Introduction
Pulmonary hypertension (PH) is a progressive, often
fatal disease that is caused by increased pulmonary vascular
resistance (PVR), which is involved in complex processes, including
abnormal vascular wall remodeling, vasoconstriction and thrombosis
(1,2). According to the 2008 WHO
classification, PH may be categorized as pulmonary arterial
hypertension (PAH), PH due to left heart disease, PH due to chronic
lung disease and/or hypoxia, chronic thromboembolic pulmonary
hypertention and miscellaneous forms (3). Among these, hypoxic pulmonary
hypertention (HPH) has higher morbidity and mortality. HPH is
caused by excessive vasoconstriction and remodeling regulated by
endothelial dysfunction. The endothelial cells modulate the
activity of smooth muscle cells by producing vasodilators, such as
prostacyclin and nitric oxide (NO), and vasoconstrictors, such as
thromboxane A2 and endothelin-1 (ET-1). Endothelial dysfunction is
a condition in which the physiological balance between vasodilator
stimuli and vasoconstrictor substances is shifted towards the
latter (4,5); this state has been clearly shown in
PH (6). NO has gained attention as
a significant mediator of PH by virtue of its ability to produce
factors that regulate blood flow and vascular tone. NO is a potent
endothelium-derived vasorelaxant substance and an inhibitor of
smooth muscle cell growth. The main function of NO is to relax
pulmonary vascular smooth muscle cells and inhibit the pulmonary
artery smooth muscle cells (PASMCs) proliferation (7). The decrease of NO production and
release under hypoxic conditions may promote the development of HPH
and pulmonary vascular remodeling. Therefore, to protect
endothelial function and promote the production of NO, the
therapeutic strategy for HPH is crucial.
The release of NO from pulmonary artery endothelial
cells mainly depends on the intercellular concentration of
Ca2+, which increases by hyperpolarization. Since
endothelial cells do not express voltage-dependent Ca2+
channels, Ca2+ influxes following receptor activation
may be facilitated by cell hyperpolarizations mediated by the
activation of K+ channels (6). An increasing number of studies have
shown that the endothelial cell hyperpolarization is mainly
controlled by specific ATP-sensitive K+
(KATP) channels. Consequently, KATP channels
may play a key role in generating the electrical activity of
endothelial cells and have profound effects in regulating the
endothelial function (8). However,
it is unclear whether the activation of KATP channels
promotes NO release by increasing the intercellular concentration
of Ca2+.
Iptakalim, a lipophilic para-amino compound with a
low molecular weight, has been demonstrated to be a new selective
KATP channel opener via pharmacological,
electrophysiological and biochemical studies, and a receptor
binding test (9,10). Our previous study revealed that
iptakalim can alleviate pulmonary artery remodeling and has the
potential to treat pulmonary arterial disorders in PH (11). Moreover, an animal study showed
that the activation of KATP channels by iptakalim can
enhance NO release in bovine aortic endothelial cells (BAECs) under
normoxic conditions (12).
However, it is unclear whether iptakalim also protects human
pulmonary artery endothelial cells (HPAECs) from hypoxia.
In the present study, to assess whether hypoxia
inhibits endothelial nitric oxide synthase (eNOS) activity and NO
production, and whether iptakalim can rescue HPAECs from
hypoxia-induced NO system dysfunction, HPAECs were cultured under
hypoxic conditions in the absence or presence of 0.1, 10 and 1,000
μM iptakalim or the combination of 10 μM iptakalim and 1, 10 and
100 μM glibenclamide for 24 h; the eNOS activity and NO levels were
measured in the conditioned medium from the HPAEC cultures.
Materials and methods
Drugs and chemicals
Iptakalim, with a purity of 99.36%, was synthesized
and provided by the Institute of Pharmacology and Toxicology,
Academy of Military Medical Sciences, China. Glibenclamide was
purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
HPAEC cultures
HPAECs were purchased from ScienCell (San Diego, CA,
USA). The cells were routinely maintained in cell culture medium
(ScienCell) at 37°C in a humidified atmosphere containing 5%
CO2. The third- to sixth-passage cultures were then
seeded onto glass-bottom culture dishes (Corning Inc., NY, USA) and
allowed to reach subconfluence in 2–3 days.
Hypoxic experiments
For the hypoxic experiments, cell cultures were
placed in a modular incubator chamber (Billups-Rothenberg, Del Mar,
CA, USA), where the hypoxic gas mixture (95% N2, 5%
CO2) was pre-analyzed and infused into airtight
incubators with in-flow and out-flow valves at a flow rate of 3
l/min for 15 min to attain a 2% O2 level. The airtight
chamber containing the cell cultures was incubated for periods of
up to 24 h at 37°C. For the normoxic cultures, the third- to
sixth-passage HPAECs were cultured for 24 h in cell culture medium
at 37°C in a humidified atmosphere containing 21% O2 and
the medium was collected for assay of NO and eNOS. The third- to
sixth-passage HPAECs were cultured under hypoxic conditions in the
absence or presence of 0.1, 10 and 1,000 μM iptakalim or the
combination of 10 μM iptakalim and 1, 10 and 100 μM glibenclamide
for 24 h; the eNOS activity and NO levels were measured in the
conditioned medium from the HPAEC cultures.
Measurement of NO and eNOS
HPAEC production of NO was determined indirectly in
HPAEC supernatants. Due to its instability in physiological
solutions, the majority of the NO is rapidly converted to nitrite
(NO2−) and further to nitrate (NO3−).
Therefore, the levels of NO2−/NO3− in the
culture medium were analyzed by a commercially available NO
detection kit (Beyotime Institute of Biotechnology, China)
according to the manufacturer's instructions. Briefly, nitrate was
converted to nitrite with aspergillus nitrite reductase, and the
total nitrite was measured with the Griess reagent. The absorbance
was determined at 540 nm with a spectrophotometer (13). The eNOS activity was measured using
a commercial kit (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the instructions provided by the
manufacturer.
Statistical analysis
Each test was performed and was then repeated six
times. Data were expressed as the means ± SD. Comparisons of the
measurement data between multiple groups were performed using the
one-way ANOVA test. The statistical process was performed with SPSS
12.0 software. Probability values were considered to indicate a
statistically significant difference at P<0.05.
Results
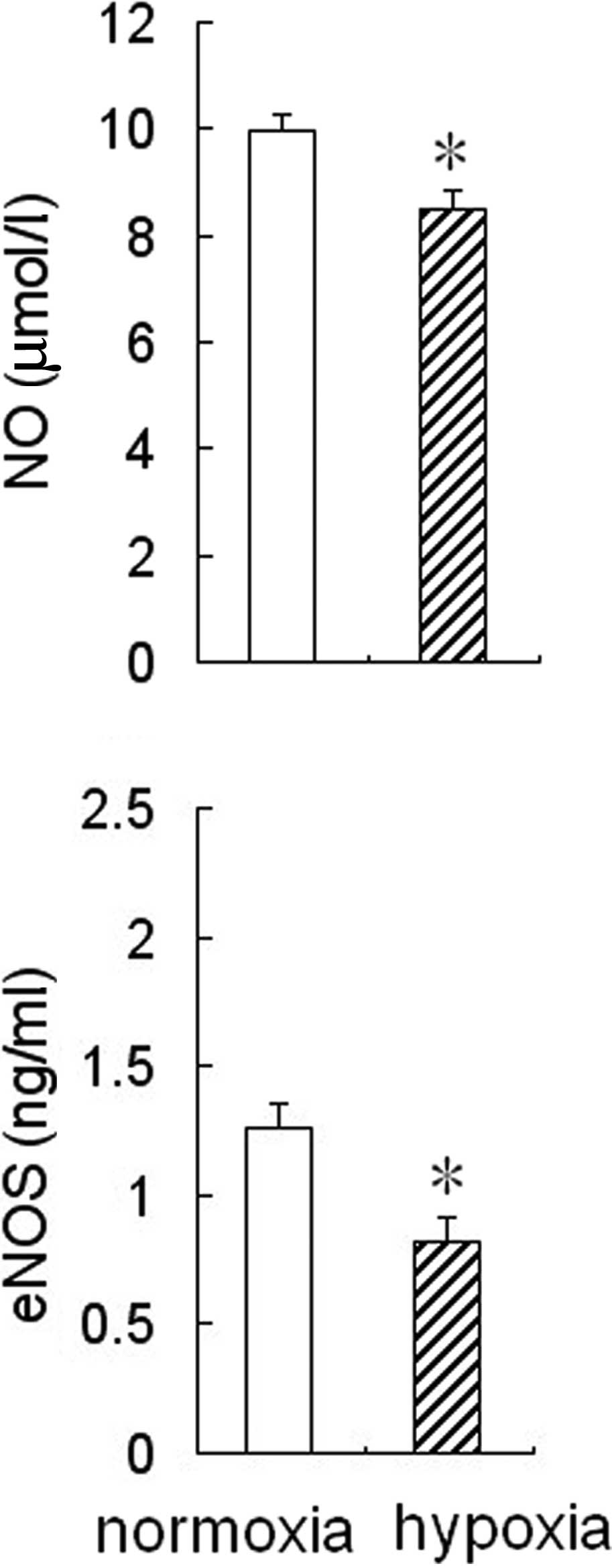
Effect of hypoxia on eNOS activity and NO
production in HPAECs
To determine whether hypoxia affected eNOS activity
and NO production in HPAECs, the cells were cultured for 24 h under
hypoxic or normoxic conditions, then the medium was collected to
measure NO levels and eNOS activity. The results showed that eNOS
activity and NO levels were reduced significantly in the
conditioned medium from HPAEC cultures under hypoxic conditions
compared to the cultures under normoxic conditions (Fig. 1).
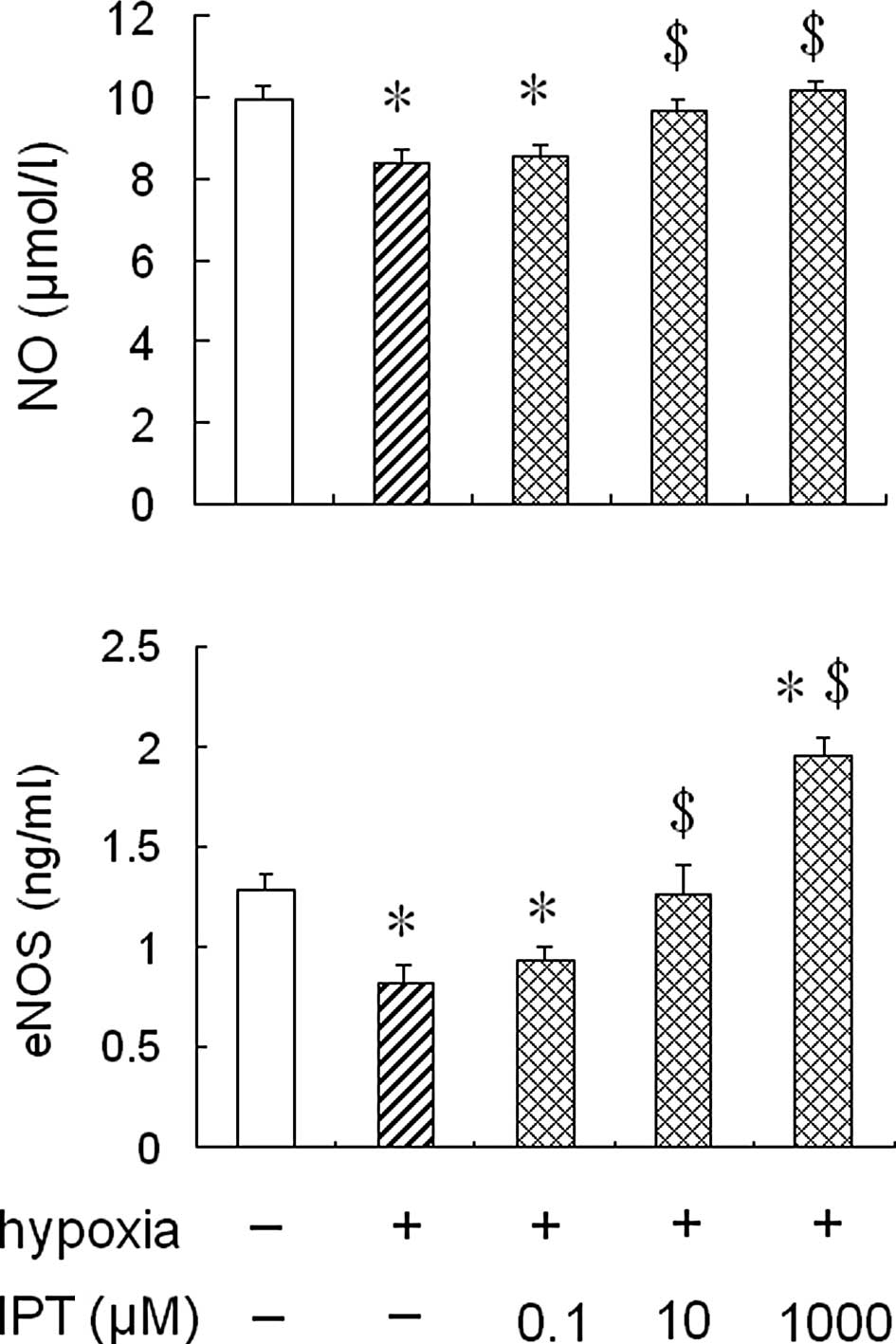
Effect of iptakalim on eNOS activity and
NO production in HPAECs under hypoxia
To determine whether the treatment with iptakalim
antagonized a hypoxia-induced reduction of eNOS activity and NO
production in HPAECs, the cells were pre-treated with 0.1 or 10 or
1,000 μM iptakalim for 1 h prior to hypoxia and cultured under
hypoxia conditions for 24 h, and eNOS activity and NO levels were
measured in the conditioned medium from the HPAEC cultures. The
results showed that eNOS activity and NO levels were increased
significantly in the conditioned medium from HPAEC cultures
pre-treated with 10 or 1,000 μM iptakalim compared to those from
hypoxic cultures alone and were the same or even higher than the
levels of the normoxic cultures (Fig.
2). However, eNOS activity and NO levels were not raised
significantly in the the conditioned medium from HPAEC cultures
pre-treated with 0.1 μM iptakalim.
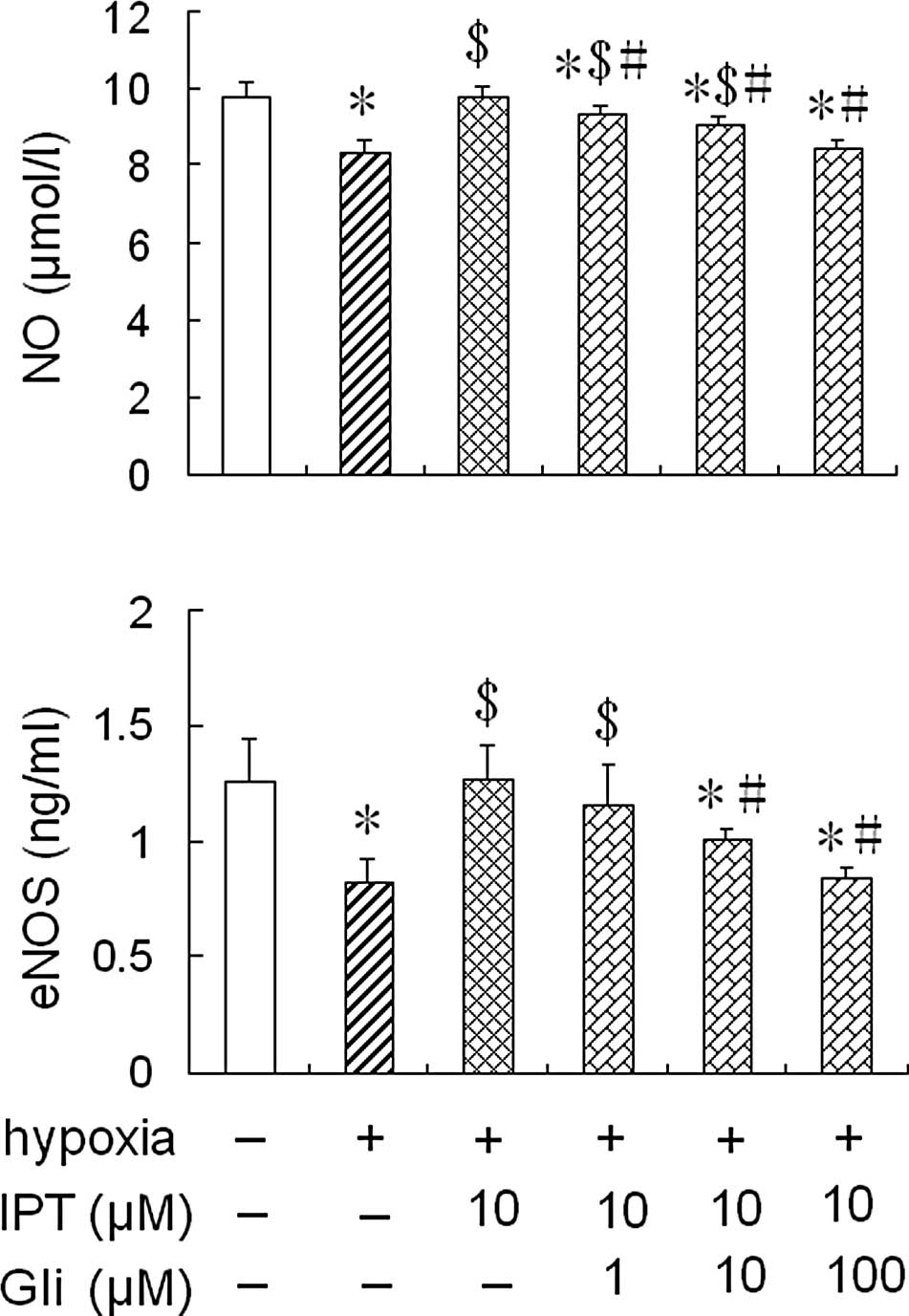
Effect of glibenclamide on eNOS activity
and NO production in HPAECs pre-treated with iptakalim under
hypoxia
To further assess whether iptakalim increases eNOS
activity and NO levels through the activation of the
KATP channel, the cells were pre-treated with 1.0 or 10
or 100 μM glibenclamide, a KATP channel blocker, for 1 h
prior to the addition of 10 μM iptakalim, and were cultured under
hypoxia conditions for 24 h; eNOS activity and NO levels were
measured in the conditioned medium from the HPAEC cultures. The
treatment of glibenclamide alone did not alter eNOS activity and NO
levels in the conditioned medium from the HPAEC cultures under
normoxic or hypoxic conditions (data not shown). However, the
increased eNOS activity and NO levels induced by the pre-treatment
with 10 μM iptakalim in the conditioned medium from the HPAEC
cultures were blocked completely by the pre-treatment of 10 or 100
μM glibenclamide. However, eNOS activity and NO levels were not
blocked completely in the conditioned medium from the HPAEC
cultures pre-treated with 1.0 μM glibenclamide (Fig. 3).
Discussion
In the present study, we found that the eNOS
activity and NO levels were reduced significantly in the
conditioned medium from the HPAEC cultures under hypoxic
conditions. Previous studies have shown that the release of NO is
decreased in bovine aortic and pulmonary endothelial cells and
human umbilical vein endothelial cells (HUVECs) by hypoxia
(13,14). It has also been shown that eNOS
activities decrease in bovine and human endothelial cells exposed
to chronic hypoxia (15–18). However, hypoxia has been found to
increase the formation of NO and its products in cultured coronary
endothelial cells (19–22). The differences in the NO release
under hypoxic conditions may be due to the endothelial cell type
and duration of hypoxic exposure. Our results indicate that hypoxia
may impair NO system function in HPAECs.
NO is an endogenous vasoactive compound that
contributes to pulmonary vascular homeostasis and is produced by
three NOS isoforms: Neuronal NOS (nNOS), inducible NOS (iNOS) and
eNOS. Though all these NOS isoforms are present in the lung, it was
thought that eNOS-derived NO plays a significant role in modulating
pulmonary vascular tone and attenuating PH (23). The vasorelaxation of smooth muscle
cells may be achieved by the release of NO from endothelial cells
in response to various stimuli (24), and the ability of the endothelium
to produce NO is essential for the maintenance of vascular
homeostasis. Reduced endothelium-derived NO production in pulmonary
arterial vessels has been implicated in the pathophysiology of PH.
It has been confirmed that NO synthase expression is reduced in
pulmonary endothelial cells from patients suffering from PAH
(25).
It has been shown that the synthesis of NO is
Ca2+-dependent (constitutive form). The Ca2+
influx in endothelial cells is controlled by the membrane potential
(26). KATP channels
are present in endothelial cells of the vascular system (6,27)
and are responsible for maintaining the resting potential of
endothelial cells and modulating the release of vasoactive
compounds. Thus, KATP channels may play a key role in
generating the electrical activity of endothelial cells and have
profound effects on endothelial function. In fact, pinacidil, a
KATP channel opener, has been shown to cause an increase
of Ca2+ influx in rat aorta and brain microvascular
endothelial cells (6). Iptakalim,
a new compound of the potassium channel opener class, is a
promising drug undergoing Phase II clinical trials to treat
pulmonary hypertension (28). By
opening the KATP channels in vascular smooth muscle
cells, iptakalim induces membrane hyperpolarization, relaxing the
vessels and reducing blood pressure.
We previously found that iptakalim antagonized the
vascular contraction evoked by ET-1 in isolated rat aorta rings
(29,30). It was also found that iptakalim
increased the intercellular concentration of Ca2+ and
promoted the NO production in BAECs under normoxic conditions.
Since the decreased eNOS/NO activities have been implicated in the
vascular remodeling and endothelial dysfunction observed in the
hypertensive models, opening the endothelial KATP
channels may have protective effects on endothelial functions under
hypoxic conditions and have certain therapeutic functions in HPH.
Indeed, in the present study, we found that iptakalim increased the
NO production and eNOS activity in HPAECs under hypoxia conditions.
Glibenclamide, a KATP channel blocker, blocked the
increased NO levels and eNOS activity caused by iptakalim under
hypoxic conditions. These results demonstrate that the effect of
iptakalim on NO production and eNOS activity in HPAECs under
hypoxic conditions occurs through the activation of the
KATP channel.
In conclusion, our results indicate that hypoxia
impairs the NO system function, whereas the ATP-sensitive K channel
opener, iptakalim, rescues HPAECs from hypoxia-induced NO system
dysfunction. Combined with previous findings where iptakalim not
only reduced the blood pressure indefinitely, but also antagonized
the proliferation of human PASMCs induced by ET-1 through the
activation of KATP channels. Our results show that
iptakalim may be effective in the treatment of PAH by reversing
human PASMCs remodeling and protecting HPAEC functions. Perhaps it
should be considered as a promising drug for the treatment of
PH.
Acknowledgements
The authors wish to thank Dr H. Wang
(Institute of Pharmacology and Toxicology, Academy of Military
Medical Sciences, Beijing, China) for the excellent technical
assistance. This study was supported by the National Natural
Science Foundation of China (No. 30971319), the ‘Six Talent Peak’
project of Jiangsu Province (No. 08-B), and the grant from Open
Project Program of the key discipline of Public Health Department
of Jiangsu Province (No. XK13_200902).
References
|
1.
|
Y ZhuS ZhangW XieQ LiY ZhouH WangIptakalim
inhibited endothelin-1-induced proliferation of human pulmonary
arterial smooth muscle cells through the activation of KATP
channelVascul Pharmacol489299200810.1016/j.vph.2008.01.001
|
|
2.
|
FA KlokMV HuismanEpidemiology and
management of chronic thromboembolic pulmonary hypertensionNeth J
Med68347351201020876914
|
|
3.
|
MM HoeperDefinition, classification, and
epidemiology of pulmonary arterial hypertensionSemin Respir Crit
Care Med30369375200910.1055/s-0029-123330619634076
|
|
4.
|
B AlanS NalbantgilGenetic, cellular and
molecular mechanisms of pulmonary arterial hypertensionAnadolu
Kardiyol Derg10Suppl 1913201010.5152/akd.2010.114
|
|
5.
|
J BauersachsJD WidderEndothelial
dysfunction in heart failurePharmacol Rep601191262008
|
|
6.
|
D JanigroGA WestEL GordonHR
WinnATP-sensitive K+ channels in rat aorta and brain
microvascular endothelial cellsAm J
Physiol265C812C82119938214038
|
|
7.
|
M ShiraiJT PearsonA ShimouchiChanges in
functional and histological distributions of nitric oxide synthase
caused by chronic hypoxia in rat small pulmonary arteriesBr J
Pharmacol139899910200310.1038/sj.bjp.0705312
|
|
8.
|
S ChatterjeeAB Al-MehdiI LevitanT
StevensAB FisherShear stress increases expression of a KATP channel
in rat and bovine pulmonary vascular endothelial cellsAm J Physiol
Cell Physiol285C959C967200310.1152/ajpcell.00511.200212826604
|
|
9.
|
LF HuS WangXR ShiATP-sensitive potassium
channel opener iptakalim protected against the cytotoxicity of
MPP+ on SH-SY5Y cells by decreasing extracellular
glutamate levelJ
Neurochem9415701579200510.1111/j.1471-4159.2005.03306.x16000145
|
|
10.
|
N MisakiX MaoYF LinIptakalim, a vascular
ATP-sensitive potassium (KATP) channel opener, closes rat
pancreatic beta-cell KATP channels and increases insulin releaseJ
Pharmacol Exp Ther322871878200710.1124/jpet.107.12112917522344
|
|
11.
|
W XieH WangH WangG HuEffects of iptakalim
hydrochloride, a novel KATP channel opener, on pulmonary vascular
remodeling in hypoxic ratsLife
Sci7520652076200410.1016/j.lfs.2004.03.03115312751
|
|
12.
|
H WangC LongZ DuanC ShiG JiaY ZhangA new
ATP-sensitive potassium channel opener protects endothelial
function in cultured aortic endothelial cellsCardiovasc
Res73497503200710.1016/j.cardiores.2006.10.00717116295
|
|
13.
|
AR WhortonDB SimondsCA
PiantadosiRegulation of nitric oxide synthesis by oxygen in
vascular endothelial cellsAm J Physiol2721161116619979227518
|
|
14.
|
JK LiaoJJ ZuluetaFS YuHB PengCG CotePM
HassounRegulation of bovine endothelial constitutive nitric oxide
synthase by oxygenJ Clin
Invest9626612666199510.1172/JCI1183328675632
|
|
15.
|
UA ArnetA McMillanJL DinermanB
BallermannCJ LowensteinRegulation of endothelial nitric-oxide
synthase during hypoxiaJ Biol
Chem2711506915073199610.1074/jbc.271.25.150698663208
|
|
16.
|
JK LiaoRho-kinase mediates hypoxia-induced
downregulation of endothelial nitric oxide
synthaseCirculation1065762200210.1161/01.CIR.0000020682.73694.AB12093770
|
|
17.
|
LP McQuillanGK LeungPA MarsdenSK KostykS
KourembanasHypoxia inhibits expression of eNOS via transcriptional
and posttranscriptional mechanismsAm J
Physiol2671921192719947526714
|
|
18.
|
MW PhelanDV FallerHypoxia decreases
constitutive nitric oxide synthase transcript and protein in
cultured endothelial cellsJ Cell
Physiol167469476199610.1002/(SICI)1097-4652(199606)167:3%3C469::AID-JCP11%3E3.0.CO;2-%238655601
|
|
19.
|
JX ChenB MeyrickHypoxia increases Hsp90
binding to eNOS via PI3K-Akt in porcine coronary artery
endotheliumLab
Invest84182190200410.1038/labinvest.370002714661033
|
|
20.
|
JM JusticeMA TannerPR MyersEndothelial
cell regulation of nitric oxide production during hypoxia in
coronary microvessels and epicardial arteriesJ Cell
Physiol182359365200010.1002/(SICI)1097-4652(200003)182:3%3C359::AID-JCP6%3E3.0.CO;2-310653602
|
|
21.
|
HY SohnF KrotzT GloeDifferential
regulation of xanthine and NAD(P)H oxidase by hypoxia in human
umbilical vein endothelial cellsRole of nitric oxide and adenosine
Cardiovasc Res58638646200312798437
|
|
22.
|
XP XuJS PollockMA TannerPR MyersHypoxia
activates nitric oxide synthase and stimulates nitric oxide
production in porcine coronary resistance arteriolar endothelial
cellsCardiovasc Res30841847199510.1016/0008-6363(95)00117-4
|
|
23.
|
L OstergaardE StankeviciusMR
AndersenDiminished NO release in chronic hypoxic human endothelial
cellsAm J Physiol Heart Circ
Physiol29328942903200710.1152/ajpheart.01230.200617720765
|
|
24.
|
S TaddeiA VirdisL GhiadoniI SudanoA
SalvettiEffects of antihypertensive drugs on endothelial
dysfunction: clinical
implicationsDrugs62265284200210.2165/00003495-200262020-0000311817973
|
|
25.
|
A GiaidD SalehReduced expression of
endothelial nitric oxide synthase in the lungs of patients with
pulmonary hypertensionN Engl J
Med333214221199510.1056/NEJM1995072733304037540722
|
|
26.
|
L KuoJD ChancellorAdenosine potentiates
flow-induced dilation of coronary arterioles by activating KATP
channels in endotheliumAm J Physiol6954154919957653618
|
|
27.
|
M Mederos y SchnitzlerC DerstJ DautR
Preisig-MüllerATP-sensitive potassium channels in capillaries
isolated from guinea-pig heartJ Physiol525307317200010835035
|
|
28.
|
H WangPharmacological characteristics of
the novel antihypertensive drug, iptakalim hydrochloride, and its
molecular mechanismsDrug Dev Res586568200310.1002/ddr.10132
|
|
29.
|
H XueYL ZhangGS LiuH WangA new
ATP-sensitive potassium channel opener protects the kidney from
hypertensive damage in spontaneously hypertensive ratsJ Pharmacol
Exp Ther315501509200510.1124/jpet.105.08972216051697
|
|
30.
|
H WangYL ZhangXC TangHS FengG HuTargeting
ischemic stroke with a novel opener of ATP-sensitive potassium
channels in the brainMol
Pharmacol6611601168200410.1124/mol.104.00317815304552
|