Introduction
High-intensity focused ultrasound (HIFU) surgery was
first used to created focal lesions deep in liver tissue and was
further developed by a group headed by William Fly in Illinois in
the 1950s (1). At the point where
the ultrasound (US) waves are focused, sudden and intense
absorption of the US beam creates a rapid elevation in temperature,
which destroys the cells located at the targeted area without
damaging tissue elsewhere in the path of the beam. HIFU has been
used to treat glaucoma in human patients (2) and for ablation of prostatic tissue in
dogs (3). Clinical studies also
explored the use of HIFU for the transrectal treatment of benign
prostatic hyperplasia and prostate cancer (4–6). The
main disadvantage of HIFU is that only a small amount of tissue is
ablated in a single exposure, since it works by focusing
high-energy US waves on a volume of tissue approximately the size
of a grain of rice. When larger amounts of tissue are to be
ablated, as in tumor therapy, it results in long treatment periods
and thereby adversely affects the patient’s quality of life.
Furthermore, the clinical applications of HIFU are limited due to
the rarity of an adequate acoustic window to access the tumors, or
inevitable injuries to the adjacent structures.
The microbubbles (Mbs) contain gas encased in a
shell, and have diameters between approximately 1 and 5 μm so that
they are capable of passing through the capillary network. When the
bubbles pass through the tissue volume and are exposed to US, they
expand and contract at the frequency of the propagating acoustic
wave due to the cyclic pressure reductions and increases associated
with the wave propagation. The bubble’s oscillation also causes the
surrounding fluid to flow (microstreaming), thus creating large
shear forces around the bubbles. In addition, the bubbles are
pushed by a radiation force in the direction of wave propagation
(7,8). Above a particular threshold, the
bubble’s oscillation becomes so intense that the inertia of the
surrounding fluid causes the bubble to collapse, inducing high
temperatures and pressures. The result is a shock wave, which
propagates at supersonic speed from the collapse site. If the
bubbles collapse near a vessel wall, they may create fluid jets,
which are likely to puncture the wall (9–11).
As a result, the bubbles absorb and concentrate energy from the US
wave into a microscopic tissue volume, reducing the US power levels
by at least two orders of magnitude from that required to induce
bio-effects without the bubbles (12).
The purpose of this study was to evaluate the
anti-tumor effect of low frequency and low intensity US radiation
combined with Mb intravenous injection on prostate tumors
subcutaneously implanted in nude mice.
Materials and methods
Experimental animals
A total of 40 male Balb-c nude mice (5 weeks old;
weight 18–25 g) were obtained from the Experimental Animal Center
of Shanghai (Shanghai, China). Animal experiments were approved by
the Ethics Committee of Laboratory Animal Welfare of Shanghai
Jiaotong University, Shanghai, China.
Animal model
The Du145 cell line was obtained from the cell
library of the Chinese Academy of Sciences (Shanghai, China). A
total of 2×107 Du145 cells in 200 μl of
phosphate-buffered saline were injected subcutaneously into the
right flank region of the nude mouse to establish a tumor model.
When the tumors grew to approximately 10 mm in maximum diameter, 40
mice were randomly divided into 4 groups of 10 each: the US+Mbs
group, US group, Mbs group and control group.
Microbubbles
SonoVue® (Bracco Company, Italy) was
used. After the plastic cap of the vial was removed, 5 ml sterile
normal saline was added into the vial. The vial was agitated
vigorously for approximately 20 sec before the milky white
suspension was ready.
US equipment and experimental
procedures
The Ultrasonics Processing FS4500 US Tumor
Therapeutic System (Fudan Institute Technology, China) was used in
this study. The parameters used were: US frequency 20 kHz, ISP 200
mW/cm2. In the US+Mbs group, 0.2 ml SonoVue was injected
slowly via tail veins, followed by rapid injection of 0.2 ml normal
saline as the power generator was turned on; the whole target tumor
was irradiated for 120 sec. In the US group, the same US was
applied to tumors as the US+Mbs group, but it was combined with
intravenous injection of 0.4 ml normal saline only. In the Mbs
group, the mice were intravenously injected with 0.2 ml SonoVue and
0.2 ml normal saline, but without US exposure. No interventions
were performed on the control group. The treatments were repeated
three times in total every other day.
Tumor challenge
From the first day of treatment, the tumor growth
was monitored, and its diameter was measured every 3rd day by a US
machine (ESAOTE MyLab 90, Genoa, Italy). The tumor volume was then
calculated using the formula: tumor volume
(mm3)=d2xD/2, where d and D are the shortest
and longest diameters of the measured tumor, respectively. Tumor
inhibition ratio was calculated using the formula: tumor volume
inhibition ratio (%)=(V1−V2)/V2,
where V1 and V2 are the tumor volume of
therapy groups and average volume of control group, respectively.
Differences were tested with analysis of variance or the Student’s
t-test, and results were considered to be statistically significant
at P<0.05.
Specimens
Two weeks following the intial treatment, all of the
mice in the four groups were sacrificed and tumors were surgically
excised. Sections (1 mm3) were sampled, double fixed by
glutaraldehyde and osmium acid, embedded in epoxy resin and
sectioned ultra-thinly. Ultra-structural changes of the targeted
tissues were observed under a transmission electron microscope. The
remaining tumor tissues were stained with hematoxylin and eosin
(H&E) and observed under a light microscope.
Immunohistochemical examination
H&E staining and immunohistochemical examination
were performed. The microvessel density (MVD) of the tumor was
calculated under microscopy by marking the tumor vessels with mouse
anti-human CD34 monoclonal antibody according to Weidner’s revised
technique (13). The expression of
vascular endothelial growth factor (VEGF) was marked with the mouse
anti-human VEGF monoclonal antibody. The whole slide was viewed at
100-times field of view (FOV) under the microscope, and the
‘hot-spot’ (i.e., the most intensive area of tumor angiogenesis)
was found. Then, under 200-times FOV, the number of tumor vessels
was calculated and averaged as MVD by viewing 5 FOV randomly on
each slide. The same method was used to calculate the average
optical density (AOD) of VEGF. The observed data were exhibited as
the mean ± standard deviation. Statistical analysis was performed
using a SPSS 13.0 statistical software package. The difference of
MVD counts and AOD of VEGF in the tumor tissue of 40 mice in 4
groups were obtained using the one-way analysis of variance.
Differences were considered to be statistically significant at
P<0.05.
Results
Gross observation
Each tumor was detected with US when the tumor
reached a diameter of approximately 0.5 cm in muscle, 14 days after
the Du145 prostate tumor cells were injected subcutaneously. A
total of 28 days after the tumor cells were implanted, the diameter
of the tumors ranged from 7 to 11 mm. The implanted tumors were
observed to be spherical-, elliptical- or nodular-shaped by US
sonography.
In the control group, the average tumor volume
markedly increased at the time of 2 weeks compared to the US+Mbs
group (P<0.05). The average tumor volume inhibition ratio of the
US+Mbs group was 62.70%, which was significantly greater than that
of the Mbs group (16.34%) and the US group (23.66%). However, the
average tumor volume of the US group and the Mbs group at 2 weeks
was similar to the control group (P>0.05).
Gross pathological findings and light
microscopy
In the US, Mbs and control groups, the tumors
resembled the appearance of gray fish meat and the cells were
encapsulated. A clear demarcation with a sharp boundary was
detected between the tumor and normal surrounding tissues. In the
US+Mbs group, there was a large amount of yellowish coagulation
necrosis inside the tumor.
On the H&E-stained slides, under a low power
lens, Du145 prostate tumor cells in the control group appeared
mass-flake-like or as an invasive cancer nest, with reduced
connective tissues and an unclear demarcation between the tumor and
mesenchymal cells. Under a high-power lens, tumor cells were large,
irregularly arranged with an irregular morphology. The nuclei were
large and deeply stained, with a large karyoplasmic ratio and
increased mitosis. The tumor tissue of the US and Mbs groups was
similar to that of the control group. The tumor cells of the US+Mbs
group were shrunk by coagulation necrosis, and their volume was
reduced. Some residual tumor cells remained in the periphery of the
tumors. The tumor cytoplasm was lightly stained with cytoplasmic
vacuoles of various sizes (Fig.
1).
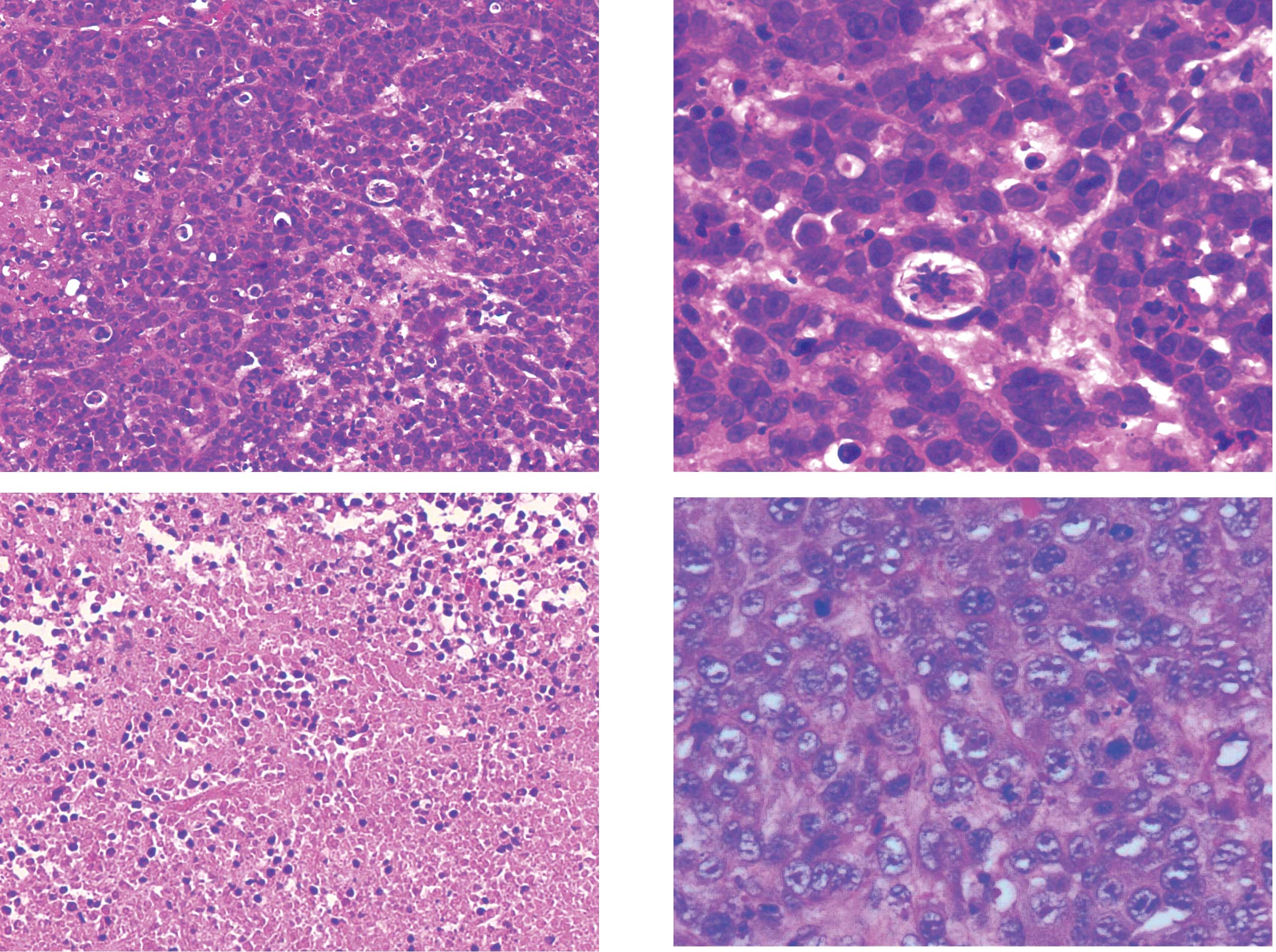 | Figure 1.Light microscopic pathology of the
targeted prostate tumor tissues of node mice in the control and
US+Mbs groups (H&E, A and C; magnification, x100; B and D;
magnification, x400). (A and B) In the control group, tumor cells
were large, irregularly arranged and with irregular morphology. The
nuclei were large and deeply H&E-stained, with a large
karyoplasmic ratio and increased mitosis. (C and D) In the US+Mbs
group, the tumor cytoplasm in all 10 mice was lightly stained, with
cytoplasmic vacuoles of various sizes, chromatin margination and
karyopyknosis. US, ultrasound; Mbs, microbubbles; H&E,
hematoxylin and eosin. |
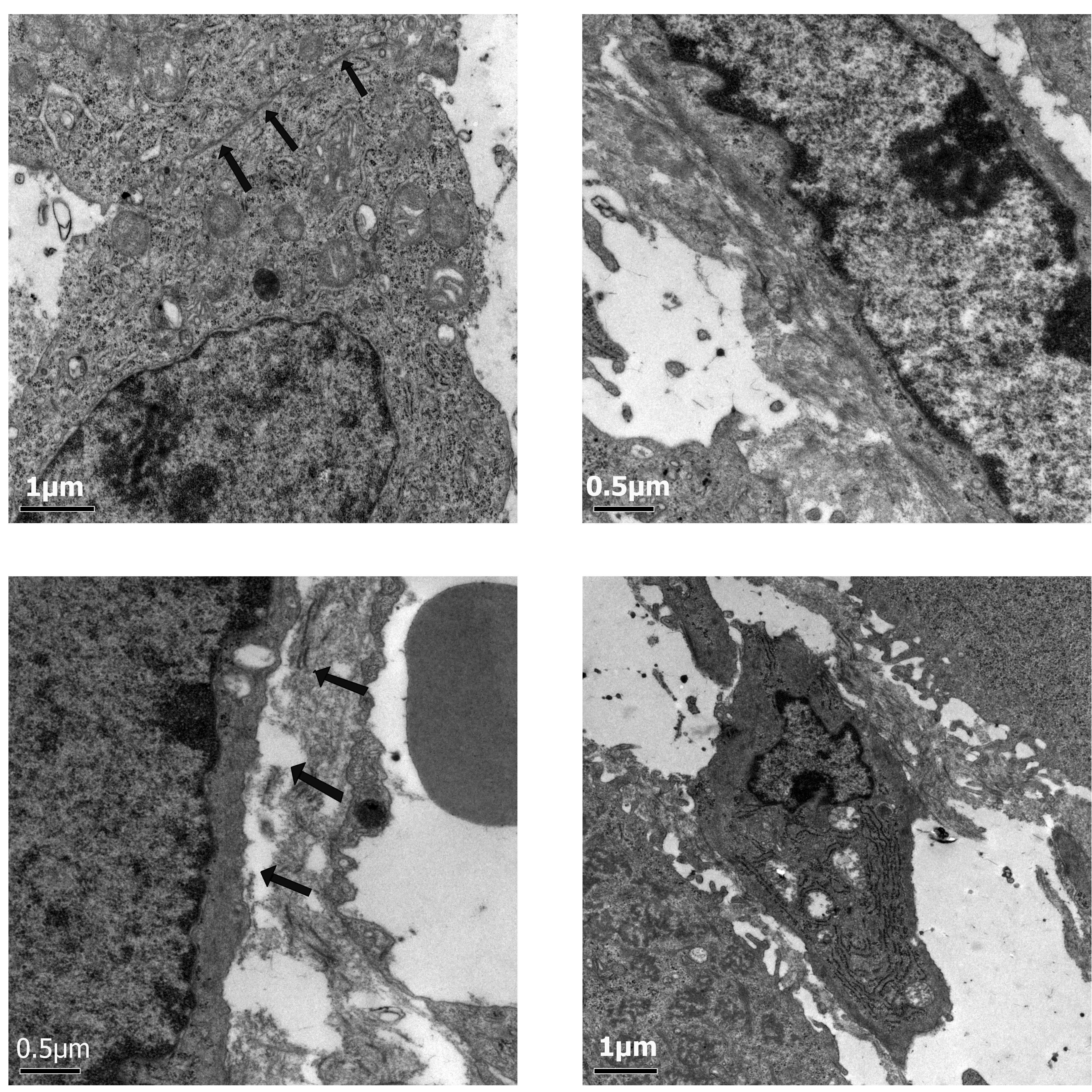
Electron microscopy
In the control group, a high magnification view of
the wall of a tumor blood vessel in the control group showed highly
attenuated vascular endothelium. The nuclei of the endothelial
cells were large and deformed, with clear nuclear membranes and
rich euchromatin. The chromatin particles were large with
intranuclear pseudo-inclusions and multiple visible nucleoli were
present. No difference was observed in the appearance of the US and
Mbs groups compared with the control group. In the US+Mbs group,
changes in the wall perimeter included small membrane blebs,
unusual vacuoles or multiple filopodia, small gaps in the
endothelial layer and regions of disrupted or missing endothelium.
The diameter of these gaps ranged from hundreds of nanometers to
several microns. Endothelial cells were reduced in size, and in
certain endothelial cells, karyopyknosis was revealed and various
vacuoles of different sizes were present in the cytoplasm (Fig. 2).
Immunohistochemical examination
CD34 expression was located on the vascular
endothelial cells of the tumor. By targeting the microvessels with
CD34 monoclonal antibody, a large amount of tumor microvessel
density was exhibited as a brown-yellow dying area on the
immunohistochemical slides in the control group. In the US+MBs
group, the microvessels of the tumor were dispersed and exiguous,
and the MVD markedly decreased compared with that of the control
group.
The expression of VEGF was mainly located in the
plasma of the tumor cells. A positive expression of VEGF appeared
as brown-yellow slender particles. A decreased expression level of
VEGF in tumor cells was observed in the US+Mbs group compared to
the control group. A small amount of the brown-yellow granular
substance was detected in part of the cytoplasm.
As shown in Table
I, the amount of CD34- and VEGF-positive expression in US+Mbs
significantly decreased when compared with that of the control, US
and MBs groups (P<0.05), suggesting that US+Mbs was capable of
inducing the inhibition of angiogenesis. However, no differences
were observed in the expression of CD34 and VEGF between the US,
Mbs and control groups, respectively (P>0.05).
 | Table I.The tumor vessel counts of CD34- and
VEGF-positive expression in the different groups. |
Table I.
The tumor vessel counts of CD34- and
VEGF-positive expression in the different groups.
| Group | MVD of CD34 | AOD of VEGF |
|---|
| Control | 32.50±3.05 | 29.34±7.70 |
| US | 26.53±6.58d | 28.4±6.77d |
| Mbs | 26.73±2.37d | 28.18±5.68d |
| US+Mbs |
3.30±1.84a,b,c |
5.35±2.85a,b,c |
Discussion
Physical therapy applications using Mbs and US
cavitation on the disruption of tumor neovasculature have drawn
much attention due to their use in gene transfection, targeted drug
delivery and release, and thrombolysis (14–20).
The ultrasonic cavitation effect is a significant physical effect
of US, besides the thermal effect. Mbs as an effective cavitation
core may induce a significant cavitation effect under appropriate
ultrasonic impulse excitation. Cavitation-released mechanical
energy (non-thermal effect) has the potential of ablating targeted
tissue. This hypothesis suggests disrupting the immature, leaky and
fragile tumor microvasculature is possible. In addition, being a
simple physical therapeutic method, Mbs enhanced US cavitation to
obstruct tumor microcirculation can be repeated with equal success
and may be capable of preventing the thermal side effects of HIFU
treatment.
In our study, the prostate tumors of nude mice
treated with low-intensity US combined with the intravenous
injection of Mbs were ablated by non-thermal effects, which have
characteristic pathological changes that are different from those
of thermal lesions. Ashush et al (21) observed the following morphological
changes after US cavitation: cell shrinkage, vacuole formation,
chromatin condensation, karyorrhexis and the formation of apoptotic
bodies. Kieran et al (22)
studied non-thermal lesions by changing the US intensity and duty
cycle. Their histological observation showed that within a certain
intensity and duty cycle, vacuoles were formed in the cells, with
blanched and dense liquid inside the vacuoles. Our study found that
neither low intensity US nor Mbs, as separate conditions, were able
to achieve a tumor ablation effect. However, when the two factors
were combined together, the tumor inhibition effect was
significant. Light microscopy showed abundant vacuoles of various
sizes in the cytoplasm and chromatin margination and karyopyknosis
in certain cells. Electron microscopic examination revealed a
presence of karyopyknosis and chromatin margination in certain
cells, intercellular space widening, and a number of vacuoles of
various sizes in the cytoplasm. These findings indicated that by
combining low frequency US with Mbs, cavitation effects may be
intensified to achieve non-thermal tumor ablation.
VEGF is known to be a potent stimulator of
endothelial cell proliferation, vascular permeability and
angiogenesis. VEGF may be stimulated by the platelet-derived growth
factor and function synergistically with the fibroblast growth
factor to stimulate new vessel growth. Inhibition of the VEGF
receptor tyrosine kinase activity has been shown to slow the tumor
growth in various tumor models, including metastatic colon cancer,
mammary and pancreatic adenocarcinomas (23–27).
It is likely that by targeting and disrupting the receptor tyrosine
kinase activity of multiple angiogenic modulators, such as VEGF,
platelet-derived growth factor and fibroblast growth factor, may
more effectively inhibit tumor growth. A distinct increase in the
expression levels of promoting factors of angiogenesis, such as
VEGF, has been observed during tumor growth and evolution. VEGF is
capable of specially binding the corresponding acceptor of vascular
endothelial cells and promoting the proliferation of vascular
endothelial cells. Moreover, it increases the permeability of
vessels and facilitates the exudation of serous protein including
fibrinogen (28). Accordingly,
during contrast-enhanced low frequency and low intensity US
therapy, US cavitation inhibited the expression of VEGF in prostate
tumors in nude mice.
Contrast-enhanced low frequency and low intensity US
cavitation produced injury of vascular endothelial cells in
prostate tumors, and inhibited the expression of VEGF in the tumor,
resulting in tumor inhibition effects. The potential for such
effects during contrast-enhanced US cavitation at 20 kHz should be
acknowledged. The major application of this study is in the target
therapy of solid tumors with abundant microvessels. Future studies
are required into certain aspects of US cavitation, such as
cavitation detection, temperature monitoring and other means to
detect non-thermal effects; how to optimize the combination between
US and Mbs exposure parameters; the means to control and monitor
cavitational lesions; and long-term outcomes of non-thermal tumor
ablation.
Acknowledgements
This study was supported by the
Natural Science Foundation of Shanghai (grant 10JC14125600).
References
|
1.
|
JG LynnRL ZwemerAJ ChickAE MillerA new
method for the generation and use of the focused ultrasound in
experimental biologyJ Gen
Physiol26179193194210.1085/jgp.26.2.17919873337
|
|
2.
|
F ValtotJ KopelJ HautTreatment of glaucoma
with high-intensity focused ultrasoundInt
Ophthalmol13167170198910.1007/BF020286592744949
|
|
3.
|
LF KincaidNT SanghviO CummingsNoninvasive
ultrasound subtotal ablation of the prostate in dogsAm J Vet
Res571225122719968836379
|
|
4.
|
R BihrleRS FosterNT SanghviFJ FryJP
DonohueHigh-intensity focused ultrasound in the treatment of
prostatic
tissueUrology432126199410.1016/0090-4295(94)90214-37509533
|
|
5.
|
S MadersbacherM PedevillaL VingersM
SusaniM MarbergerEffect of high-intensity focused ultrasound on
human prostate cancer in vivoCancer Res553346335119957542168
|
|
6.
|
G VallancienE Chartier-KastlerM HarouniD
ChopinJ BougaranFocused extracorporeal pyrotherapy: experimental
study of feasibility in manSemin Urol117919937682004
|
|
7.
|
K HynynenN McDannoldN VykhodtsevaFocal
disruption of the blood-brain barrier due to 260-kHz ultrasound
bursts: a method for molecular imaging and targeted drug deliveryJ
Neurosurg105445454200610.3171/jns.2006.105.3.44516961141
|
|
8.
|
P DaytonA KlibanovG BrandenburgerK
FerraraAcoustic radiation force in vivo: a mechanism to assist
targeting of microbubblesUltrasound Med
Biol2511951201199910.1016/S0301-5629(99)00062-910576262
|
|
9.
|
RE ApfelAcoustic cavitation: A possible
consequence of biomedical use of ultrasoundBr J Cancer
Suppl514014619826950749
|
|
10.
|
LA CrumJB FowlkesAcoustic cavitation
generated by microsecond pulses of
ultrasoundNature3195254198610.1038/319052a0
|
|
11.
|
MA MargulisSonochemistry of
CavitationGordon and Breach PublishersLuxembourg1995
|
|
12.
|
K HynynenN McDannoldN VykhodtsevaFA
JoleszNoninvasive MR imaging-guided focal opening of the
blood-brain barrier in
rabbitsRadiology220640646200110.1148/radiol.220200180411526261
|
|
13.
|
N WeidnerTumor vascularity and
proliferation: clear evidence of a close relationshipJ
Pathol189297299199910.1002/(SICI)1096-9896(199911)189:3%3C297::AID-PATH434%3E3.0.CO;2-O10547589
|
|
14.
|
S MayerPA GrayburnMyocardial contrast
agents: recent advances and future directionsProg Cardiovasc
Dis443344200110.1053/pcad.2001.2643811533925
|
|
15.
|
EC UngerE HershM VannanTO MatsunagaT
McCreeryLocal drug and gene delivery through microbubblesProg
Cardiovasc Dis444554200110.1053/pcad.2001.2644311533926
|
|
16.
|
TR PorterF XieTherapeutic ultrasound for
gene
deliveryEchocardiography18349353200110.1046/j.1540-8175.2001.00349.x11415508
|
|
17.
|
JR LindnerS KaulDelivery of drugs with
ultrasoundEchocardiography18329337200110.1046/j.1540-8175.2001.00329.x11415506
|
|
18.
|
K TachibanaS TachibanaThe use of
ultrasound for drug
deliveryEchocardiography18323328200110.1046/j.1540-8175.2001.00323.x11415505
|
|
19.
|
EC UngerTO MatsunagaT McCreeryP SchumannR
SweitzerR QuigleyTherapeutic applications of microbubblesEur J
Radiol42160168200210.1016/S0720-048X(01)00455-711976013
|
|
20.
|
RJ PriceS KaulContrast ultrasound targeted
drug and gene delivery: an update on a new therapeutic modalityJ
Cardiovasc Pharmacol
Ther7171180200210.1177/10742484020070030712232566
|
|
21.
|
H AshushLA RozenszajnM BlassApoptosis
induction of human myeloid leukemic cells by ultrasound
exposureCancer Res6010141020200010706118
|
|
22.
|
K KieranTL HallJE ParsonsRefining
histotripsy: defining the parameter space for the creation of
nonthermal lesions with high intensity, pulsed focused ultrasound
of the in vitro kidneyJ
Urol178672676200710.1016/j.juro.2007.03.09317574617
|
|
23.
|
DJ HicklinLM EllisRole of the vascular
endothelial growth factor pathway in tumor growth and angiogenesisJ
Clin Oncol2310111027200510.1200/JCO.2005.06.08115585754
|
|
24.
|
IJ FidlerLM EllisThe implications of
angiogenesis for the biology and therapy of cancer
metastasisCell79185188199410.1016/0092-8674(94)90187-27525076
|
|
25.
|
G BergersLE BenjaminTumorigenesis and the
angiogenic switchNat Bev Cancer3401410200310.1038/nrc1093
|
|
26.
|
D HanahanRA WeinbergThe hallmark of
cancerCell1005770200010.1016/S0092-8674(00)81683-9
|
|
27.
|
P NybergL XieR KalluriEndogenous
inhibitors of angiogenesisCancer
Res6539673979200510.1158/0008-5472.CAN-04-242715899784
|
|
28.
|
N FerraraHP GerberJ LeCouterThe biology of
VEGF and its receptorsNat
Med9669676200310.1038/nm0603-66912778165
|