates (Table III). The MST of the females was 19
months (95% CI; 13.8–24.3), the MST of the males was 9 months (95%
CI; 7.2–10.8) and the difference was statistically significant
(p=0.0022). As shown in Table III,
adenocarcinoma, a PS of 0–1 and absence of smoking history, as well
as female gender, were favorable prognostic factors in the
univariate analysis. The MST of the patients who received gefitinib
as first-line therapy, second-line therapy and third- or later line
therapy was 10 (95% CI, 0–26.3 months), 16 (95% CI, 12.6–19.4
months), and 9 months (95% CI, 6.0–12.0 months), respectively, and
there were no statistically significant differences in survival
time according to the therapy line (p=0.8907). A multivariate
analysis of the significant prognostic factors identified in the
univariate analysis was carried out using Cox’s proportional hazard
model, and the results showed that female gender [hazard ratio
(HR), 0.575; 95% CI, 0.374–0.886; p<0.012] and a PS of 0–1 (HR,
0.543; 95% CI, 0.351–0.840; p<0.006) were independent
statistically significant prognostic factors (Table IV).
 | Table IIIUnivariate analysis of clinical
features. |
Table III
Univariate analysis of clinical
features.
| Variable | N | MST (months) | 95% CI | P-value |
|---|
| Gender | | | | 0.0022 |
| Female | 77 | 19 | 13.8–24.3 | |
| Male | 64 | 9 | 7.2–10.8 | |
| Histology | | | | 0.0304 |
|
Adenocarcinoma | 121 | 16 | 12.0–20.0 | |
|
Non-adenocarcinoma | 20 | 6 | 2.7–9.3 | |
| Female | | | | 0.0694 |
|
Adenocarcinoma | 73 | 22 | 16.1–27.9 | |
|
Non-adenocarcinoma | 4 | 2 | 0.0–4.9 | |
| Male | | | | 0.5602 |
|
Adenocarcinoma | 48 | 10 | 6.2–13.8 | |
|
Non-adenocarcinoma | 16 | 8 | 5.7–10.3 | |
| Smoking status | | | | 0.0243 |
| Smoker | 70 | 8 | 6.3–9.7 | |
| Non-smoker | 69 | 17 | 10.8–23.2 | |
| Female | | | | 0.7518 |
| Smoker | 17 | 19 | 9.2–28.8 | |
| Non-smoker | 58 | 22 | 15.6–28.4 | |
| Male | | | | 0.1544 |
| Smoker | 53 | 8 | 6.3–9.7 | |
| Non-smoker | 11 | 15 | 11.0–19.0 | |
| PS | | | | 0.0006 |
| 0–1 | 96 | 17 | 9.6–24.4 | |
| 2–4 | 45 | 8 | 5.6–10.6 | |
| Female | | | | 0.0001 |
| 0–1 | 55 | 32 | 20.7–43.3 | |
| 2–4 | 22 | 7 | 2.6–11.5 | |
| Male | | | | 0.5012 |
| 0–1 | 41 | 8 | 4.3–11.3 | |
| 2–4 | 23 | 9 | 6.7–11.3 | |
| Gefitinib Tx
line | | | | 0.8907 |
| First | 25 | 10 | 0.0–26.3 | |
| Second | 57 | 16 | 12.6–19.4 | |
| Third | 59 | 9 | 6.0–12.0 | |
| Tumor response | | | | <0.0001 |
| CR/PR | 53 | 24 | 13.5–34.5 | |
| SD | 36 | 17 | 8.3–25.7 | |
| PD | 25 | 4 | 1.6–6.5 | |
 | Table IVCOX analysis proportional hazards
model of survival. |
Table IV
COX analysis proportional hazards
model of survival.
| Variable | HR | 95% CI | P-value |
|---|
| Female/male | 0.575 | 0.374–0.886 | 0.012 |
| PS 0,1/2–4 | 0.543 | 0.351–0.840 | 0.006 |
Toxicity
The risks of ILD are shown in Table II. ILD adverse events greater than
grade 1 were reported in 5 (3.5%) of the 141 patients, but there
was no mortality due to ILD (no cases of grade 5 toxicity). The
subgroup analysis according to adenocarcinoma histology, gender and
smoking history showed a lower incidence of ILD in the non-smoking
group (0–1.8%) than in the smoking group (5.3–6.3%).
EGFR mutation and clinical features
EGFR mutation data for 35 patients were available
from only 2 institutions, as the cost of testing for EGFR mutations
was not reimbursed by health insurance at the time of this study.
As shown in Table V, a mutation
was detected in 60% of the females, 52.6% of those with no smoking
history and 37% of those with adenocarcinoma (34.3% of the patients
overall). There were significant differences in the EGFR mutation
rate according to gender (p=0.0055) and smoking status (p=0.0127)
based on the results of the Chi-square test. The tumor response
rate in the mutation-positive group was higher than in the
mutation-negative group, but the difference was not significant
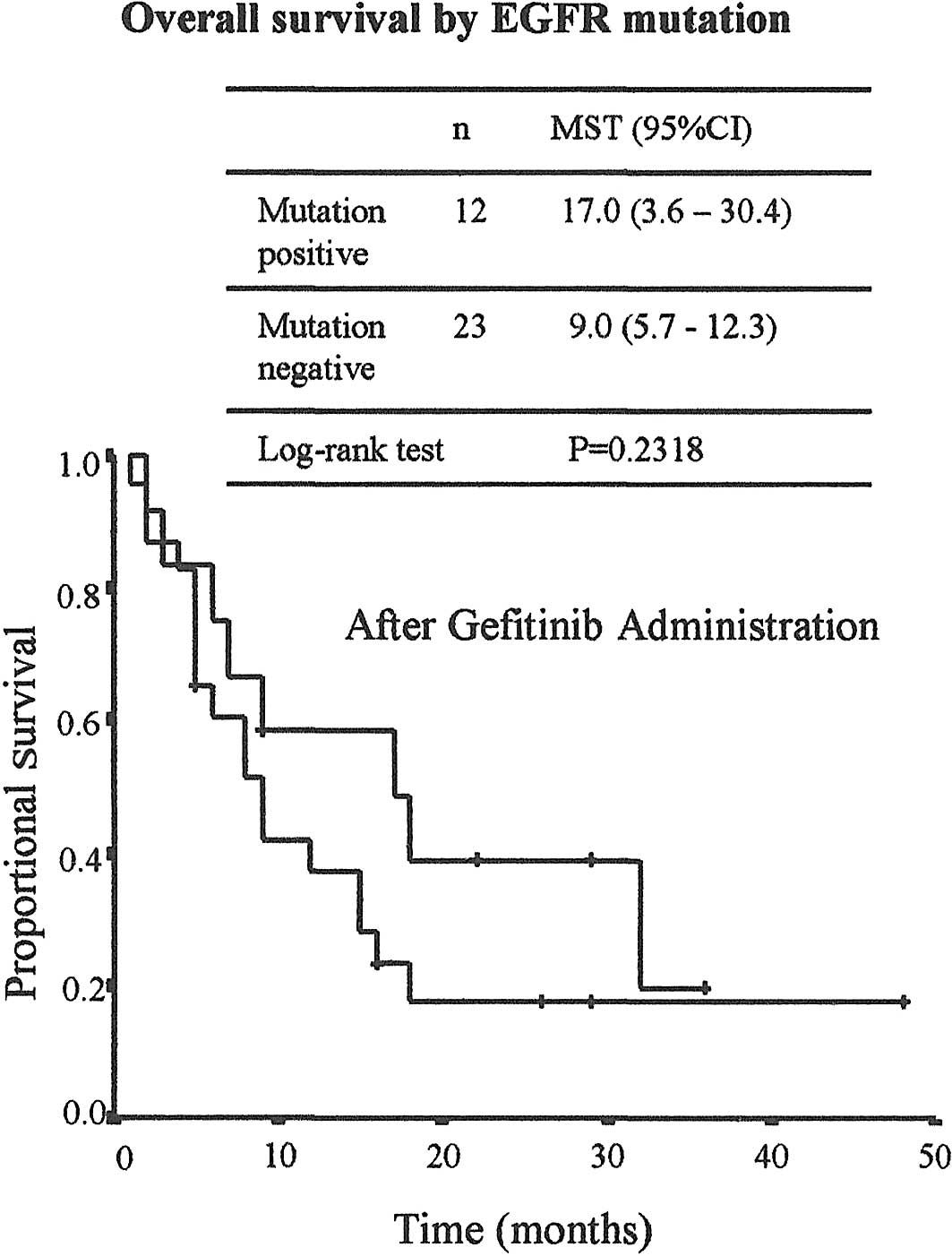
(50% vs. 26.1%, p<0.1572). As shown in Fig. 2, overall survival following the
initiation of gefitinb therapy differed according to mutation
status. MST was 17 months (95% CI; 3.6–30.4 months) in the
mutation-positive group and 9 months (95% CI; 5.7–12.3 months) in
the wild-type group, but due to the small number of patients, the
difference was not statistically significant (p=0.2318).
 | Table VEGFR mutation and clinical features
(n=35). |
Table V
EGFR mutation and clinical features
(n=35).
| Mutant
| Wild-type
| |
|---|
| Variable | n | % | n | % | P-value |
|---|
| Gender | | | | | 0.0055 |
| Female | 9 | 60.0 | 6 | 40.0 | |
| Male | 3 | 15.0 | 17 | 85.0 | |
| Smoking status | | | | | 0.0127 |
| Smoker | 2 | 12.5 | 14 | 87.5 | |
| Non-smoker | 10 | 52.6 | 9 | 47.4 | |
| Histology | | | | | 0.5287 |
|
Adenocarcinoma | 10 | 37.0 | 17 | 63.0 | |
|
Non-adenocarcinoma | 2 | 25.0 | 6 | 75.0 | |
| Response | | | | | 0.1572 |
| CR/PR | 6 | 50.0 | 6 | 26.1 | |
| SD/PD | 6 | 50.0 | 17 | 73.9 | |
| Total | 12 | 34.3 | 23 | 65.7 | |
Discussion
Molecularly targeted therapy with gefitinib has
already become a treatment option similar to chemotherapy in cases
of recurrence following surgical resection as well as in inoperable
advanced cases in general clinical practice in Japan. However,
since the efficacy of chemotherapeutic agents or gefitinib in cases
of recurrence following surgery and advanced inoperable cases may
differ, we evaluated the effectiveness of gefitinib in recurrent
NSCLC following surgery. The survival time and survival rate after
the initiation of gefitinib therapy in the group with recurrence
following surgery in this population-based study were improved as
opposed to the group with advanced inoperable NSCLC (MST, 12 vs. 7
months; two-year survival, 28.9 vs. 13%). In 2001, prior to the
approval of gefitinib in Japan, Yoshino et al reviewed the
cases of a total of 118 patients with recurrence following surgery
for NSCLC and showed that systemic chemotherapy tended to prolong
survival (MST, 12 months; two-year survival, 19% after recurrence),
but the difference between the group that received chemotherapy and
the control group was not statistically significant (p=0.0928)
(9). Our results for survival
following the initiation of gefitinib therapy, but not after the
diagnosis of recurrence, showed improved survival (MST, 12 months;
two-year survival, 28.9%) compared to the study by Yoshino et
al. The data obtained in our study suggest that treatment
including gefitinib is superior to chemotherapy alone without
gefitinib in NSCLC patients with recurrence following surgery.
Therefore, we should try to treat patients with recurrence
following surgery with gefitinib.
As the effectiveness of gefitinib against
adenocarcinoma was particularly well known at the time it was
released on the market based on the data from the IDEAL-1 and -2
studies (1,2), general practitioners were already
prescribing gefitinib for 86% of the patients with adenocarcinoma
in this study. Initially, general practitioners may attach
importance to adenocarcinoma histology rather than gender or
smoking habit, since the numbers of males or females, and of
smokers or non-smokers in this study were almost the same. Since a
number of recent clinical studies of gefitinib in inoperable NSCLC
have shown a correlation between gefitinib response and a number of
clinical factors, including histological diagnosis of
adenocarcinoma, no history of smoking, female gender, Asian
ethnicity and EGFR-mutation-positive status (3–7),
general practitioners have started to consider some of these
factors, such as female gender and EGFR-mutation-positive status.
Mitsudomi et al demonstrated that patients with recurrence
following resection who were female, non-smokers and had
adenocarcinoma tended to respond better to gefitinib therapy
(7). The subgroup analysis in our
own study showed a longer MST in the adenocarcinoma, female gender
and non-smoker subgroups. Interestingly, the female patients had a
favorable MST of more than 20 months regardless of smoking status,
while the male smokers had a poor MST of only 7 months. Gefitinib
should be considered a treatment option for women even when they
are smokers. Women with a PS of 0–1 had a longer MST than women
whose PS was 2–3. By contrast, male patients with a PS of 0–1 did
not have a longer MST than males whose PS was 2–3. PS does not seem
to be a significant clinical prognostic factor in male patients.
However, while smoking history, gender and PS were highly
confounded with each other, gefitinib is expected to be effective
in female non-smokers with a PS of 0–1, the same as in previously
reported clinical trials.
A cohort and nested case-control study concluded
that ILD was relatively common in Japanese patients with NSCLC who
were being treated with gefitinib, and that its incidence was
higher in older, smoking patients with preexisting interstitial
pneumonitis or poor PS (10). The
incidence of ILD in our own study was 3.5% overall, and almost the
same as in previous reports (10,11).
The incidence was higher in smokers regardless of the histological
type of their NSCLC.
Although the addition of gefitinib to standard
first-line chemotherapy does not provide a clinical benefit over
chemotherapy alone in patients with advanced or metastatic NSCLC
(12,13), recent studies have demonstrated the
usefulness of first-line monotherapy with gefitinib in advanced
NSCLC. The results of the Iressa Pan-Asia Study (IPASS) showed
superior progression-free survival (PFS) in the gefitinib group
than in a group treated with a combination of carboplatin and
paclitaxel as first-line treatment of East Asian patients who were
non- or light-smokers and had a histological diagnosis of
adenocarcinoma (14). A subgroup
analysis in that study showed that the presence of a mutation of
the EGFR gene in the tumor is a strong predictor of an improved
outcome with gefitinib (HR for progression or death, 0.48; 95% CI,
0.36–0.64; P<0.001). Also, in a Japanese study of Iressa, the
combined survival analysis of the mutation positive (I-CAMP) study
group identified seven eligible trials for NSCLC patients with EGFR
mutations who were treated with first-line gefitinib (15). The median PFS after the start of
first-line therapy was significantly longer in the gefitinib-first
group than in the chemotherapy-first group (10.7 vs. 6 months;
p<0.001) in the I-CAMP study. The International Expert Panel
Meeting on the first-line treatment of advanced NSCLC organized by
the Italian Association of Thoracic Oncology recommended that every
effort be made to obtain adequate tumor tissue prior to initiation
of treatment (16).
An EGFR mutation analysis should be performed in
subgroups of patients characterized by a higher prevalence of
sensitizing mutations (Asians, never-smokers, females and patients
with adenocarcinoma), and first-line treatment with a single-agent
EGFR tyrosine-kinase inhibitor may be considered when a mutation is
present. In our own retrospective study, there were no significant
differences in survival between the first-line, second-line, and
third- or later-line groups. Although first-line treatment with
gefitinib is considered for patients with poor PS who cannot
tolerate systemic chemotherapy, since gefitinib was administered to
64% of the patients with a PS of 0–1, clinical factors other than
poor PS, such as the presence of complications or patient refusal
of systemic chemotherapy, may affect the selection of first-line
gefitinib treatment in general clinical practice.
A number of studies on recurrent NSCLC following
surgery that was treated with gefitinib reported ORRs of 33–56.5%,
EGFR mutation rates of 44.1–58.8%, and response rates in
EGFR-mutation-positive groups of 65–92%. MST in the
mutation-positive cases was 16–31 months, and the significant
prognostic factors according to the results of a multivariate
analysis were EGFR mutation and disease-free interval (7,17,18).
Our data regarding the percentage of mutations (34.3%) and response
rate of mutations (50%) were slightly lower than those data. The
differences may be explained by the fact that there were fewer EGFR
mutation tests in our study, as their cost was not reimbursed by
health insurance at the time of this study. The other data in our
study (ORR, 37.6%; MST in mutations, 17 months) were similar to the
data obtained in other studies. Significant prognostic factors
identified in the multivariate analysis were female gender and a PS
of 0–1. However, we did not include EGFR mutation in the analysis
as so few patients were tested. The Nagoya University Group
analyzed primary tumor specimens obtained from lung cancer patients
at initial surgery for the presence of EGFR mutations, and
investigated whether the response of recurrent tumors to gefitinib
depended on the presence of the activating mutation (19). The results of their study suggested
that analyzing specimens obtained at surgery for EGFR mutations may
be useful in selecting the appropriate treatment for patients with
recurrent lung cancer.
In the present study, gefitinib therapy for the
recurrence of NSCLC following surgical resection was found to be
superior in terms of survival to gefitinib therapy for inoperable
advanced NSCLC in general clinical practice, and gefitinib therapy
should be considered for patients with postoperative recurrent
NSCLC who are female, who have adenocarcinoma, a PS of 0–1, are
non-smokers, and whose cancer is EGFR mutation-positive, similar to
published clinical phase studies conducted on highly selected
patients. Gefitinib is a feasible treatment for patients with
recurrence of NSCLC following surgery, and a good response and
longer survival may be obtained. Since treatments that included
gefitinib were demonstrated to be more useful than cytotoxic
chemotherapy regimens alone in patients with postoperative
recurrent NSCLC in this study, gefitinib should be considered for
the treatment of recurrent NSCLC following surgery. Further
prospective investigation of EGFR mutations may support the
findings in this study.
Acknowledgements
The authors of this study all belong
to the Ibaraki Advanced Lung Cancer Study Group. The authors are
indebted to Mr Roderick J. Turner and Associate Professor Raoul
Breugelmans of the Department of International Medical
Communications of Tokyo Medical University for their review of this
manuscript.
References
|
1.
|
M FukuokaS YanoG
GiacconeMulti-institutional randomized phase II trial of gefitinib
for previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial)J Clin
Oncol2122372246200310.1200/JCO.2003.10.03812748244
|
|
2.
|
MG KrisRB NataleRS HerbstEfficacy of
gefitinib, an inhibitor of the epidermal growth factor receptor
tyrosine kinase, in symptomatic patients with non-small cell lung
cancer: a randomized
trialJAMA29021492158200310.1001/jama.290.16.214914570950
|
|
3.
|
SW HamTY KimKH LeeClinical predictors
versus epidermal growth factor receptor mutation in gefitinib-treat
non-small-cell lung cancer patientsLung
Cancer54201207200610.1016/j.lungcan.2006.07.00716956694
|
|
4.
|
GC ChangCM TsaiKC ChenPredictive factors
of gefitinib antitumor activity in East Asian advanced non-small
cell lung cancer patientsJ Thorac
Oncol1520525200610.1097/01243894-200607000-0000417409911
|
|
5.
|
T ItayaN YamaotoM AndoInfluence of
histological type, smoking history and chemotherapy on survival
after first-line therapy in patients with advanced non-small cell
lung cancerCancer
Sci98226230200710.1111/j.1349-7006.2006.00379.x17233840
|
|
6.
|
TJ LynchDW BellR SordellaActivating
mutations in the epidermal growth factor receptor underlying
responsiveness of non-small-cell lung cancer to gefitinibN Eng J
Med35021292139200410.1056/NEJMoa04093815118073
|
|
7.
|
T MitsudomiT KosakaH EndohMutations of the
epidermal growth factor receptor gene predict prolonged survival
after gefitinib treatment in patients with non-small-cell lung
cancer with postoperative recurrenceJ Clin
Oncol23251320200510.1200/JCO.2005.00.992
|
|
8.
|
K HayashibaraH SatohY ShinoharaA
population-based study of gefitinib in patients with non-small cell
lung cancerMed
Oncol26222227200910.1007/s12032-008-9110-y18975151
|
|
9.
|
I YoshinoT YohenaM KitajimaC UshijimaK
NishiokaY IchinoseK SugimachiSurvival of non-small cell lung cancer
patients with postoperative recurrence at distant organsAnn Thorac
Cardiovasc Surg7204209200111578260
|
|
10.
|
S KudohH KatoY NishiwakiInterstitial lung
disease in Japanese patients with lung cancer: a cohort and nested
case-control studyAm J Respir Crit Care
Med17713481357200810.1164/rccm.200710-1501OC18337594
|
|
11.
|
M NakagawaT NishimuraS
TeramukaiInterstitial lung disease in gefitinib-treated Japanese
patients with non-small cell lung cancer - a retrospective
analysis: JMTO LC03-02BMC Res
Notes2157200910.1186/1756-0500-2-15719656374
|
|
12.
|
G GiacconeRS HerbstC ManegoldGefitinib in
combination with gemcitabine and cisplatin in advanced
non-small-cell lung cancer: a phase III trial--INTACT 1J Clin
Oncol22777784200410.1200/JCO.2004.08.00114990632
|
|
13.
|
RS HerbstG GiacconeJH SchillerGefitinib in
combination with paclitaxel and carboplatin in advanced
non-small-cell lung cancer: a phase III trial-INTACT 2J Clin
Oncol22785794200410.1200/JCO.2004.07.21514990633
|
|
14.
|
TS MokYL WuS ThongprasertGefitinib or
carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J
Med361947957200910.1056/NEJMoa081069919692680
|
|
15.
|
S MoritaI OkamotoK KobayashiCombined
survival analysis of prospective clinical trials of gefitinib for
non-small cell lung cancer with EGFR mutationsClin Cancer
Res1544934498200910.1158/1078-0432.CCR-09-039119531624
|
|
16.
|
C GridelliA ArdizzoniJY DouillardRecent
issues in first-line treatment of advanced non-small-cell lung
cancer: Results of an International Expert Panel Meeting of the
Italian Association of Thoracic OncologyLung
Cancer68319331201010.1016/j.lungcan.2009.11.01820036027
|
|
17.
|
F ShojiT YanoI YoshinoD MoriF YamasakiH
KohnoY MaeharaThe characteristics and failure pattern of gefitinib
responders with postoperative recurrence of pulmonary
adeno-carcinomaEur J Surg
Oncol348993200810.1016/j.ejso.2007.03.00517449217
|
|
18.
|
J OkamiK TaniguchiM HigashiyamaPrognostic
factors for gefitinib-treated postoperative recurrence in non-small
cell lung cancerOncology72234242200710.1159/00011294718176089
|
|
19.
|
M KondoT YokoyamaT FukuiMutations of
epidermal growth factor receptor of non-small cell lung cancer were
associated with sensitivity to gefitinib in recurrence after
surgeryLung
Cancer50385391200510.1016/j.lungcan.2005.06.00816140420
|
Appendices
Appendix
The following principal investigators and
institutions also belonged to the Ibaraki Advanced Lung Cancer
Study Group and participated in this study: Saito T, Ibaraki
Higashi Hospital; Kurishima K, Ohtsuka M, Hizawa N, Gotoh Y,
Onizuka M, and Satoh Y, University of Tsukuba Hospital; Shinohara
Y, Tsuchiura Kyodo General Hospital; Ishikawa H, Tsukuba Medical
Center Hospital; Yamamoto Y, Hitachi General Hospital; Nawa T,
Hitachi General Hospital; Funayama Y, Tsukuba Gakuen General
Hospital; Matsumura T, Ibaraki Seinan General Hospital; Kagohashi
K; Mito Kyodo General Hospital; Endo T, Mito Medical Center
Hospital; Sumi M, Kensei General Hospital; Kashimoto T, Mito
Saiseikai Hospital.