Introduction
Although gastrointestinal stromal tumors (GIST) are
rare, they are the most common primary mesenchymal tumor of the
gastrointestinal tract (1). GIST
expresses the tyrosine kinase receptor, KIT, which is the protein
product of the KIT protooncogene. GIST is generally characterized
by gain-of-function mutations of KIT (2). Moreover, recent studies have
described mutations of PDGFRA in certain populations of GIST
(3,4). However, 12% of GIST cases do not have
mutations of either KIT or PDGFRA. The mechanism of GIST genesis is
not yet fully understood. As for the biological behavior, GIST was
classified into two groups based upon the clinical outcome by
long-term follow-up. Tumors that developed recurrence or metastasis
were judged as malignant, including those which caused patient
mortality. Tumors with peripheral invasive growth microscopically
were also diagnosed as malignant. The other cases without the above
evidence of malignancy were classified as benign. GIST has a wide
spectrum of biological behavior ranging from benign to malignant.
Due to its specific biological behavior, there is not a standard
definition of benign and malignant GIST once the patient is
diagnosed at an early stage. According to the consensus approach at
the National Institutes of Health (NIH) in 2001, the use of risk
assessment in predicting GIST behavior has been recommended, in
preference to trying to draw a sharp line between benign and
malignant lesions. They categorized GIST into 4 groups: very low
risk, low risk, intermediate risk and high risk (Table I) (5). Although this system is useful in
predicting GIST behavior, it is only based on the experience of a
wide range of experts on GIST.
 | Table I.National Institutes of Health system
of risk grading for gastrointestinal stromal tumors. |
Table I.
National Institutes of Health system
of risk grading for gastrointestinal stromal tumors.
| Tumor size
(cm) | Mitotic count |
|---|
| Very low risk | <2 | ≤5/50 HPF |
| Low risk | 2–5 | ≤5/50 HPF |
| Intermediate
risk | ≤5 | >5 to ≤10
HPF |
| >5 to ≤10 | ≤5/50 HPF |
| High risk | >5 | >5/50 HPF |
| >10 | Any mitotic
rate |
| Any size | >10/50 HPF |
To explore other prognostic factors in GIST, a
number of studies have completed research concerning cell-cycle
regulatory proteins. p53, one of the cell-cycle regulatory
proteins, has been implicated in the pathogenesis and tumor
progression of various types of tumors. As in other neoplasms, it
was assumed that the overexpression of p53 protein in GIST may be
essential for tumorigenesis and therefore significant in predicting
patient prognosis, particularly as it is known that when the genome
is damaged, p53 suppresses the cell growth cycle by activating the
transcription of genes that cause arrest in the G1 phase. This
regulatory function may be lost in most neoplasms that have p53
overexpression and GIST is no exception. A number of studies have
been designed to test the relationship between p53 and GIST
behavior, with conflicting results partially due to the relatively
small sample size in each of the published studies. Therefore, we
performed a meta-analysis of the published studies to derive a more
precise estimation of the association.
Materials and methods
Publication search
Two electronic databases (PubMed and Embase) were
searched (last search was updated on 1 June 2010, using the search
terms: ‘gastrointestinal stromal tumor’ and ‘p53’). All eligible
studies were retrieved, and their bibliographies were checked for
other relevant publications. Review articles and bibliographies of
other relevant studies identified were hand-searched to find
additional eligible studies. Only published studies with full-text
articles were included. When more than one of the same patient
populations was included in a number of publications, only the most
recent or complete study was used in this meta-analysis.
Inclusion criteria
The inclusion criteria were as follows: a)
evaluation of the p53 expression in GIST and biological behavior;
b) benign (non-aggressive)-malignant (aggressive) study or NIH risk
study; and c) sufficient published data for estimating an odds
ratio (OR) with a 95% confidence interval (CI).
Data extraction
Information was carefully extracted from all
eligible studies by two of the authors (Z.L. and C.P.), according
to the inclusion criteria listed above. The following data were
collected from each study: first author’s surname, publication
date, category method, total number of benign cases and malignant
cases, number of positive p53 patients in the benign group and the
malignant group, total number of patients in the NIH very low risk
group, low risk group, intermediate risk group and high risk group,
and number of patients with positive p53 in each NIH risk group,
respectively. Data were extracted separately according to the
category for subgroup analyses. We did not define a minimum number
of patients required to include a study in our meta-analysis.
Statistical analysis
ORs with 95% CI were used to assess the predictive
value of p53 expression in the risk of malignant GIST, according to
the method of Woolf. Heterogeneity assumption was calculated by the
χ2-based Q-test. A P-value >0.10 for the Q-test
indicates a lack of heterogeneity among studies, so the OR estimate
of each study was calculated by the fixed-effects model (the
Mantel-Haenszel method). Otherwise, the random-effects model (the
DerSimonian and Laird method) was used. The significance of the
pooled OR was determined by the Z-test and a value of P>0.05 was
considered to be statistically significant. Sensitivity analyses
were carried out to investigate whether modification of the
inclusion criteria of this meta-analysis affected the final
results. An estimate of potential publication bias was carried out
by the funnel plot, in which the OR of each study was plotted
against its log (OR). An asymmetric plot suggests a possible
publication bias. Funnel plot asymmetry was assessed by the method
of Egger’s linear regression test, a linear regression approach to
measure funnel plot asymmetry on the natural logarithm scale of the
OR. The significance of the intercept was determined by the t-test,
suggested by Egger (P<0.05 was considered representative of
statistically significant publication bias). All the statistical
tests were performed with Review Manager Version 4.2 (The Cochrane
Collaboration, Oxford, England) and STATA version 9.2 (Stata
Corporation, College Station, TX, USA).
Results
Study characteristics
A total of 19 publications met the inclusion
criteria (6–24). The studies by Chou et al,
Padilla et al, Romeo et al and Kwon et al were
excluded due to insufficient information to calculate an OR
(25–28), and the study by Sakurai et
al was also excluded since they used telomerase activity as the
criteria for measuring the malignant risk of GIST (29). Similarly, the studies by Wang et
al and Tsai et al were excluded as the subsequent
articles contained the same patient population (30,31).
The study by Wong et al was excluded since they focused on
proving that the mitotic count remained the best predictor of GIST
(32). Hence, a total of 19 groups
including 1163 patients were used in the pooled analyses. Table II lists the studies identified and
their main characteristics. Of the 19 groups, sample sizes ranged
from 11 to 343. Almost all of the patients with GIST were confirmed
by histology and immunohistochemistry. No significant differences
were found in the age distributions and gender differences among
all the studies.
 | Table II.Main characteristics of all studies
included in the meta-analysis. |
Table II.
Main characteristics of all studies
included in the meta-analysis.
| Author/(Refs.) | Category | B/M or NIH (VL+
L/I+H) | Age
distribution | Gender
(male/female) | Size |
|---|
| Feakins (6) | NIH | 48/57 | No report | No report | 105 |
| Gumurdulu et
al (7) | NIH | 3/22 | 62.3±11.18 | 16/9 | 25 |
| Hu et al
(8) | NIH | 14/35 | 59.2±12.1 | 25/24 | 49 |
| Lopes et al
(9) | NIH | 60/283 | 59 (22–92) | 255/258 | 343 |
| Nakamura et
al (10) | NIH | 22/58 | 63.4 (20–93) | 39/41 | 80 |
| Neves et al
(11) | NIH | 8/32 | 56 (22–84) | 21/19 | 40 |
| Pauser et al
(12) | NIH | 35/65 | 62 (24–90) | 45/59 | 100 |
| Ryu et al
(13) | NIH | 42/83 | 58 (28–83) | 71/54 | 125 |
| Yang et al
(14) | NIH | 13/8 | 48 (36–84) | 11/10 | 21 |
| Takeyama et
al (15) | NIH | 18/9 | 63.0±13.1 | 16/16 | 27 |
| Al-Bozom (16) | NIH | 5/10 | 57 (29–79) | 7/8 | 15 |
| Sabah et al
(17) | NIH | 2/21 | 59 (19–93) | 11/12 | 23 |
| Aoyagi et al
(18) | NIH | 5/6 | 61.0±9.7 | 8/3 | 11 |
| Yalcinkaya et
al (19) | NIH | 5/36 | 52.8±14.0 | 25/16 | 41 |
| Chang et al
(20) | B/M | 11/13 | 48 (23–95) | 15/9 | 24 |
| Meara (et al
21) | B/M | 6/8 | 58 (17–84) | 7/7 | 14 |
| Wang et al
(22) | B/M | 38/35 | No report | 42/31 | 73 |
| Ozdamar et
al (23) | B/M | 9/13 | 48.8±12.9 | 11/11 | 22 |
| Panizo-Santos et
al (24) | B/M | 10/15 | 52.6 (30–80) | 18/14 | 25 |
Meta-analysis results
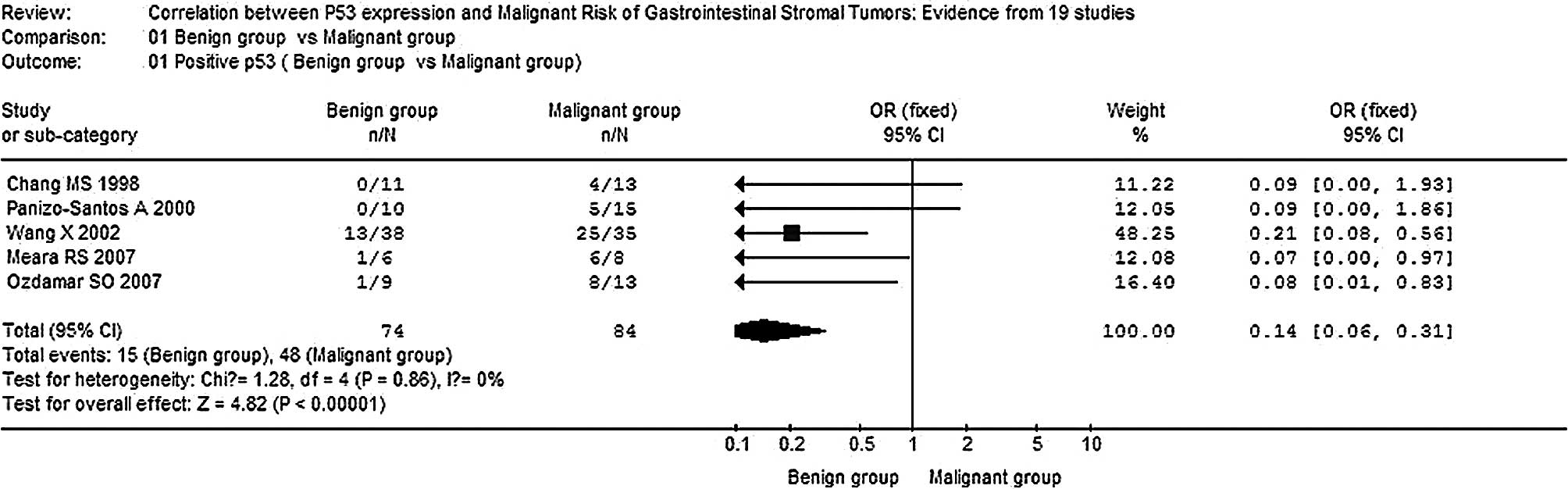
The overall OR for the positive rate of p53 in the
malignant group vs. the benign group revealed that significantly
elevated risks of positive p53 in the malignant group were achieved
(OR, 0.14; 95% CI, 0.06–0.31; P<0.00001,
Pheterogeneity=0.86) (Fig.
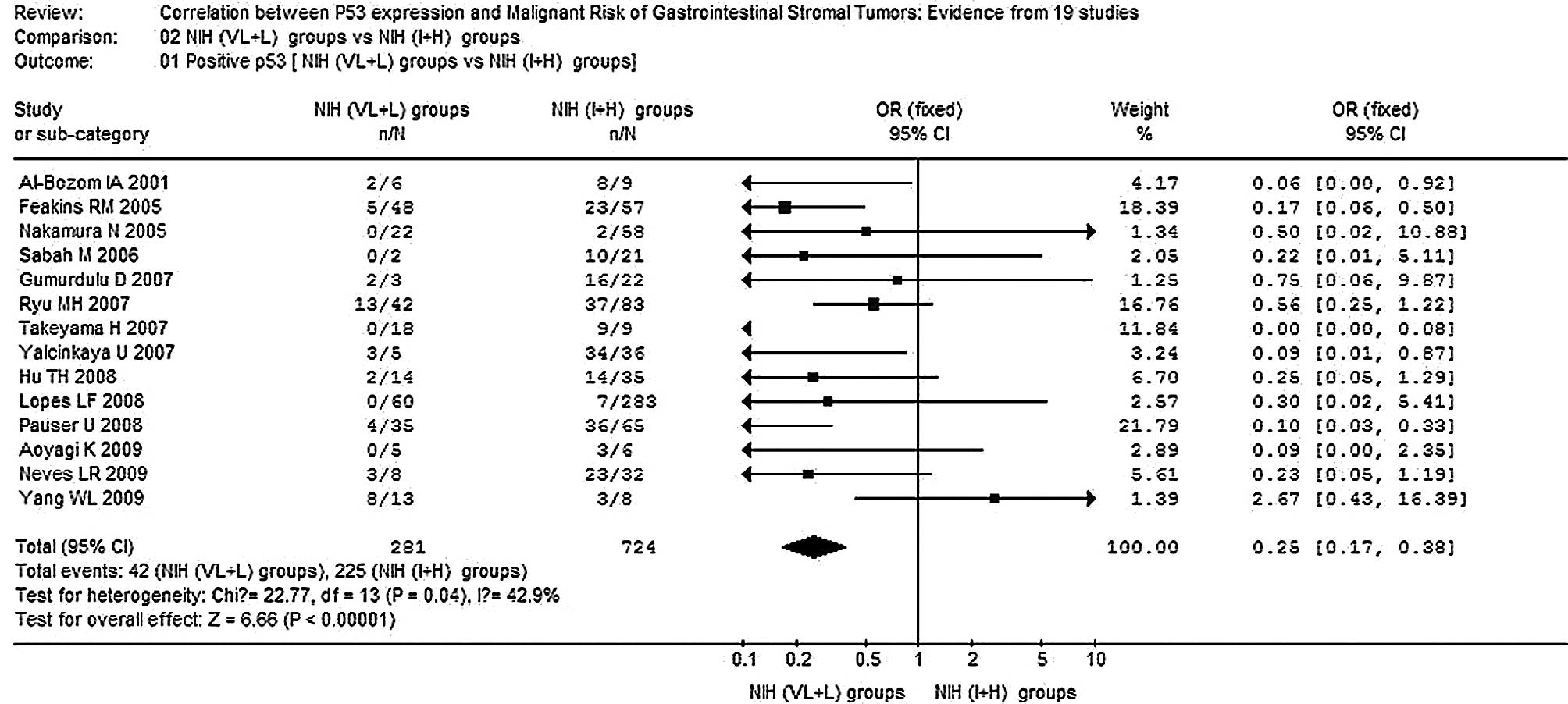
1). Moreover, significantly elevated risks of correlation
between p53 expression and the NIH intermediate risk + high risk
(I+H) group were achieved in the comparison of the NIH very low
risk + low risk (VL+L) group vs. the NIH I+H group (OR, 0.25; 95%
CI, 0.17–0.38; P<0.00001; Pheterogeneity=0.04)
(Fig. 2). The only heterogeneity
existed in a comparison of those 14 combined studies of the NIH
VL+L group vs. the NIH I+H group (P<0.10). In this analysis,
although the p53-positive rate in the study of Yang et al
(18) did not follow the tendency
of other studies, the corresponding pooled OR was not materially
altered with or without including both of them. No other single
study affected the pooled OR qualitatively as indicated by
sensitivity analyses (data not shown).
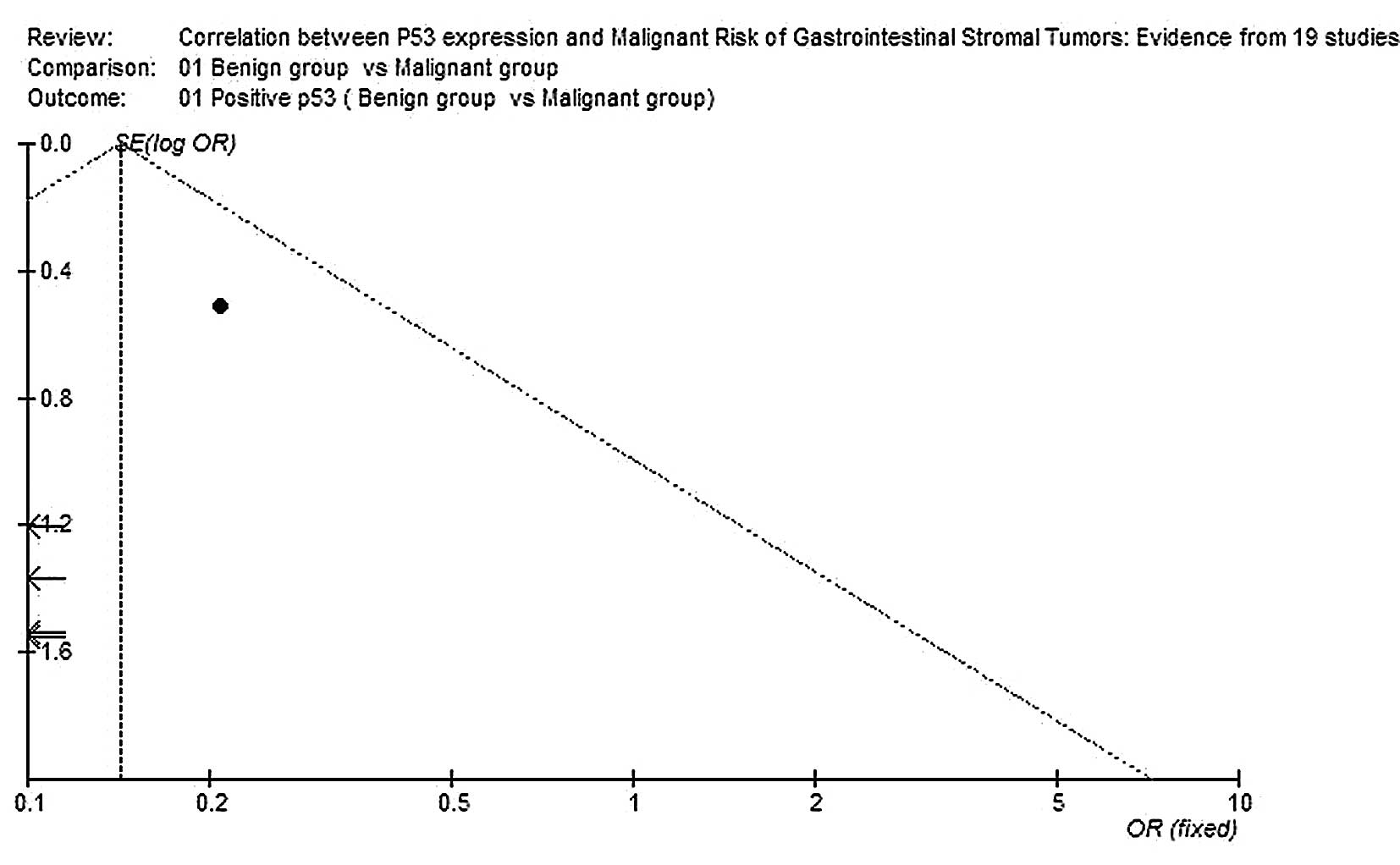
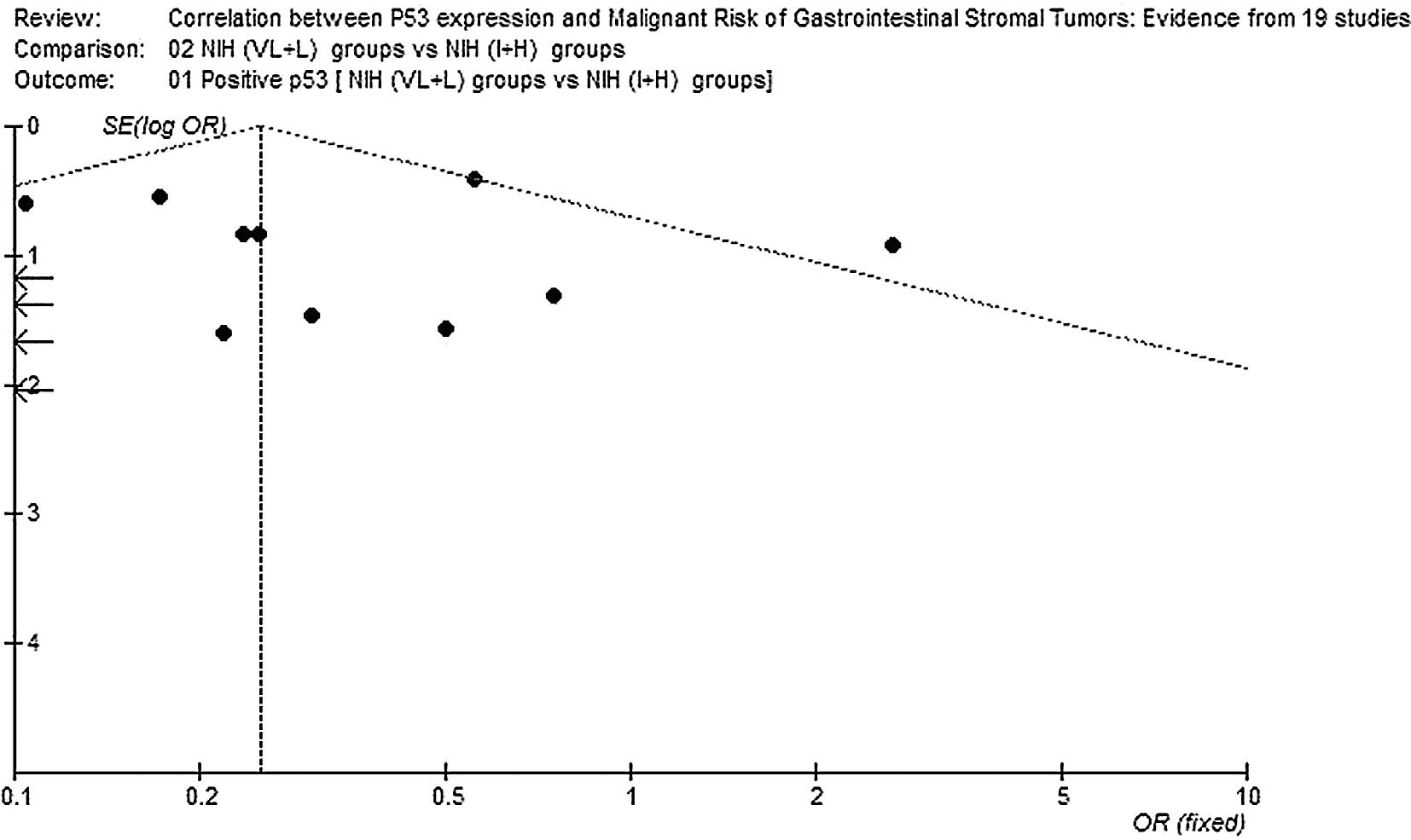
Publication bias
Begg’s funnel plot was performed to assess the
publication bias of the literature. The shapes of the funnel plots
did not reveal any evidence of marked asymmetry (Figs. 3 and 4).
Discussion
To date, scientists have been looking for various
criteria to determine the biological behavior of GIST and only two
classification methods have been widely applied and admitted. The
most direct way is by classifying the GIST patients into two groups
(a malignant group and a benign group), based on clinical outcome
by follow-up, to satisfy the criteria as follows: i) Malignant
definition: peripheral invasive growth, lymph node metastasis,
metastasis to another organ, recurrence or mortality; ii) other
cases without evidence of malignancy are classified as benign. This
malignant-benign system is used to achieve the guaranteed result of
the biological behavior of GIST by long-term follow-up. Therefore,
it is difficult to predict the malignant behavior of GIST before
any standard system is established. On the other hand, a number of
studies have suggested that tumor stage at presentation, tumor size
and mitotic activity are significant clinicopathological markers.
Accordingly, the NIH system, based on tumor size and mitotic
activity, has been established to predict GIST behavior by using
risk assessment (very low risk, low risk, intermediate risk, and
high risk), rather than attempting to draw a sharp line between
benign and malignant lesions. Moreover, the NIH system as a
prognostic tool is supported by the guaranteed evidence from
certain follow-up studies (24).
Activating mutations of the genes, c-kit and PDGFRα,
characterize the tumor entity GIST. The mutation status is
important for prognosis and a predictive factor for the response to
therapy with the tyrosine kinase receptor inhibitor, imatinib
(33).
Altered cell cycle regulation may underlie the
tumorigenesis and/or the progression of human malignancies.
Regarding p53 expression in GIST, certain studies have been carried
out, with conflicting results. Cai et al evaluated p53
expression in 55 GIST patients and concluded that p53 expression
may be associated with the transformation of leiomyoma into
leiomyosarcoma, and may be used as a predictive marker for
prognosis (35). Hillemanns et
al found four out of five metastasizing GIST cases to be p53
positive and concluded that positivity may indicate a more
aggressive course (34). Chang
et al studied 31 intestinal tumors divided into two groups,
clinically aggressive and clinically benign (20). They found p53 expression in 31% of
aggressive cases and 0% of benign cases and concluded that p53
expression, in conjunction with other parameters such as
cellularity, MI, tumor size, degree of necrosis and pleomorphism,
is important in predicting malignancy. By contrast, Lopes et
al studied 33 cases of GIST and did not find this correlation
between p53 and behavior, although in their study, 8 out of 14
cases with tumor size <5 cm in diameter and 3 out of 19 cases
with tumor size >5 cm showed some positivity with p53, which
they ignored and considered statistically insignificant (36).
Whether p53 expression is a prognostic or predictive
marker in malignant GIST has attracted considerable attention. With
a goal to explore the possible association between p53 and the
biological behavior of GIST, we performed this meta-analysis of the
published studies to derive an overall pooled estimation. Our
meta-analysis showed that p53 expression appeared more often in
recurred or metastasized GIST (malignant group) than in tumors with
disease-free follow-up (benign group). Furthermore, p53 expression
was significantly associated with the established prognostic
criteria (NIH system), and was consistent with most previous GIST
studies (6,11–13,15,16–19).
NIH I+H showed more positivity with p53 than NIH VL+L tumors.
These data indicate the impact of the tumor
suppressor gene, p53, on GIST progression. Our results confirmed
p53 as a powerful immunohistochemical marker for predicting the
risk of malignancy in GIST and having a close correlation with NIH
I+H. However, a small sample size, varied clones of antibodies
tested and potential heterogeneity, limit us to conclude more
precise results. Lopes et al studied p53 expression in 343
GIST patients and found expression only in 2.6% of cases, of which
all belonged to the high-risk group for aggressive behavior
according to the NIH consensus approach (9). They revealed that p53 expression
exists with a lower positive rate but is not a common phenomenon
for the specified group. Therefore, mitotic count and tumor size
are still the most significant prognostic criteria for the
classification of GIST, and in conjunction with p53 expression are
important in predicting malignancy, particularly for the NIH I+H
group.
Immunohistochemical staining should be positive for
wild-type p53 as well as for mutant-type p53, but wild-type p53 is
barely detectable by immunohistochemistry. However, most positive
cells represent mutant p53 since the half-life of the wild-type p53
protein is very short, and mutant-type p53 is altered in structure
with a longer half-life and greater stability. At this point there
should be a molecular incidence of p53 mutation driving the
progression of GIST to more malignant behavior in theory.
Notably, c-kit and PDGFRA mutations represent the
primary genetic alteration found in the majority of cases of GIST.
Carcinogenesis and tumor progression are favored by the
accumulation of genetic events.
El-Rifai et al demonstrated that malignant
GIST contains more genetic alterations than tumors of a benign
nature (37). We assumed that p53
mutation may be one of the significant incidents in the progression
of GIST. p53, a tumor suppressor gene, is mapped on chromosome 17p
and has a crucial function in DNA repair and in the regulation of
apoptosis. Mutation of p53 leads to disruption of these pathways
and results in a selective growth advantage for tumor cells. At
present, studies focusing on p53 mutation are still few in number.
However, it is necessary to conduct large trials to explore the
correlation of the p53 mutation genotype with the biological
behavior of GIST. Moreover, p53 mutation may be a molecular
incident in the progression of GIST. The p53 gene also requires
further investigation with regard to resistance to imatinib and
prognosis in metastatic GIST. Molecular p53-targeting agents, such
as small-molecule MDM2 antagonists, termed nutlins, and PRIMA-1,
which are able to restore the DNA-binding property of a wide range
of mutant p53 proteins, may be developed and put into clinical use.
Furthermore, the combination of such p53-targeting agents and
imatinib may improve outcomes in GIST patients with a p53
mutation.
References
|
1.
|
S GeorgeJ DesaiManagement of
gastrointestinal stromal tumors in the era of tyrosine kinase
inhibitorsCurr Treat Options
Oncol3489496200210.1007/s11864-002-0068-212392638
|
|
2.
|
S HirotaK IsozakiY MoriyamaK HashimotoT
NishidaS IshiguroK KawanoM HanadaA KurataM TakedaGain-of-function
mutations of c-kit in human gastrointestinal stromal
tumorsScience279577580199810.1126/science.279.5350.5779438854
|
|
3.
|
MC HeinrichCL CorlessA DuensingL
McGreeveyCJ ChenN JosephS SingerDJ GriffithA HaleyA TownGD
DemetriCD FletcherJA FletcherPDGFRA activating mutations in
gastrointestinal stromal
tumorsScience299708710200310.1126/science.107966612522257
|
|
4.
|
S HirotaA OhashiT NishidaK IsozakiK
KinoshitaY ShinomuraY KitamuraGain-of-function mutations of
platelet-derived growth factor receptor alpha gene in
gastrointestinal stromal
tumorsGastroenterology125660667200310.1016/S0016-5085(03)01046-112949711
|
|
5.
|
CD FletcherJJ BermanC CorlessF GorsteinJ
LasotaBJ LongleyM MiettinenTJ O’LearyH RemottiBP RubinDiagnosis of
gastrointestinal stromal tumors: a consensus approachHum
Pathol33459465200210.1053/hupa.2002.12354512094370
|
|
6.
|
RM FeakinsThe expression of p53 and bcl-2
in gastrointestinal stromal tumours is associated with anatomical
site, and p53 expression is associated with grade and clinical
outcomeHistopathology46270279200510.1111/j.1365-2559.2005.02071.x15720412
|
|
7.
|
D GumurduluS ErdoganF KayaselcukG
SeydaogluCK ParsakO DemircanI TuncerExpression of COX-2, PCNA,
Ki-67 and p53 in gastrointestinal stromal tumors and its
relationship with histopathological parametersWorld J
Gastroenterol13426431200710.3748/wjg.v13.i3.42617230613
|
|
8.
|
TH HuMH TaiSK ChuahHH ChenJW LinHY HuangYP
ChouLN YiCM KuoCS ChangchienElevated p21 expression is associated
with poor prognosis of rectal stromal tumors after resectionJ Surg
Oncol98117123200810.1002/jso.2109418521824
|
|
9.
|
LF LopesEB OjopiCE BacchiGastrointestinal
stromal tumor in Brazil: Clinicopathology, immunohistochemistry,
and molecular genetics of 513 casesPathol
Int58344352200810.1111/j.1440-1827.2008.02235.x
|
|
10.
|
N NakamuraH YamamotoT YaoY OdaK NishiyamaM
ImamuraT YamadaH NawataM TsuneyoshiPrognostic significance of
expressions of cell-cycle regulatory proteins in gastrointestinal
stromal tumor and the relevance of the risk gradeHum
Pathol36828837200510.1016/j.humpath.2005.03.01216084954
|
|
11.
|
LR NevesCT OshimaR Artigiani-NetoG
YanaguibashiLG LourençoNM ForonesKi67 and p53 in gastrointestinal
stromal tumors - GISTArq
Gastroenterol46116120200910.1590/S0004-2803200900020000819578612
|
|
12.
|
U PauserN Schmedt Auf der GünneG KlöppelH
MerzAC FellerP53 expression is significantly correlated with high
risk of malignancy and epithelioid differentiation in GISTs. An
immunohistochemical study of 104 casesBMC
Cancer8204200810.1186/1471-2407-8-20418651966
|
|
13.
|
MH RyuYK KangSJ JangTW KimH LeeJS KimYH
ParkSS LeeBY RyooHM ChangPrognostic significance of p53 gene
mutations and protein overexpression in localized gastrointestinal
stromal
tumoursHistopathology51379389200710.1111/j.1365-2559.2007.02797.x17727479
|
|
14.
|
WL YangJR YuYJ WuKK ZhuW DingY GaoQY
ShenKZ LvQ ZhangXJ YangDuodenal gastrointestinal stromal tumor:
clinical, pathologic, immunohistochemical characteristics, and
surgical prognosisJ Surg Oncol100606610200910.1002/jso.21378
|
|
15.
|
H TakeyamaH FunahashiH SawaiH TakahashiM
YamamotormY AkamoT ManabeExpression of α6 integrin subunit is
associated with malignancy in gastric gastrointestinal stromal
tumorsMed Sci Monit13CR51562007
|
|
16.
|
IA Al-Bozomp53 expression in
gastrointestinal stromal tumorsPathol
Int51519523200110.1046/j.1440-1827.2001.01233.x11472564
|
|
17.
|
M SabahR CumminsM LeaderE KayAltered
expression of cell cycle regulatory proteins in gastrointestinal
stromal tumors: markers with potential prognostic implicationsHum
Pathol37648655200610.1016/j.humpath.2006.01.023
|
|
18.
|
K AoyagiK KouhujiS YanoM MiyagiT ImaizumiJ
TakedaK ShirouzuMalignant potential of gastrointestinal stromal
tumor of the stomachInt Surg9419200920099418
|
|
19.
|
U YalcinkayaO YerciEU KocSignificance of
p53 expression in gastrointestinal stromal
tumorsHepatogastroenterology54140143200717419248
|
|
20.
|
MS ChangG ChoeWH KimYI KimSmall intestinal
stromal tumors: A clinicopathologic study of 31 tumorsPathol
Int483417199810.1111/j.1440-1827.1998.tb03916.x9704340
|
|
21.
|
RS MearaJ CangiarellaA SimsirD HortonI
EltoumDC ChhiengPrediction of aggressiveness of gastrointestinal
stromal tumours based on immunostaining with bcl-2, Ki-67 and
p53Cytopathology18283289200710.1111/j.1365-2303.2007.00505.x17883690
|
|
22.
|
X WangI MoriW TangH UtsunomiyaM NakamuraY
NakamuraG ZhouK KakudoGastrointestinal stromal tumors:
Clinicopathological study of Chinese casesPathol
Int51701706200110.1046/j.1440-1827.2001.01260.x11696173
|
|
23.
|
SO OzdamarS BektaşS Erdem OzdamarG
GedikoğluB Doğan GünB BahadirNuclear morphometric analysis in
gastrointestinal stromal tumors: A preliminary studyTurk J
Gastroenterol187176200717602353
|
|
24.
|
A Panizo-SantosI SolaF VegaE de AlavaMD
LozanoMA IdoateJ Pardo-MindánPredicting metastatic risk of
gastrointestinal stromal tumors: role of cell proliferation and
cell cycle regulatory proteinsInt J Surg
Pathol8133144200010.1177/10668969000080020811493978
|
|
25.
|
YP ChouJW LinCC WangYC ChiuCC HuangSK
ChuahMH TaiLN YiCM LeeCS ChangchienTH HuThe abnormalities in the
p53/p21WAF1 pathway have a significant role in the pathogenesis and
progression of gastrointestinal stromal tumorsOncol
Rep194956200818097575
|
|
26.
|
D PadillaP MenéndezM GarcíaP VillarejoT
CuboD GambíR PardoJ MartínImmunohistochemical expression of
epidermal growth factor and its prognostic value for
gastrointestinal stromal tumorsRev Esp Enferm
Dig100752757200819222333
|
|
27.
|
S RomeoM Debiec-RychterM van GlabbekeH van
PaassenP ComiteR van EijkJ OostingJ VerweijP TerrierU SchneiderR
SciotJY BlayPC HogendoornEuropean Organization for Research and
Treatment of Cancer Soft Tissue and Bone Sarcoma GroupCell
cycle/apoptosis molecule expression correlates with imatinib
response in patients with advanced gastrointestinal stromal
tumorsClin Cancer
Res1541914198200910.1158/1078-0432.CCR-08-329719509155
|
|
28.
|
MJ KwonES NamSJ ChoHR ParkHS ShinJH ParkCH
ParkWJ LeeComparison of tissue microarray and full section
inimmunohistochemistry of gastrointestinal stromal tumorsPathol
Int59851856200910.1111/j.1440-1827.2009.02465.x20021609
|
|
29.
|
S SakuraiM FukayamaY KaizakiK SaitoK
KanazawaM KitamuraY IwasakiT HishimaY HayashiM KoikeTelomerase
activity in gastrointestinal stromal
tumorsCancer8320602066199810.1002/(SICI)1097-0142(19981115)83:10%3C2060::AID-CNCR3%3E3.0.CO;2-%239827709
|
|
30.
|
X WangI MoriW TangH UtsunomiyaM NakamuraY
NakamuraG ZhouK KennichiHelpful parameter for malignant potential
of gastrointestinal stromal tumors (GIST)Jpn J Clin
Oncol32347351200210.1093/jjco/hyf07412417600
|
|
31.
|
MC TsaiJW LinSE LinHH ChenCM LeeTH
HuPrognostic analysis of rectal stromal tumors by reference of
national institutes of health risk categories and
immunohistochemical studiesDis Colon
Rectum5115351543200810.1007/s10350-008-9370-918633679
|
|
32.
|
NA WongR YoungRD MalcomsonAG NayarLA
JamiesonVE SaveFA CareyDH BrewsterC HanA Al-NafussiPrognostic
indicators for gastrointestinal stromal tumours: a
clinicopathological and immunohistochemical study of 108 resected
cases of the
stomachHistopathology43118126200310.1046/j.1365-2559.2003.01665.x12877726
|
|
33.
|
P ChenL ZongW ZhaoL ShiEfficacy evaluation
of imatinib treatment in patients with gastrointestinal stromal
tumors: a meta-analysisWorld J
Gastroenterol1642274232201010.3748/wjg.v16.i33.422720806443
|
|
34.
|
M HillemannsS PasoldK BottcherH
HoflerPrognostic factors of gastrointestinal stromal tumors of the
stomachVerh Dtsch Ges Pathol82261266199810095444
|
|
35.
|
J CaiY JiangY ZhangG LuX ZhangQ GaoL
ZuoQuantitation of p53 protein expression in gastrointestinal
smooth muscle tumors, clinicopathological correlation and
prognostic significanceChin Med J1086696731995
|
|
36.
|
JM LopesP SilvaM SeixasL CirnesR
SerucaMicrosatellite instability is not associated with degree of
malignancy and p53 expression of gastrointestinal stromal
tumorsHistopathology3357658719939870157
|
|
37.
|
W El-RifaiM Sarlomo-RikalaLC AnderssonS
KnuutilaM MiettinenDNA sequence copy number changes in
gastrointestinal stromal tumors: tumor progression and prognostic
significanceCancer Res6038993903200010919666
|