Introduction
Lung cancer is a major cause of mortality among
malignant diseases due to its high incidence and malignant behavior
as well as a lack of major advancements in treatment strategy
(1). Lung cancer was the leading
indication for respiratory surgery (46.7%) in 2007 in Japan
(2), and more than 25,000 patients
underwent surgery for lung cancer at Japanese institutions in the
same year (2). The clinical
behavior of lung cancer is largely associated with its stage. The
cure of the disease by surgery is only achieved in cases
representing an early stage of lung cancer (3).
Sox2 belongs to a family of evolutionarily conserved
transcription factors containing a Sry-related high mobility group
(HMG) box (4,5). Sox proteins are recognized as key
players in the regulation of embryonic development and
determination of cell fate and maintenance (6–9).
Later in development, Sox2 is expressed in the endodermal
epithelium of the tongue, esophagus, trachea and lung, and plays
crucial roles in the differentiation and morphogenesis of these
organ systems (5,10–12).
Recently, Sox2 amplifications were investigated using
large-scale single nucleotide polymorphism (SNP) arrays in
esophageal and lung cancer (13).
Sox2 is highly expressed in squamous cell carcinoma (SCC) of the
gastrointestinal tract (14).
Although Sox2 expression was also investigated in small cell lung
cancers (15) and lung SCC (mRNA)
(16), the correlation between
Sox2 gene status and lung cancer in Japan has not been
previously reported.
To determine the Sox2 copy number status in
Japanese lung carcinoma patients for screening purposes, we
investigated the Sox2 copy number using real-time polymerase
chain reaction (real-time PCR) amplifications. The findings were
compared against the clinicopathological features of lung
cancer.
Patients and methods
Patients
The study group included 127 lung cancer patients
who had undergone surgery at the Department of Surgery II, Nagoya
City University Medical School, Japan, between 2001 and 2008. All
tumor samples were immediately frozen and stored at −80°C until
assayed. The clinical and pathological characteristics of the 127
lung cancer patients were as follows: 78 cases at stage I, 24 at
stage II, and 25 at stages III–IV. The mean age was 66.0 years
(range, 29–86). Among the patients, 30 (23.6%) were non-smokers, 94
(74.0%) were male and 87 (68.5%) were diagnosed as having SCC (this
study focused mainly on SCC). The samples from these patients had
previously been sequenced for EGFR (17–20).
PCR assays for Sox2
Genomic DNA was extracted from lung cancer tissues
using the Wizard SV Genomic DNA Purification System (Promega,
Madison, WI, USA) according to the manufacturer’s instructions. The
DNA concentration was determined using a NanoDrop spectrophotometer
(NanoDrop Technologies, Inc., Rockland, DE, USA) and adjusted to a
concentration of 2.5 ng/ml.
We then used 5 μl of each DNA for the PCR
assays. The Sox2 copy number was analyzed by quantitative
real-time PCR and performed on the 7500 Real-Time PCR System
(Applied Biosystems, Foster City, CA, USA) using a QuantiTect
SYBR-Green kit (Qiagen, Inc., Valencia, CA, USA) (21,22).
The PCR run was performed in triplicate for each patient. The
Sox2 primers used for amplification were 5′-CAAAGAAAAA
CGAGGGAAAT-3′ and 5′-ATGGGATTGGTGTTCTCTTT-3′. Total DNA content was
estimated by assaying LINE-1 elements for each sample using
the primers 5′-AAAGCCGCTCAA CTACATGG-3′ and
5′-TGCTTTGAATGCGTCCCAGAG-3′.
The cycling conditions were as follows: initial
denaturation at 95°C for 15 min, followed by 40 cycles at 94°C for
15 sec, 56°C for 30 sec, and 72°C for 34 sec.
Immunohistochemistry
Sox2 and p63 protein expression were evaluated by
immunohistochemistry (IHC) using an anti-Sox2 antibody (rabbit
polyclonal; Thermo Scientific, Rockford, IL, USA) and a p63
ready-to-use antibody (mouse, clone 4A4; Dako, Carpinteria, CA,
USA). We used a standard protocol for the immunostaining of the
samples. Sections (4 μm) were cut from paraffin tissue
blocks from the non-small cell lung cancer tumors. The slides were
treated with xylene then dehydrated in alcohol. For epitope
retrieval, specimens were exposed to 10 mM citrate buffer (pH 6.0)
and heated to approximately 10 min in a microwave. Endogenous
peroxidase activity was blocked with H2O2 in
methanol. Sections were incubated with blocking solution (10% Block
Ace) and then reacted with anti-Sox2 (x250) or p63 antibody
overnight at 4°C. After washing out the excess antibody with
phosphate-buffered saline (PBS), samples were incubated with a
peroxidase-conjugated anti-mouse antibody (Mouse HRP EnVision™+;
Dako) for 45 min. After washing out the excess antibody with PBS,
3,3′-diaminobenzidine (DAB) substrate (10 min) was used to
visualize the antibody binding, and the sections were
counterstained with hematoxylin. Sox2 and p63 staining was
evaluated under a light microscope at x400 magnification. Tumor
nuclear staining intensity was graded on a scale of 1–4. The
percentage of positive tumor nuclei was evaluated and a proportion
score was attributed (1, <5%; 2, 5–25%; 3, 25–50% and 4,
>50%), as previously described (23,24).
Statistical analysis
Statistical analyses were performed using the
Mann-Whitney U-test for unpaired samples and Wilcoxon’s signed rank
test for paired samples. Linear relationships between variables
were determined by means of simple linear regression. Correlation
coefficients were determined by rank correlation using the
Spearman’s test and the χ2 test. The overall survival of
lung cancer patients was evaluated by the Kaplan-Meier method, and
differences were determined by the log-rank test. All analyses were
performed using the StatView software package (Abacus Concepts
Inc., Berkeley, CA, USA), and p<0.05 was considered
significant.
Results
Sox2 gene status in Japanese lung cancer
patients
Using the primer sets for the Sox2 gene, 42
of the 127 lung cancer patients were found to have more than 4
copies of the Sox2 gene. The clinicopathological background
of the patients is shown in Table
I. The Sox2 gene copy status was significantly
correlated with gender (male, 40.4% vs. female, 12.1%; p=0.0026),
tobacco smoking (non-smokers, 6.6% vs. smokers, 41.2%; p=0.0003)
and pathological subtype (squamous cell carcinoma, 44.8% vs.
non-squamous cell carcinoma, 7.5%; p<0.0001), but not with
pathological stage (stage I vs. II–IV, p=0.3878) or age (≤65 vs.
>65; p=0.8052). The increased Sox2 gene copy number was
observed in 2/13 (15.4%) of the adenosquamous cell carcinomas and
1/26 (3.8%) of the adenocarcinomas. There was a tendency towards a
higher degree of differentiation within the normal Sox2 copy
number group when compared with the increased Sox2 copy
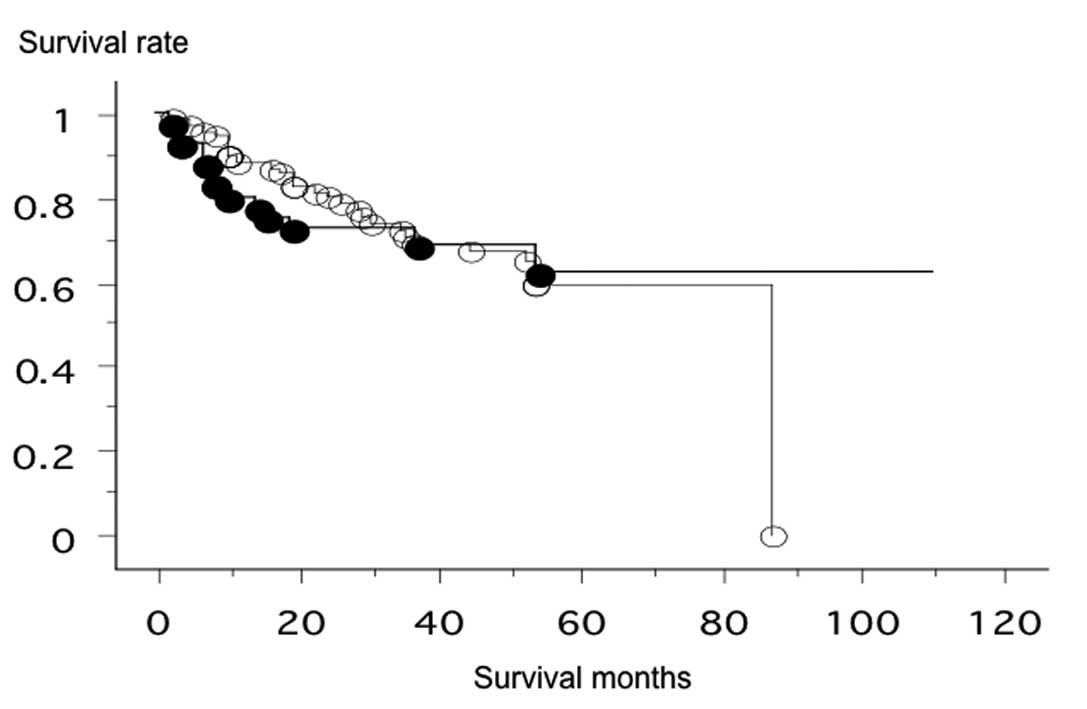
number group (p=0.1046). The overall survival of the 127 lung
cancer patients from Nagoya City University, who were followed-up
until December 31 2009, was studied in reference to the Sox2
gene status. The survival rate of patients with an increased
Sox2 gene copy number (n=42, 13 were deceased) and patients
with a normal Sox2 copy number (n=85, 27 were deceased) was
not significantly different (log-rank test, p=0.9299; Fig. 1).
 | Table IClinicopathological data of 127 lung
cancer patients. |
Table I
Clinicopathological data of 127 lung
cancer patients.
| Factors | | Sox2 gene
status
| p-value |
|---|
| Amplified Patients
(n=42) | Normal Patients
(n=85) |
|---|
| Mean age
(years) | 66.0±10.2 | 67.1±8.7 | 65.5±10.8 | 0.4134 |
| Stage | | | | |
| I | | 26 (61.9%) | 52 (61.2%) | 0.9999 |
| II–IV | | 16 (38.1%) | 33 (38.8%) | |
| Lymph node
metastasis | | | | |
| N0 | | 29 (55.8%) | 56 (65.9%) | 0.2779 |
| N(+) | | 23 (44.2%) | 29 (34.1%) | |
| Smoking status | | | | |
| Never smoker | | 2 (4.8%) | 28 (32.9%) | 0.0003 |
| Smoker | | 40 (95.2%) | 57 (67.1%) | |
|
Differentiation | | | | |
|
Well-differentiated | | 6 (15.4%) | 21 (31.3%) | 0.1046 |
|
Moderate/poor | | 33 (64.6%) | 46 (68.7%) | |
| Pathological
subtype | | | | |
| Squamous | | 39 (92.9%) | 48 (56.5%) | |
| Non-squamous | | 3 (7.1%) | 37 (43.5%) | <0.0001 |
| Age | | | | |
| ≤65 | | 18 (42.9%) | 39 (45.9%) | 0.8501 |
| >65 | | 24 (57.1%) | 46 (54.1%) | |
| Gender | | | | |
| Male | | 38 (90.5%) | 56 (65.9%) | 0.0026 |
| Female | | 4 (9.5%) | 29 (34.1%) | |
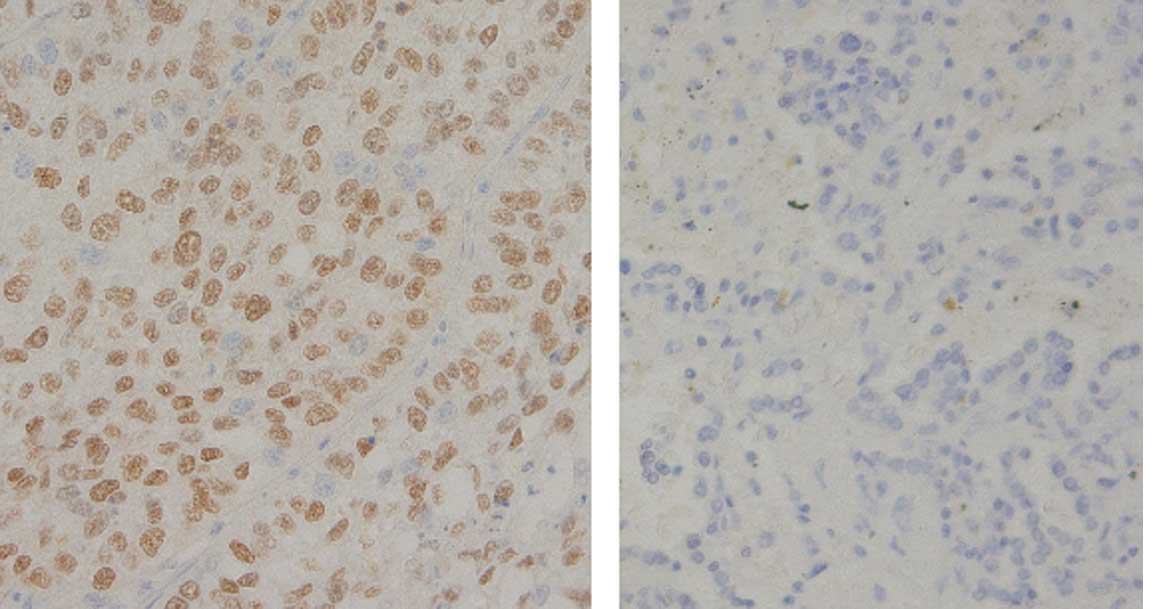
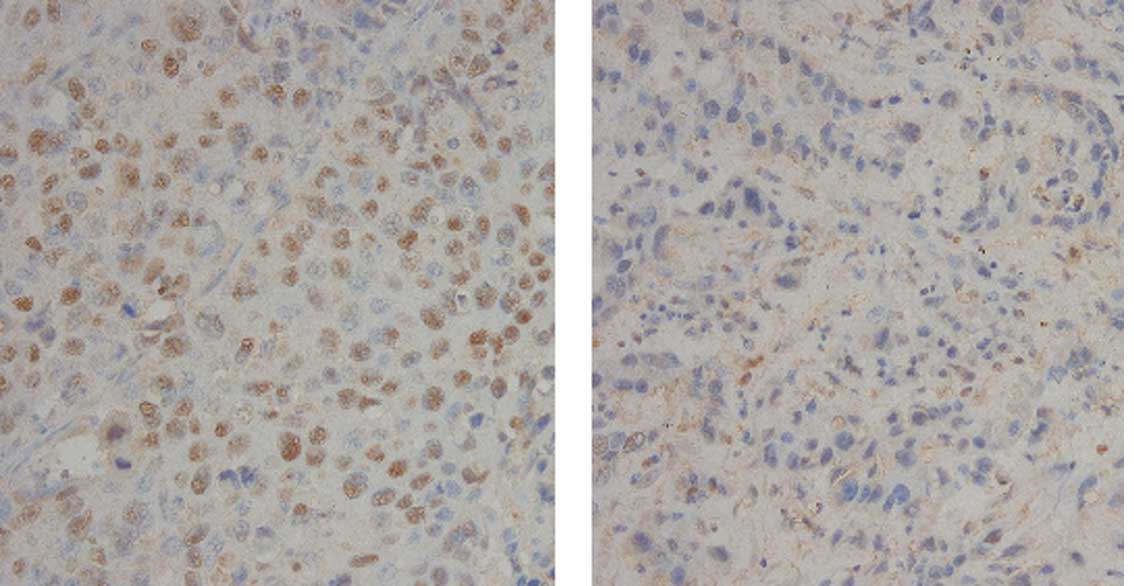
Immunohistochemistry
The immunohistochemical evaluation was performed
according to the scoring system described in Materials and methods.
IHC was performed for 72 patients, since the tissue blocks were not
available for the other patients. The p63-positive (≥4) ratio
(Fig. 2) was more closely
correlated with squamous histology (adenosquamous + SCC, 95.2% vs.
adenocarcinoma, 60%; p=0.0004) than the Sox2-positive ratio
(adenosquamous + SCC, 85.4% vs. adenocarcinoma, 45.5%; p=0.001)
(Fig. 3). The p63 IHC was
correlated with an increased Sox2 copy number (p=0.0226).
The Sox2-positive IHC cases had a tendency towards an increased
Sox2 copy number (p=0.1035). However, the p63 IHC status
(p=0.8188) and Sox2 IHC status (p=0.3394) did not correlate with
the prognosis of lung cancers.
Discussion
In this analysis, we found an increased Sox2
gene copy number in 33% of the Japanese lung cancer patients. The
Sox2 gene statuses were mainly correlated with squamous
histology.
The HMG domain is a DNA-binding motif that was
originally discovered in abundant nonhistone components of
chromatin. The proteins containing this motif are considered as
architectural components in the assembly of nucleoprotein
complexes, and they regulate transcription by interacting with the
minor groove of the DNA helix and modulating DNA structure by
bending the DNA helix (25). At
least 20 Sox genes have so far been identified in mammals
and more in other vertebrates (26–28),
and they have been implicated in the regulation of a variety of
developmental processes. Sox2 has a recognized role in lung
branching morphogenesis and differentiation in embryonic
development. Mice engineered to overexpress Sox2 in the developing
lung epithelium have a marked reduction in the number of airways
(5). Sox2 is modulated in concert
during the course of tracheal and esophageal development (12).
Sox2 is highly expressed in SCC of the
gastrointestinal tract (14). Sox2
was expressed in 81% of esophageal SCC cases and 91% of anal canal
SCC cases, compared with 13% and 17% of esophageal and rectal
adenocarcinoma cases, respectively (14). In the upper gastrointestinal tract,
Sox2 is expressed in the developing foregut endoderm and is thought
to play a role in establishing the boundary between the
keratinized, squamous-lined esophagus and the glandular hindstomach
(12). The high correlation
between p63 and Sox2 expression was reported in esophageal cancer
and raised the issue of a possible contribution of Sox2 to squamous
cell carcinogenesis, perhaps via disturbed differentiation and
patterning in the context of other procarcinogenic events.
Copy number increase in the 3q26-qter region is
observed in various squamous cell cancers including lung,
esophagus, head and neck and cervix (13). Two studies have reported the
identification of Sox2 as a novel oncogene in lung and esophageal
SCC (13,29). The knockdown experiments
demonstrated that Sox2 was necessary for lung squamous cell
viability by protecting cells from apoptosis (13,29).
The suppression of Sox2 with shRNA constructs reduced proliferation
and colony formation in the 3q26.33-amplified cell lines but not in
the controls (13). Although Sox2
itself was not transforming, the combination of Sox2 with the FOXE1
or FGFR2 isoforms promoted anchorage-independent growth in cancers
(13).
Sholl et al demonstrated that Sox2 IHC
expression was more common in poorly differentiated adenocarcinoma
of the lung (23), and this study
also demonstrated that there was a tendency towards a higher degree
of differentiation within the normal Sox2 copy number group
compared to the increased Sox2 copy number group (p=0.1046).
However, Hussenet et al addressed the relationship between
the Sox2 expression level and histological differentiation status
in lung SCC and found no significant changes (29). They found that Sox2 is highly
expressed in both aggressive and non-aggressive tumors with no
statistically significant differences between the two groups
(29). This result is consistent
with the very high expression levels of Sox2 protein observed in
the majority of primary SCC cases (13,23,29).
Acknowledgements
The authors would like to thank Mrs.
Akiha Kuramoto for her excellent technical assistance. This study
was supported by Grants-in-Aid for scientific research from the
Japan Society for the Promotion of Science (JSPS) (nos. 23659674,
21390394 and 21591820) and a grant for cancer research of the
Program for Developing the Supporting System for Upgrading
Education and Research (2009) from the Ministry of Education,
Culture, Sports, Science and Technology of Japan.
References
|
1.
|
RJ GinsbergMK KrisJG ArmstrongCancer of
the lungPrinciples and Practice of Oncology4th
editionLippincottPhiladelphia6736821993
|
|
2.
|
Y UedaY FujiiH KuwanoThoracic and
cardiovascular surgery in Japan during 2007. Annual report by the
Japanese association for thoracic surgeryGen Thorac Cardiothorac
Surg57488200710.1007/s11748-009-0460-y19756938
|
|
3.
|
PE PostmusChemotherapy for non-small cell
lung cancer: the experience of the Lung Cancer Cooperative Group of
the European Organization for Research and Treatment of
CancerChest11328S31S199810.1378/chest.113.1_Supplement.28S9438687
|
|
4.
|
J QueX LuoRJ SchwartzMultiple roles for
Sox2 in the developing and adult mouse
tracheaDevelopment13618991907200910.1242/dev.03462919403656
|
|
5.
|
C GontanA de MunckM VermeijSox2 is
important for two crucial processes in lung development: Branching
morphogenesis and epithelial cell differentiationDev
Biol317296309200810.1016/j.ydbio.2008.02.03518374910
|
|
6.
|
Y KamachiM UchikawaH KondohPairing SOX
off: with partners in the regulation of embryonic developmentTrends
Genet16182187200010.1016/S0168-9525(99)01955-110729834
|
|
7.
|
V LefebvreB DumitriuA Penzo-MendezControl
of cell fate and differentiation by Sry-related high-mobility group
box (Sox) transcription factorsInt J Biochem Cell
Biol3921952214200710.1016/j.biocel.2007.05.01917625949
|
|
8.
|
G SchepersR TeasdaleP KoopmanTwenty pairs
of sox, extent, homology, and nomenclature of the mouse and human
sox transcription factor gene familiesDev Cell3167200212194848
|
|
9.
|
M WilsonP KoopmanMatching SOX: partner
proteins and co-factors of the SOX family of transcriptional
regulatorsCurr Opin Genet
Dev12441446200210.1016/S0959-437X(02)00323-412100890
|
|
10.
|
Y IshiiM RexPJ ScottingRegion-specific
expression of chicken Sox2 in the developing gut and lung
epithelium: regulation by epithelial-mesenchymal interationsDev
Dyn213464475199810.1002/(SICI)1097-0177(199812)213:4%3C464::AID-AJA11%3E3.0.CO;2-Z9853967
|
|
11.
|
T OkuboLH PevnyBL HoganSox2 is required
for development of taste bud sensory cellsGenes
Dev2026542659200610.1101/gad.145710617015430
|
|
12.
|
J QueT OkuboJR GoldenringMultiple
dose-dependent roles for Sox2 in the patterning and differentiation
of anterior foregut
endodermDevelopment13425212531200710.1242/dev.00385517522155
|
|
13.
|
AJ BassH WatanabeCH MermelSOX2 is an
amplified lineage-survival oncogene in lung and esophageal squamous
cell carcinomasNature Genet4112381242200910.1038/ng.46519801978
|
|
14.
|
KB LongJL HornickSOX2 is highly expressed
in squamous cell carcinomas of the gastrointestinal tractHum
Pathol4017681773200910.1016/j.humpath.2009.06.00619716157
|
|
15.
|
MJ TitulaerR KloosterM PortmanSOX
antibodies in small-cell lung cancer and Lambert-Eaton myasthenic
syndrome: frequency and relation with survivalJ Clin
Oncol2742604267200910.1200/JCO.2008.20.616919667272
|
|
16.
|
P YuanH KadaraC BehrensSex determining
region Y-box 2 is a potential cell-lineage gene highly expressed in
the pathogenesis of squamous cell carcinomas of the lungPLoS
One5e9112201010.1371/journal.pone.000911220161759
|
|
17.
|
JG PaezPA JanneLC LeeEGFR mutations in
lung cancer: correlation with clinical response to gefitinib
therapyScience30414971500200410.1126/science.109931415118125
|
|
18.
|
H SasakiS ShimizuK EndoEGFR and erbB2
mutation status in Japanese lung cancer patientsInt J
Cancer118180184200610.1002/ijc.2130116003726
|
|
19.
|
K EndoA KonishiH SasakiEpidermal growth
factor receptor gene mutation in non-small cell lung cancer using
highly sensitive and fast TaqMan PCR assayLung
Cancer50375384200510.1016/j.lungcan.2005.08.00916199108
|
|
20.
|
H SasakiK EndoA KonishiEGFR mutation
status in Japanese lung cancer patients: genotyping analysis using
LightCyclerClin Cancer
Res1129242929200510.1158/1078-0432.CCR-04-190415837743
|
|
21.
|
K EndoH SasakiM YanoEvaluation of the
epidermal growth factor receptor gene mutation and copy number in
non-small cell lung cancer with gefitinib therapyOncol
Rep16533541200616865253
|
|
22.
|
TL WangC MaierhoferMR SpeicherDigital
karyotypingProc Natl Acad Sci
USA991615616161200210.1073/pnas.20261089912461184
|
|
23.
|
LM ShollKB LongJL HornickSox2 expression
in pulmonary non-small cell and neuroendocrine carcinomasAppl
Immunohistochem Mol
Morphol185561201010.1097/PAI.0b013e3181b16b8819661786
|
|
24.
|
D NonakaDifferentiatial expression of SOX2
and SOX17 in testicular germ cell tumorsAm J Clin
Pathol131731736200910.1309/AJCP7MNCNBCRN8NO19369635
|
|
25.
|
R GrosschedlK GieseJ PagelHMG domain
proteins: Architectual elements in the assembly of nucleoprotein
structuresTrends
Genet109499199410.1016/0168-9525(94)90232-18178371
|
|
26.
|
P DennyS SwiftN BrandA conserved family of
genes related to the testis determining gene, SRYNucleic Acids
Res202887199210.1093/nar/20.11.28871614875
|
|
27.
|
C HudsonD ClementsRV FridayXsox17-α and -β
mediate endoderm formation in XenopusCell913974051997
|
|
28.
|
SRH RussellN Sanchez-SorianoCR WrightM
AshburnerThe dechaete gene of Drosophila melanogaster
encodes a SOX-domain protein required for embryonic
segmentationDevelopment122366936761996
|
|
29.
|
T HussenetS DaliJ ExingerSOX2 is an
oncogene activated by recurrent 3q26.3 amplification in human lung
squamous cell carcinomasPLoS
One58960201010.1371/journal.pone.000896020126410
|