Introduction
The optimal treatment of squamous cell carcinoma of
the esophagus remains to be elucidated (1,2). As
the esophagus is not surrounded by a serosal lining, infiltration
into adjacent mediastinal structures, including the aorta,
tracheobronchial tree and vertebral column, occurs easily (3). It is extremely important to
accurately evaluate tumor invasion of the adjacent mediastinal
structures in order to prevent unnecessary surgery in patients with
inoperable tumors (4,5).
At the time of diagnosis, fewer than half the
patients have locally advanced tumors that are resectable (6). In these patients, induction
therapies, such as pre-operative chemotherapy and
chemoradiotherapy, offer a survival advantage compared to surgery
alone (7). However, in patients
who respond unfavorably, the inefficient induction therapy should
be discontinued and surgery should not be delayed. On the other
hand, patients who respond favorably to the induction therapy may
benefit from additional pre-operative treatment. Thus, there is a
need for a method that may be used to reliably predict the
pathological response to the induction therapy in order to prevent
wastage of time and unnecessary surgery.
Currently, endoscopic ultrasound (EUS) is used to
assess the local tumor extent in esophageal cancer (8,9);
unfortunately, many patients with locally advanced cancer have too
narrow a lumen to allow passage of the endoscope (3). Alternatively, prediction of aortic
invasion has also been evaluated with computed tomography (CT). The
overall circumference of contact between the tumor and the aortic
wall has been shown to be a useful predictor, with an interface arc
greater than 90 degrees, suggesting invasion, as reported by Picus
et al (10). Currently used
multidetector CT (MDCT) scanners enable thinner collimation and
faster scanning, which markedly improves imaging resolution and
enables rapid handling of image reconstruction (11). Therefore, we hypothesized that the
evaluation of intervening tissues between the tumor and aortic wall
visualized by MDCT may enable assessment of the induction therapy
response and aortic invasion.
In the present study, we retrospectively evaluated
MDCT attenuation values between the tumor and aortic wall prior to
and following the induction therapy, and examined whether
attenuation values could be used to assess aortic invasion in
patients with advanced esophageal cancer.
Materials and methods
Patients
A total of 162 consecutive patients underwent
transthoracic esophagectomy for esophageal cancer at the National
Defense Medical College Hospital (Japan) from January 2005 to May
2010. Out of 162 patients, 56 who were suspected to have a tumor
invading the adventitia (T3) without any distant metastasis
underwent an induction therapy, chemotherapy or chemoradiotherapy,
and were enrolled in this study. The pathological and clinical
stages of the tumors in these patients were determined according to
the 5th edition of the tumor-node-metastasis (TNM) Classification
of Malignant Tumors of the International Union Against Cancer
(12). Pathological criteria for
the effects of chemotherapy or chemoradiotherapy are described
based on the Japanese Classification of Esophageal Cancer, 10th
edition (13): grade 1a, viable
cancer cells account for two thirds or more of the tumor tissue,
but there is some evidence of degeneration of cancer tissue or
cells; grade 1b, viable cancer cells account for one third or more
of the tumor tissue, but less than two thirds of tumor tissue;
grade 2, viable cancer cells account for less than one third of the
tumor tissue, while other cancer cells are severely degenerated or
necrotic. Surgical findings regarding the extension of the tumor
were described according to the medical records. All surgeries were
performed by three experts who had more than 10 years of experience
in performing esophagectomies (H.T., T.I. and S.A.).
The chemotherapeutic regimens included two courses
of chemotherapy of cisplatin (CDDP; 80 mg/m2 of
intravenous drip infusion, day 1) and 5-fluorouracil (5-FU; 800
mg/m2 of continuous infusion, days 1–5). For the
chemoradiotherapy, the chemotherapeutic regimens included the
administration of CDDP (70 mg/m2 of intravenous drip
infusion, day 1) and 5-FU (700 mg/m2 of continuous
infusion, days 1–4), and the concurrent radiation therapy was
planned to be administered in daily fractions of 2 Gy for a total
dose of 30–40 Gy (median 30.9). The chemotherapy or
chemoradiotherapy as an induction therapy was chosen, taking risk
and benefit into consideration. Written informed consent was
obtained from all individuals prior to initiation of the study.
Evaluation of CT attenuation values
between the tumor and aorta
All studies were performed using a 64-detector row
CT (Aquilion™ 64; Toshiba Medical, Tokyo, Japan). A total of 300 ml
of non-ionic contrast agents were intravenously administered at a
speed of 3 ml/sec, and CT was performed 120 sec following
injection. To adjust the CT attenuation values, water and air
calibrations were performed quarterly and weekly, respectively. The
scanning parameters included 120 kVp, 0.5-sec tube rotation time,
27 mm/rotation helical pitch, 55 mm table speed, 0.5-sec gantry
rotation time and 2.5-mm thick reconstructed sections. The images
were reviewed on a workstation (Zio workstation; AMIN Inc., Tokyo,
Japan). These were independently and retrospectively evaluated by
two surgeons who had more than 10 years of experience in performing
esophagectomies and were blinded to complaints, specific medical
history and findings of physical examination, surgery, laboratory
evaluation and imaging.
The maximum tumor size in a horizontal section,
tumor location and CT attenuation value before and after induction
therapies were described. The overall circumference of contact
between the tumor and aortic wall (Picus’ angle) was determined, as
previously described (10). To
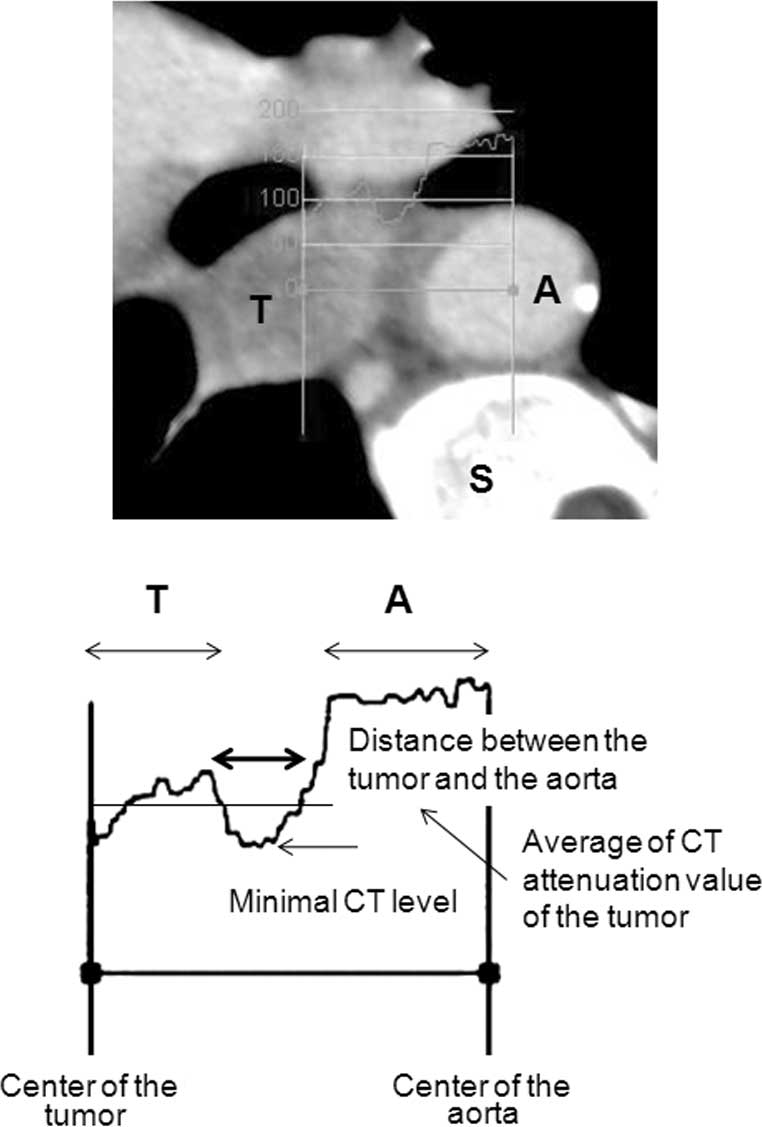
determine the CT attenuation value between the tumor and aorta,
consecutive CT values between the center of the tumor and the
center of the aorta were determined using a Zio workstation. We
examined the average CT attenuation value of the tumor and
determined the distance between the intersections of this average
with the lower CT attenuation value of the inclusion tissues
(Fig. 1). In our previous
examinations, which included 101 patients with advanced esophageal
cancer, serial cut-off values were inserted around the inflection
points on the receiver operating characteristic (ROC) curve for the
pathological aortic invasion of the esophageal cancer (Fig. 1). We also determined the minimum CT
attenuation value between the tumor and aorta.
Statistical analysis
The data are expressed as the means ± standard
deviation (SD). Comparison between the two groups was analyzed
using the Mann-Whitney U test or Wilcoxon signed rank test. These
data were analyzed using MedCalc version 9 statistical software
package (MedCalc software, Mariakerke, Belgium). A p-value of
<0.05 was considered to be statistically significant.
Results
The demographic data of the patients are presented
in Table I. There were no
differences in the distance and minimum CT value between the tumor
and aorta, and Picus’ angle due to age, gender and body mass index
(data not shown). The potentially curative resections were achieved
in 78.6%. A total of 27 patients received pre-operative
chemotherapy and 29 patients received pre-operative
chemoradiotherapy. A total of 7 patients (12.5%) were suspected to
have tumor invasion of the aortic wall during surgery, and 6
patients (10.7%) were pathologically confirmed to have tumor
invasion of the aortic wall. The result of the pathological
response to the induction therapy was 39 patients
(chemotherapy:chemoradiotherapy, 23:16) with grade 1a disease, 6
patients (2:4) with grade 1b disease and 11 patients (2:9) with
grade 2 disease.
 | Table I.Demographic data of patients who were
suspected to have a tumor invading the adventitia (T3) without any
distant metastasis. |
Table I.
Demographic data of patients who were
suspected to have a tumor invading the adventitia (T3) without any
distant metastasis.
| Characteristic | No. of patients
(n=56) |
|---|
| Age (years) | 64.3±7.5 |
| Gender | |
| Male | 52 |
| Female | 4 |
| Location | |
| Upper thoracic | 4 |
| Middle
thoracic | 27 |
| Lower thoracic | 15 |
| Abdominal
esophagus | 10 |
| Histology | |
| SCC | 51 |
| Well | 4 |
| Moderate | 41 |
| Poor | 8 |
| Adenocarcinoma | 3 |
| Tumor depth | |
| pT2 | 6 |
| pT3 | 40 |
| pT4 | 10 |
| Aorta | 6 |
| Lung | 2 |
| Trachea | 1 |
| Pericardiac
membrane | 1 |
|
Lymphadenectomy | |
| 2-field | 24 |
| 3-field | 32 |
| Residual tumor | |
| R0 | 44 |
| R1 | 5 |
| R2 | 7 |
| Induction
therapy | |
| Chemotherapy | 27 |
| Chemoradiation
therapy | 29 |
| Pathological
response | |
| Grade 1a | 39 |
| Grade 1b | 6 |
| Grade 2 | 11 |
| Aortic
invasion | |
| Surgical | |
| Yes | 7 |
| No | 49 |
| Pathological | |
| Yes | 6 |
| No | 50 |
The tumor-to-aorta distance following induction
therapy was significantly higher than that prior to induction
therapy (2.4±1.1 vs. 1.8±0.9 mm). The maximum tumor sizes and
Picus’ angles following the induction therapy were significantly
reduced compared to those prior to the induction therapy (30.5±9.9
vs. 34.8±8.6 mm, and 68.9±31.6 vs. 75.0±25.6 degrees,
respectively). However, there were no differences in the minimum CT
attenuation value between the tumor and aorta prior to and
following the induction therapy (65.4±32.3 vs. 61.4±29.9 Hounsfield
units).
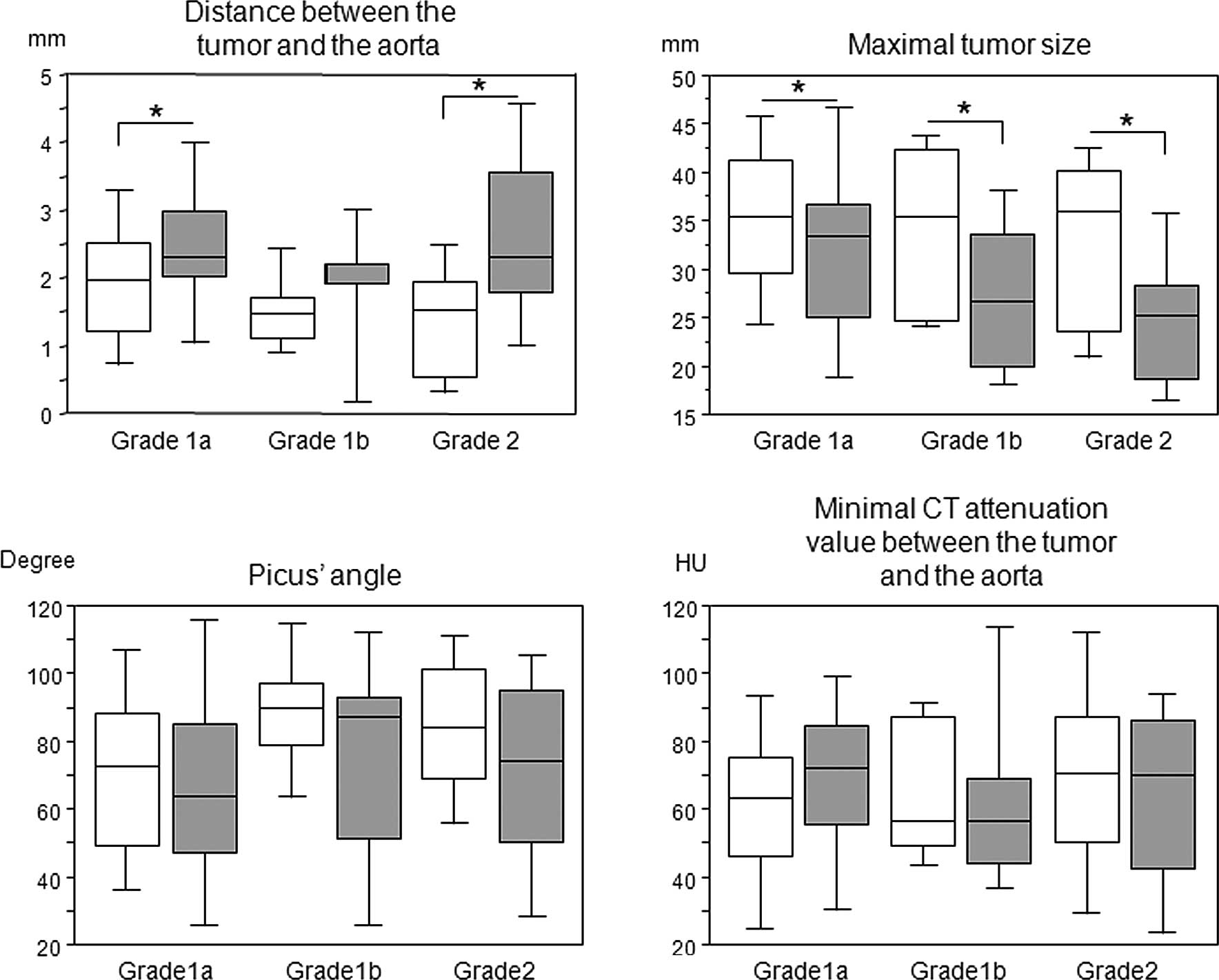
We compared each parameter before and after the
induction therapy according to the pathological response (Fig. 2). There were significant
differences in the tumor-to-aorta distance and maximum tumor size
among the pathological responses, whereas such differences were not
observed in the Picus’ angles and minimum CT attenuation values
between the tumor and aorta. We then compared each parameter prior
to and following the induction therapy according to the therapeutic
approach, such as chemotherapy and chemoradiotherapy (Table I). The tumor-to-aorta distance
following the induction therapy was significantly higher than that
prior to the induction therapy in patients who underwent
chemotherapy, while Picus’ angle following the induction therapy
was significantly reduced compared to that prior to the induction
therapy in patients who underwent chemoradiotherapy. The maximum
tumor size was significantly reduced following both induction
therapies, and we were unable to find any differences in the
minimum CT attenuation value before and after the induction therapy
(Table II).
 | Table II.Distance and minimum CT attenuation
value between the tumor and aorta, Picus’ angle and maximum tumor
size before and after the induction therapy. |
Table II.
Distance and minimum CT attenuation
value between the tumor and aorta, Picus’ angle and maximum tumor
size before and after the induction therapy.
| Before | After | p-value |
|---|
| Distance | | | |
| Chemotherapy | 1.9±0.9 | 2.8±1.2 | 0.0001 |
|
Chemoradiotherapy | 1.7±1.0 | 2.1±1.0 | 0.0846 |
| Total | 1.8±0.9 | 2.4±1.1 | 0.0001 |
| Minimal CT | | | |
| Chemotherapy | 53.2±33.6 | 56.6±36.3 | 0.9291 |
|
Chemoradiotherapy | 69.7±23.6 | 73.7±26.2 | 0.5903 |
| Total | 61.4±29.9 | 65.4±32.3 | 0.6594 |
| Picus’ angle | | | |
| Chemotherapy | 65.9±25.0 | 63.3±32.9 | 0.2795 |
|
Chemoradiotherapy | 84.1±23.2 | 74.0±30.0 | 0.0044 |
| Total | 75.0±25.6 | 68.9±31.6 | 0.0049 |
| Maximal tumor
size | | | |
| Chemotherapy | 34.2±7.8 | 29.7±9.6 | 0.0014 |
|
Chemoradiotherapy | 35.4±9.5 | 31.2±10.3 | 0.0003 |
| Total | 34.8±8.6 | 30.5±9.9 | 0.0001 |
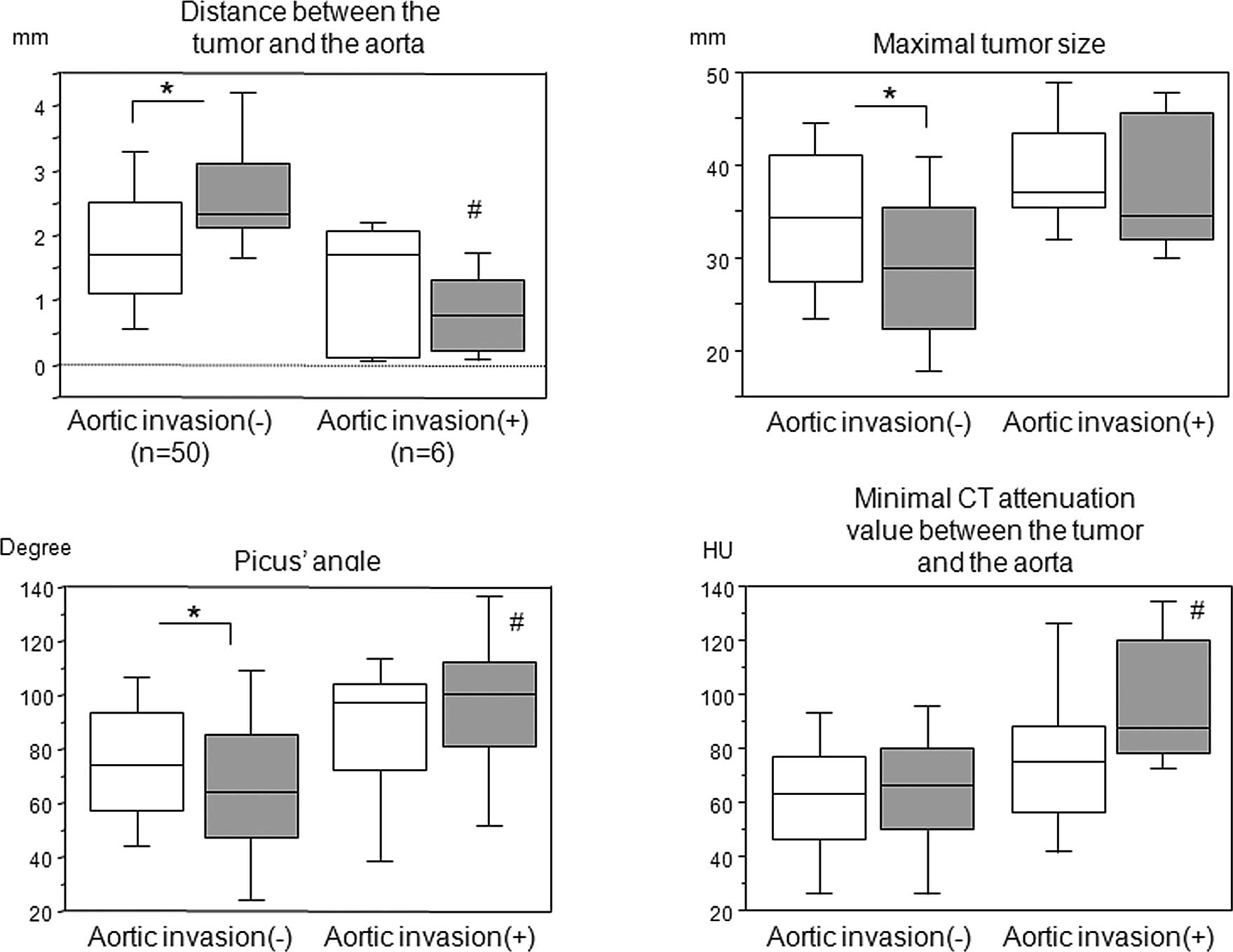
Since 6 patients were pathologically confirmed to
have tumor invasion of the aortic wall, we compared each parameter
between patients with and without aortic invasion (Fig. 3). There were significant
differences in the tumor-to-aorta distance, maximum tumor size and
Picus’ angle before and after the induction therapy in patients
without aortic invasion; however, such differences were not
observed in patients with aortic invasion and in the minimum CT
attenuation value between the tumor and aorta prior to and
following the induction therapy. Notably, in patients with aortic
invasion, the tumor-to-aorta distance following the induction
therapy was shorter than that prior to the induction therapy,
albeit not significantly. Furthermore, there were significant
differences following the induction therapy in the distance and
minimum CT attenuation values between the tumor and aorta, and
Picus’ angles between patients with and without aortic invasion;
however, this was not observed in the maximum tumor size.
In our previous examinations, which included 101
patients with advanced esophageal cancer, when the cut-off value of
the tumor-to-aorta distance was set at <1.3 mm, the sensitivity,
specificity and accuracy for the pathological aortic invasion were
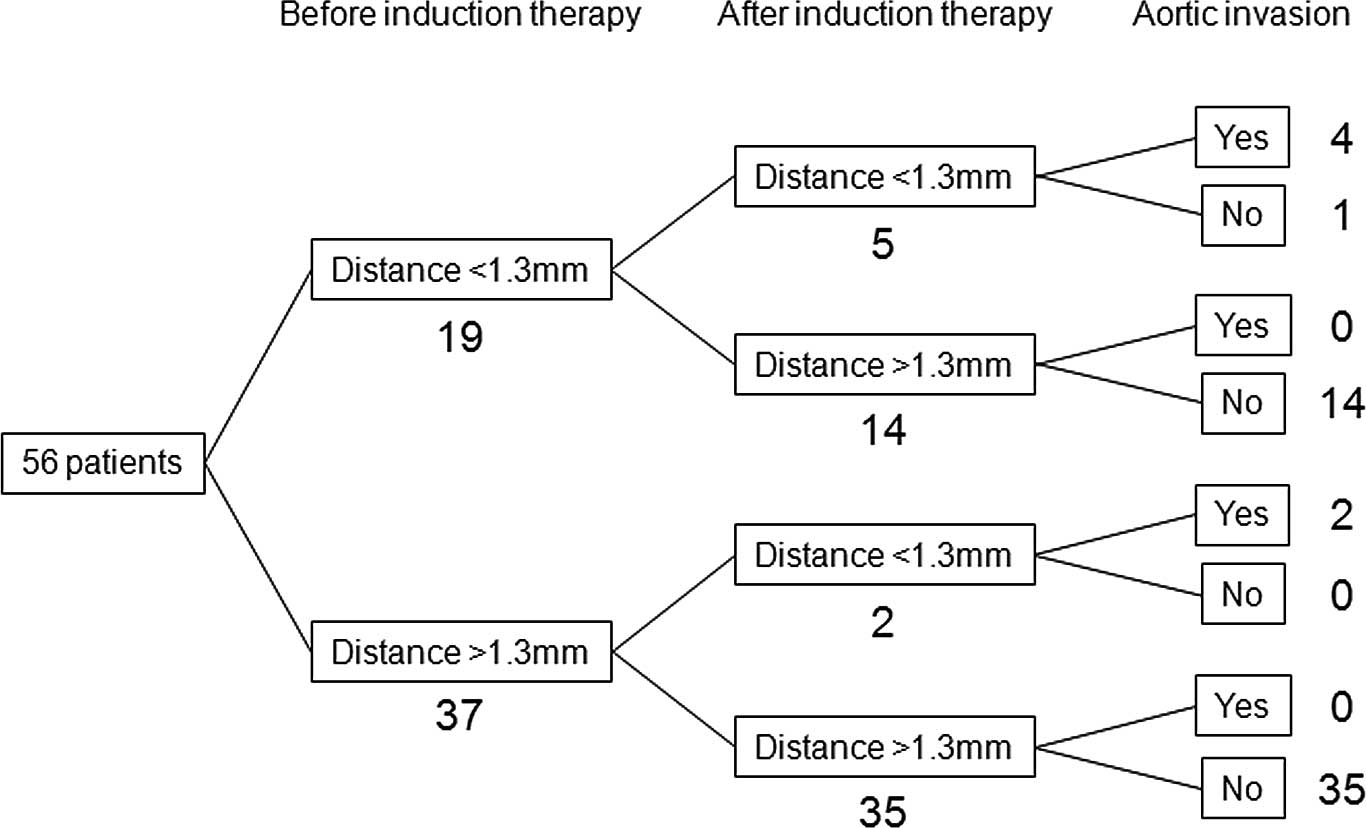
87.5, 91.4 and 91.1%, respectively (Fig. 1). A total of 19 patients had a
tumor-to-aorta distance of <1.3 mm prior to the induction
therapy, and in 14 out of the 19 patients, the tumor-to-aorta
distance increased to >1.3 mm following the induction therapy;
none of these patients had aortic invasion (Fig. 4). The remaining 5 patients
continued to have a tumor-to-aorta distances of <1.3 mm
following the induction therapy; 4 of these 5 patients had aortic
invasion. However, 2 out of 37 patients in whom tumor-to-aorta
distance was >1.3 mm prior to the induction therapy had a
decrease in this distance (<1.3 mm) following the induction
therapy; the two patients had aortic invasion and a grade 1a
pathological response.
Discussion
In the present study, we evaluated the CT
attenuation value between the tumor and aorta in response to the
induction therapy for advanced esophageal cancer using MDCT. We
demonstrated that the tumor-to-aorta distance and maximum tumor
size reflect the pathological response to the induction therapy. In
addition, the induction therapy may increase the tumor-to-aorta
distance, and decrease the maximum tumor size and Picus’ angle in
patients with esophageal cancer.
Conventionally, CT scans and 18-F-fluorodeoxyglucose
positron emission tomography have been widely employed to evaluate
the tumor response to chemotherapy and/or radiation therapy
(5). However, there has been
significant heterogeneity in the sensitivity and specificity of
these modalities (14). In
addition, the assessment of tumor size or metabolic status of the
tumor does not always reflect the resectability of esophageal
cancer.
An intervening fat plane between the esophageal
tumor and adjacent structures in the mediastinum accurately
indicates a lack of invasion. However, the lack of a fat plane does
not necessarily indicate invasion, neither in cachectic patients
nor in those of normal body weight (15). Many surgeons have employed the
overall circumference of the contact area between the tumor and
aortic wall (Picus’ angle) to predict aortic invasion in esophageal
cancer (10). However, this sign
may be considered to be unreliable (3). Indeed, the accuracy of this angle for
aortic invasion was only 78.6%, which is inferior to the accuracy
of 94.6% in the tumor-to-aorta distance when the cut-off value was
set at <1.3 mm in this study.
In the present study, we demonstrated that induction
therapy may increase the tumor-to-aorta distance and decrease the
maximum tumor size and Picus’ angle in each pathological response
to induction therapy, which was more evident in patients without
aortic invasion. Furthermore, the tumor-to-aorta distance following
induction therapy in patients with aortic invasion was
significantly reduced compared to patients without aortic invasion.
The Picus’ angle and minimum CT attenuation value between the tumor
and aorta following induction therapy in patients with aortic
invasion were significantly greater than in those without aortic
invasion. When the tumor-to-aorta distance cut-off value was set at
<1.3 mm, 19 patients were suspected to have aortic invasion
prior to the induction therapy, 14 of these 19 patients could have
a cut-off value >1.3 mm and were eligible to undergo potentially
curative resection. Conversely, 2 out of 37 patients had a
tumor-to-aorta distance of <1.3 mm following the induction
therapy; those who had a tumor-to-aorta distance of >1.3 mm
prior to the induction therapy were suspected to have progressed
tumor invasion of the aortic wall during the induction therapy.
Thus, the evaluation of the tumor-to-aorta distance may be a useful
indicator to predict the response to the induction therapy and
aortic wall invasion.
The tumor-to-aorta distance following pre-operative
chemotherapy was significantly greater than that prior to
chemotherapy; however, such a difference was not observed in
patients with chemoradiotherapy. This may be due to enhanced
inflammation of the connective tissue caused by radiation, which
may affect higher CT values.
This study has certain limitations. When the
interface between tumor and aorta was not well defined on the
pre-treatment study, this method was not applicable. Furthermore,
the patients had undergone surgical treatment of esophageal cancer.
We selected these patients to enable the exact comparisons of
surgical and pathological samples; however, as a result, highly
advanced T4 cases were excluded from the study group, which may
have increased the number of cases that were almost at the boundary
of T3 and T4. Thus, it is necessary to conduct a multicenter,
prospective, randomized study in order to verify our current
results.
In conclusion, the assessment of the MDCT
attenuation value between the esophageal tumor and the aorta is
simple, it objectively assesses the response to the induction
therapy, and should be a surrogate marker for aortic invasion and
the outcome in esophageal cancer. This method should be applied to
predict the response to induction therapy, prevent unnecessary
surgery in patients with inoperable tumors involving the aortic
wall, and to prevent the withholding of curative surgery due to the
suggestion of a false-positive result.
Acknowledgements
The authors greatly thank Yoshihisa
Yaguchi, Isao Kumano, Risa Takahata, Kazumichi Yoshida, Hiroyuki
Horiguchi and Shinsuke Nomura from the Department of Surgery,
National Defense Medical College, for their contribution to data
collection and for their critical revision.
References
|
1.
|
JW Entwistle IIIM GoldbergMultimodality
therapy for resectable cancer of the thoracic esophagusAnn Thorac
Surg7310091015200210.1016/S0003-4975(01)03148-411899954
|
|
2.
|
Z GamlielMJ KrasnaMultimodality treatment
of esophageal cancerSurg Clin North
Am85621630200510.1016/j.suc.2005.01.01115927656
|
|
3.
|
S DiederichStaging of oesophageal
cancerCancer Imaging7Spec No
AS63S66200710.1102/1470-7330.2007.9003
|
|
4.
|
Y YamabeY KurokiT IshikawaK MiyakawaS
KurokiR SekiguchiTumor staging of advanced esophageal cancer:
combination of double-contrast esophagography and contrast-enhanced
CTAm J Roentgenol191753757200810.2214/AJR.07.358118716105
|
|
5.
|
K HiguchiW KoizumiS TanabeCurrent
management of esophageal squamous-cell carcinoma in Japan and other
countriesGastrointest Cancer Res3153161200919742141
|
|
6.
|
PC EnzingerRJ MayerEsophageal cancerN Engl
J Med34922412252200310.1056/NEJMra03501014657432
|
|
7.
|
V GebskiB BurmeisterBM SmithersK FooJ
ZalcbergJ SimesSurvival benefits from neoadjuvant chemoradiotherapy
or chemotherapy in oesophageal carcinoma: a meta-analysisLancet
Oncol8226234200710.1016/S1470-2045(07)70039-617329193
|
|
8.
|
A ChakM CantoH GerdesPrognosis of
esophageal cancers preoperatively staged to be locally invasive
(T4) by endoscopic ultrasound (EUS): a multicenter retrospective
cohort studyGastrointest
Endosc42501506199510.1016/S0016-5107(95)70001-3
|
|
9.
|
J ChoiSG KimJS KimHC JungIS SongComparison
of endoscopic ultrasonography (EUS), positron emission tomography
(PET), and computed tomography (CT) in the preoperative
locoregional staging of resectable esophageal cancerSurg
Endosc2413801386201010.1007/s00464-009-0783-x
|
|
10.
|
D PicusDM BalfeRE KoehlerCL RoperJW
OwenComputed tomography in the staging of esophageal
carcinomaRadiology146433438198310.1148/radiology.146.2.68490896849089
|
|
11.
|
BH BurmeisterET WalpoleN D’ArcyA phase II
trial of chemoradiation therapy with weekly oxaliplatin and
protracted infusion of 5-fluorouracil for esophageal cancerInvest
New Drugs27275279200910.1007/s10637-008-9178-418841327
|
|
12.
|
LH SobinID FlemingTNM Classification of
Malignant Tumors, 5th edition (1997). Union Internationale Contre
le Cancer and the American Joint Committee on
CancerCancer8018031804199710.1002/(SICI)1097-0142(19971101)80:9%3C1803::AID-CNCR16%3E3.0.CO;2-99351551
|
|
13.
|
Japan Esophageal SocietyJapanese
Classification of Esophageal Cancer, tenth edition: part
1Esophagus6125200910.1007/s10388-009-0169-0
|
|
14.
|
RM KweePrediction of tumor response to
neoadjuvant therapy in patients with esophageal cancer with use of
18F FDG PET: a systematic
reviewRadiology254707717201010.1148/radiol.0909132420177086
|
|
15.
|
LE QuintStaging work-up of patients with
esophageal cancerCancer
Imaging7128129200710.1102/1470-7330.2007.001717766208
|