Introduction
Breast cancer is the most common malignant disease
in the world affecting women. In these patients, it is not the
primary tumor but its metastases at distant sites that are the main
cause of death. Clinical trials suggest that most cases of breast
cancer may be systemic, and in approximately 25–30% of breast
cancer patients in whom axillary lymph nodes test negative, distant
metastases appear within 5 years after surgery, sometimes resulting
in death (1). Systemic adjuvant
therapy can help eradicate breast cancer cells that may have
already spread to distant sites by the time of diagnosis and could
remain avascular for a long period of time but retain the potential
to multiply and induce metastasis if they became active (2). Human mammaglobin (hMAM) is expressed
at a lower level in normal breast epithelium, but at a higher level
in breast cancer tissue, and is not expressed in normal blood and
bone marrow (BM). We detected the expression of hMAM mRNA in the BM
of patients with breast cancer, and analyzed the relationships
between micrometastasis in the BM and clinicopathological
parameters as well as the prognosis of breast cancer. In addition,
using this method, we were able to make a judgment regarding the
likelihood of breast cancer BM micrometastasis and discuss its
relationships with ER, PR, Cath-D and other markers. Our purpose
was to study the regulation of breast cancer metastasis, in order
to provide markers to predict prognosis and provide the foundation
for individualized treatment.
Materials and methods
Patients
Of the breast cancer patients treated in our
hospital from 2002 to 2003, 102 cases were chosen stochastically.
All were female, diagnosed by pathology, and were aged from 30 to
76 years (49.2 average). Twelve patients were at stage I, 59 at
stage II, and 31 at stage III. All patients were treated
surgically, without radiotherapy or chemotherapy prior to surgery.
Fifty-three cases were infiltrating ductal carcinoma (IDC), 35 were
infiltrating lobular carcinoma (ILC), and 14 were other categories.
Ten cases of benign breast disease were used as the control group,
and these included hyperplasia of the mammary glands and
fibroadenoma. Ten milliliters of bone marrow was obtained
preoperatively by bone marrow aspiration at the anterior superior
iliac spine, with informed consent, and stored at −70°C until used
for detection.
All patients were followed up from 13 to 60 months
after surgery, 47 months on average. At the final follow-up, 8
cases had been diagnosed with distant metastases or had died, while
all others survived disease-free.
RT-PCR, primers and amplification product
evaluation
The hMAM primer was purchased from
Shanghai-Sheng-Gong Bioengineering Co. The upstream primer was -AGC
ACT GCT ACG CAG GCT CT-3′ and the downstream primer, 5′-ATA AGA AAG
AGA AGG TGT GG-3′; amplification product length 329 bp. The
inner-reference β-actin upstream primer was 5′-ATC TGG CAC CAC ACC
TTC TAC AAT GAG CTG CG-3′ and the downstream primer, 5′-CGT CAT ACT
CCT GCT TGC TGA TCC ACA TCT GC-3′; amplification product length 838
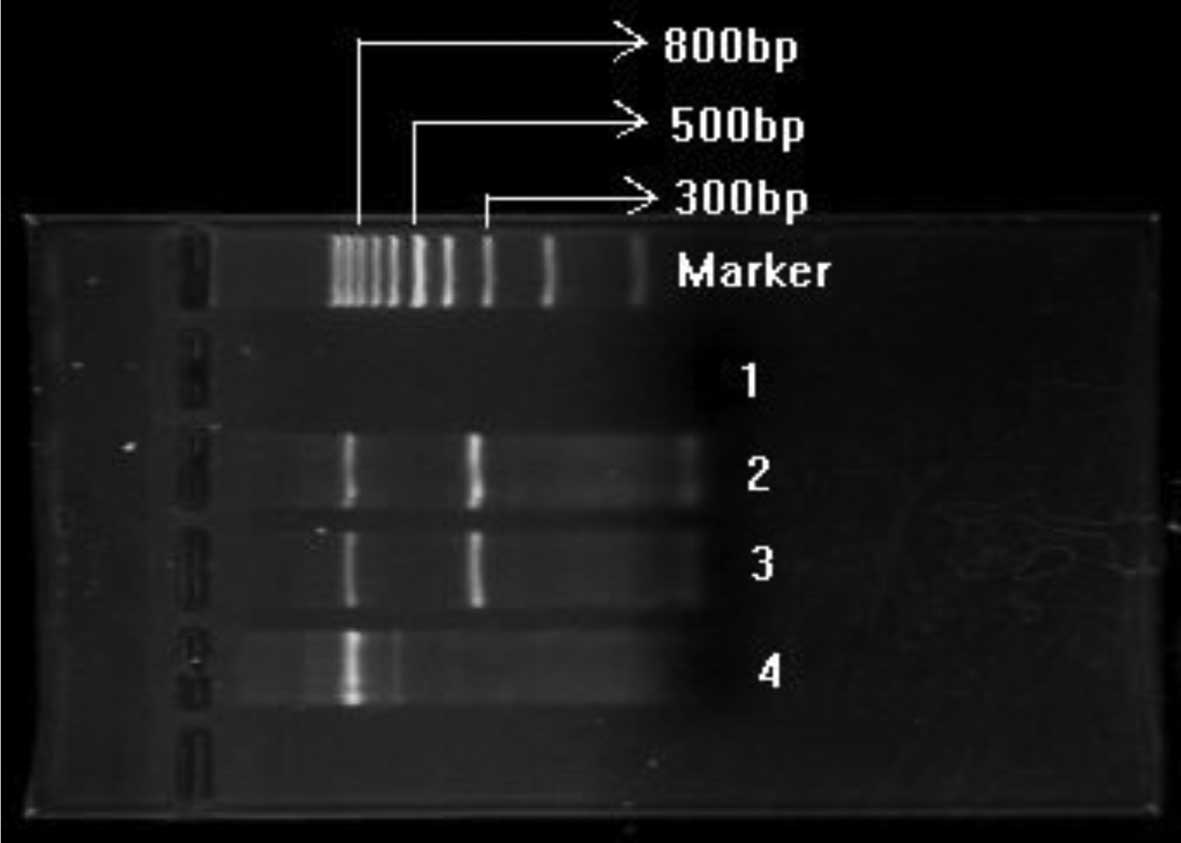
bp (Fig. 1).
TRIzol was used to lyse the cells, and the
guanidinium thiocyanate-phenol-chloroform one-step extraction
method was used to obtain total-RNA. The RNA extracted was
electrophoresed on a 1.5% agarose gel; the width and brightness of
the 28S RNA band was double the 18S band as determined under an
ultraviolet transilluminator, which indicated good integrity of the
extracted RNA.
The reaction parameters were as follow: 37°C reverse
transcription for 50 min to obtain cDNA for PCR amplification,
followed by cycling conditions of: 95°C denaturation, 5 min, 94°C,
melting, 45 sec, 56°C annealing, 1 sec, 72°C extension, 1 sec, 35
cycles, with a final 5 min extension; β-actin and hMAM were
amplified in the same tube.
We reached a positive diagnosis of BM metastasis
when a clear and specific 329-bp hMAM band and an 838-bp β-actin
band was found simultaneously. When the internal standard β-actin
was positive but hMAM was negative, the case was diagnosed as BM
metastasis-negative (Fig. 1).
Immunohistochemical technique and
evaluation of the results
Streptavidin-peroxidase (S-P) immunohistochemistry,
using a kit from Beijing Zhongshan Biotechnology Company, was
performed using primary antibodies at the following dilutions: ER
1:240; PR 1:400; Cath-D 1:80. Both negative and positive controls
were run alongside the samples; for the negative control PBS was
used in place of the antibody, while for the positive control,
directions supplied in the kit were followed.
Twenty high-power fields were selected using a light
microscope, and at least 2,000 cells were counted, and then
aggregated to analyze the positive cell population and staining
intensity of the entire slice, which was judged according to the
positive intensity as specified in the different kits. Levels of
staining are indicated by (−) to represent negative staining, while
positive staining was classified as (+), (++) or (+++).
Statistical analysis
Results were analyzed using SPSS 13.0 software, by
the RxC Chi-square test and the quadruple tabular form exact
probability test. Statistical significance was defined as
P<0.05.
Results
hMAM mRNA expression in the BM of breast
cancer patients
No mRNA expression of hMAM was found in the BM of 10
patients with benign breast disease. In contrast, positive
expression of hMAM was found in the BM of 39 of 102 breast cancer
patients; a positivity rate of 38.2%. Statistical analysis revealed
a significance level of P=0.014.
Relationship between hMAM mRNA expression
in the BM and size of the tumor
The expression of hMAM in the BM was greatly
increased with increasing tumor size. The expression rate in
patients with a tumor of size <2, 2–5 and >5 cm was
12.5% (2/16), 33.3% (23/69) and 76.5% (14/17), respectively;
comparison among the three groups achieved a statistical
significance (χ2=19.20, P=0.001) (Table I).
 | Table I.Relationship between expression of
hMAM in the bone marrow of breast cancer patients and tumor size,
metastasis to the lymph nodes, clinical stage, patient age,
pathological types, histological grading and expression of ER, PR
and Cath-D. |
Table I.
Relationship between expression of
hMAM in the bone marrow of breast cancer patients and tumor size,
metastasis to the lymph nodes, clinical stage, patient age,
pathological types, histological grading and expression of ER, PR
and Cath-D.
| Parameters | n | hMAM mRNA expression
| Positive rate
(%) |
χ2-value | P-value |
|---|
| − | + |
|---|
| Tumor size | | | | | | |
| <2 cm | 16 | 14 | 2 | 12.5 | 19.20 | 0.001 |
| 2–5 cm | 69 | 46 | 23 | 33.3 | | |
| >5 cm | 17 | 4 | 14 | 82.4 | | |
| Lymphatic
metastasis | | | | | | |
| 0 | 51 | 39 | 12 | 23.5 | 14.050 | 0.001 |
| 1–3 | 26 | 16 | 10 | 61.5 | | |
| ≥4 | 25 | 8 | 17 | 68.0 | | |
| Clinical stage | | | | | | |
| I | 12 | 11 | 1 | 8.3 | 15.101 | 0.001 |
| II | 59 | 41 | 18 | 30.5 | | |
| III | 31 | 11 | 20 | 64.5 | | |
| Age (years) | | | | | | |
| ≤50 | 61 | 40 | 21 | 34.4 | 1.056 | 0.304 |
| >50 | 41 | 23 | 18 | 43.9 | | |
| Pathological
type | | | | | | |
| Infiltrating
ductal carc. | 53 | 29 | 24 | 45.3 | 6.892 | 0.032 |
| Infiltrating
lobular carc. | 35 | 21 | 14 | 40.0 | | |
| Others | 14 | 13 | 1 | 7.1 | | |
| Histological
grading | | | | | | |
| 1 | 30 | 25 | 5 | 16.7 | 8.522 | 0.014 |
| 2 | 44 | 24 | 20 | 45.5 | | |
| 3 | 28 | 14 | 14 | 50.0 | | |
| ER | | | | | | |
| − | 27 | 11 | 16 | 59.3 | 11.80 | 0.003 |
| + | 22 | 11 | 11 | 50.0 | | |
| ++, +++ | 53 | 41 | 12 | 22.6 | | |
| PR | | | | | | |
| − | 31 | 15 | 16 | 51.6 | 8.759 | 0.013 |
| + | 26 | 13 | 13 | 50.0 | | |
| ++, +++ | 45 | 35 | 10 | 22.2 | | |
| Cath-D | | | | | | |
| − | 13 | 9 | 4 | 30.8 | 6.623 | 0.036 |
| + | 26 | 21 | 5 | 19.2 | | |
| ++, +++ | 63 | 33 | 30 | 47.6 | | |
Relationship between hMAM mRNA expression
in the BM and clinical stage
The rate of hMAM expression increased with advancing
clinical stage; the expression rate for stage I was 8.3% (1/12),
stage II, 30.5% (18/59) and stage III, 64.5% (20/31)
(χ2=15.101, P=0.001) (Table
I).
Relationship between hMAM mRNA expression
in the BM and axillary lymph node metastasis
The expression rate of hMAM in the BM was higher in
the group positive for axillary lymph node metastasis (52.5%;
27/51) than in the negative group (23.5%; 12/51); the result
achieved statistical significance (χ2=14.050, P=0.001)
(Table I). The positive group was
further separated into two subgroups: A, those with 3 or fewer
involved lymph nodes; and B, the remaining patients, with 4 or
more. The results of ambi-comparisons showed that the rate of
micrometastasis in the BM was higher in group B (≥4) than in the
negative group or group A (≤3), this result was also statistically
significant (χ2=4.464, 14.060 respectively; P=0.035,
0.001 respectively).
Relationship between hMAM mRNA expression
in BM and pathology
hMAM mRNA expression was much higher in the group
with higher potential malignancy. The rate in patients with
infiltrating ductal carcinoma was 45.3% (24/53), and the rate in
patients with infiltrating lobular carcinoma was 40.0% (14/35). In
contrast, in cases with a lower potential malignancy, the rate of
hMAM expression was 7.1% (1/14); this group consisted of 4 cases of
typical medullary carcinoma, 4 cases of tubular adenocarcinoma, 2
cases of atypical hyperplasia with canceration, 2 cases of mucinous
adenocarcinoma, and 2 cases of Paget’s disease. The rate of
expression in infiltrating ductal carcinoma was close to that in
infiltrating lobular carcinoma (χ2=0.240, P=0.624).
However, the difference between these two diseases and the group of
low potential malignant diseases was statistically significant
(χ2=6.892, P=0.032) (Table
I).
Relationship between hMAM mRNA expression
in the BM and histological differentiation
The rate of hMAM mRNA expression in the BM increased
with increasing histological grade. The rate of positive expression
in grade 1 was 16.7% (5/30), in grade 2, 45.5% (20/44), and in
grade 3, 50.0% (14/28) (χ2=8.522, P=0.014) (Table I).
Relationship between BM micrometastasis
and expression of ER and PR
The BM micrometastasis rate in the ER(−) group was
59.3% (16/27) and the rate in the ER(+) group was 50.0% (11/22),
while in the ER(++,+++) group it was 22.6% (12/53),
(χ2=11.800, P=0.003). Likewise, the BM micrometastasis
rate in the PR(−) group was 51.6% (16/31) and in the PR(+) group it
was 50.0% (13/26), while in the PR(++,+++) group the rate was 22.2%
(10/45) (χ2=8.759, P=0.013) (Table I).
Relationship between BM micrometastasis
and expression of Cath-D in breast cancer tissue
The expression rate of hMAM mRNA in the BM of the
102 patients with breast cancer increased concomitantly with the
expression of Cath-D in the breast cancer tissues. The BM
micrometastasis rate of the Cath-D(−) group was 30.8% (4/13); and
the BM micrometastasis rate in the Cath-D(+) group was 19.2%
(5/26), and this rate in the Cath-D(++,+++) group was 47.6% (30/63)
(χ2=6.623, P=0.036) (Table
I).
Relationship between hMAM mRNA expression
in the BM and patient age
There was no correlation between hMAM expression and
patient age (χ2=1.056, P=0.304) (Table I).
Relationship between hMAM mRNA expression
in the BM and distant metastasis
Positive expression of hMAM mRNA in the BM before
surgery was found in all 7 of the 8 patients who later developed
distant metastases or who died. The quadruple tabular form exact
probability test was used to examine the incidence of distant
metastasis between the hMAM-positive and -negative groups
(P=0.009).
Discussion
There are at least three conditions which must be
fulfilled to define a perfect tumor marker. These are tumor
specificity, tissue specificity and sensitivity. We found that a
pseudo-gene was present when we detected the expression of CK-19 by
RT-PCR (3), which made the
micrometastasis rate higher than it really was. Therefore, it is
difficult but rather important to choose a suitable marker.
hMAM is on chromosome 11q12–13, and the amino
acid sequence of its translated protein is similar to that of a
protein excreted by the epithelium, which belongs to the
uteroglobulin family. They are all encoded by the genome in the
same position on the chromosome. hMAM is expressed at lower levels
in normal breast epithelium, but at higher levels in breast cancer
tissue while hMAM is not expressed in normal blood and BM.
Marchetti et al (4)
researched micrometastasis to the lymph nodes in breast cancer
patients and found that hMAM was more characteristic than the other
7 markers studied, such as CEA, CK-19 and MUC-1. The results of our
study showed that the incidence rate of micrometastasis to the BM
(hMAM-positive) was 38.2% in the group of 102 patients with primary
breast cancer, while no micrometastasis was found in the group with
benign breast diseases.
Since the 1980s, many animal experiments and
clinical studies have found an intimate relationship between
micrometastasis in the BM and poor prognosis of patients with
breast cancer. Braun et al (5) carried out research on 552 patients
with breast cancer, classified from grade 1 to grade 3. He found
that the rate of incidence of micrometastasis in the BM was 36.1%.
After a 4-year follow-up, 49 of 199 patients with micrometastasis
to the BM died from cancer metastasis. In contrast, only 22 of 353
patients who were micrometastasis-negative died of cancer, which
indicates that distant metastasis is positively correlated with the
mortality rate of breast cancer patients who have micrometastasis
to the BM.
The size of the tumor, the presence of axillary
lymph node metastasis and clinical stage are all important with
regard to the choice of therapy and prediction of prognosis. The
common view is that prognosis gets worse in patients with larger
tumors, metastasis to a greater number of axillary lymph nodes, or
a more advanced clinical stage. Braun et al (6) carried out aggregate analysis of data
from 4,199 breast cancer cases and obtained a rate of
micrometastasis to the BM of 30.6%. Substratificational analysis
concluded that micrometastasis to the BM was correlated with the
size of the tumor, the condition of axillary lymph node metastasis
and histological classification (P<0.001). The 10-year follow-up
found that the total death rate in the BM micrometastasis-positive
cases was higher than that of BM micrometastasis-negative group
(P<0.001). The mortality rate was greatly increased in the BM
micrometastasis-positive group, who also did not accept adjunctive
therapy (P<0.001). The analysis indicated that the presence of
micrometastasis in the BM was an independent predictor of
prognosis. Furthermore, the impact on prognosis was much more
obvious in the group in which the lymph nodes were
metastasis-negative. Our results showed that the rate of
micrometastasis to the BM of breast cancer patients increased as
the tumor size increased or as the clinical stage increased. This
indicates that the larger the tumor, the more invasive it is, which
can promote the spreading of cancer cells to distant sites.
However, nearly 10% of patients whose tumors were smaller than 2 cm
or at stage I still expressed hMAM in their BM. At the same time,
our results showed that the patients who were axillary lymph node
metastasis-positive (52.9%) had a higher expression of hMAM mRNA
than the negative group (23.5%), and this result was statistically
significant (χ2=14.050, P=0.001). This shows that there
is a clear relationship between micrometastasis in the BM and
axillary lymph node metastasis. We separated the positive group
into two subgroups: A, those with 3 or fewer positive lymph nodes;
and B, the remainder with 4 or more positive nodes. The results of
ambi-comparisons showed that the rate of micrometastasis in the BM
was higher in group B (≥4) than in the negative group and group A
(≤3), a result which was statistically significant
(χ2=4.464, 14.060 respectively; P=0.035, 0.001
respectively). That is to say the more metastasis-positive lymph
nodes a patient has, the higher the rate of micrometastasis to the
BM. However, there was no obvious difference between the negative
group and group A, and the difference was not statistically
significant (χ2=1.881, P=0.170). Previous clinical
studies have found that approximately 20–30% of patients
with no metastasis in the lymph nodes suffer from metastasis and
recurrence, which leads to death within the first 5 years after
surgery. Our study also found that various patients with no
metastasis to the lymph nodes, and several of those with fewer than
3 positive lymph nodes, also expressed hMAM mRNA in their BM; the
rate of expression being 23.5 and 61.5%, respectively. These
findings show that breast cancer may be a systemic disease, and
that hematogenous metastasis can occur even during the initial
stage. Our team also reached this conclusion by detecting
micrometastasis in the BM by means of CK-19 (7). Clinical studies have found that
certain patients with small tumors and at initial stages die soon
after surgery as a result of metastasis; the reason may have some
relationship with this finding. These observations emphasize the
need for these patients to receive systemic adjunctive therapy to
improve their long-term survival rate. We also consider that the
detection of axillary lymph node metastasis is insufficient to
judge prognosis and to direct general treatments, especially when
the patients have no metastasis in the axillary lymph nodes or have
fewer positive lymph nodes. Therefore, it is necessary to detect
micrometastasis in the BM during routine examination. Assessment of
the level of metastasis throughout the body much earlier and more
precisely is critical. As a result, we can then offer patients with
micrometastasis active systemic therapy, and avoid over-treatment,
even in cases in which the clinical stage is more favorable, tumor
size is smaller or the axillary lymph nodes are
metastasis-negative.
Micrometastasis in the BM of breast cancer patients
has a certain relationship with histological classification. The
rate of hMAM mRNA expression in the BM of patients was higher in
poorly differentiated cases than in those with good
differentiation. The rate of micrometastasis in BM was higher in
the group with infiltrating ductal carcinoma and infiltrating
lobular carcinoma than the group with types of breast cancer with
lower malignant potential. Thus, the types of breast cancer with
poor differentiation and higher potential of malignancy have much
greater abilities to proliferate, invade and migrate.
Stathopoulou et al (9) and Ooka et al (10) found that detection of
micrometastasis was correlated with disease-free and total
survival, providing a reference with which to judge prognosis. Our
results showed that the 7 patients who later suffered distant
metastasis and death were all hMAM mRNA-positive before surgery.
Statistical analysis indicated a strong correlation between the
detection of hMAM and metastasis in patients.
The present study found that the BM micrometastasis
rate was high in the group that were ER, PR negative expression and
the group that were ER, PR low expression, however the BM
micrometastasis rate declined as the expression of ER and PR
increased, P<0.05. According to Braun et al (6) the BM micrometastasis rate was high in
the ER negative expression group. This indicated that the
hematogeneous metastasis risk would increase in cases which were
ER, PR negative expression or ER, PR low expression, and that cells
identified as ER, PR negative expression, possibly, had greater
invasive ability. Breast cancer cases identified as ER, PR low
expression had a poor prognosis in comparison with ER, PR positive
expression cases.
Cath-D is a type of lysosomal enzyme; its
physiologic function is to degrade protein in the acid environment
of the lysosomes. By accelerating tumor cell growth, decreasing the
activity of tumor-inhibiting factor (TIF), and degrading basal
lamina, Cath-D increases the propensity of the tumor cells to
invade and metastasize. Wu et al (11) found that Cath-D hyper-expression
indicated poor prognosis. Our study showed that the BM
micrometastasis rate rose as the expression of Cath-D increased
(P<0.05).
In conclusion, the detection of micrometastasis in
the BM can be one way to assess the prognosis of breast cancer.
Using dynamic detection, we can quickly obtain information
concerning therapeutic efficacy, monitor changes in the patient’s
condition, adjust the therapeutic regimen and evaluate progress.
This molecular prognostic method can play an active part in
clinical research, and it can provide a basis for an individual
therapeutic regimen for each patient with breast cancer.
Acknowledgements
This study has supported by the Key
Oncologic Subject Foundation of Hebei Province (no. 200552), the
Natural Science Foundation of Hebei Province.
References
|
1.
|
P CarcoforoL BergossiE BasagliaPrognostic
and therapeutic impact of sentinel node micrometastasis in patients
with invasive breast cancerTumori88S4S5200212365385
|
|
2.
|
G GebauerT FehmE MerkleEP BeckN LangW
JägerEpithelial cells in bone marrow of breast cancer patients at
time of primary surgery: clinical outcome during long-time
follow-upJ Clin Oncol1936693674200111504748
|
|
3.
|
YM FengXS HaoY YuLM WangX LiThe pseudogene
interference of Ck-19 as marker to detect the gene of
micrometastasis carcinoma and the means to solve the
interferenceChin J Oncol233303312001(In Chinese)
|
|
4.
|
A MarchettiF ButtittaG BertaccamRNA
markers of breast cancer nodal metastases: comparision between
mammaglobin and carcinoembryonic antigen in 248 patientsJ
Pathol195186190200110.1002/path.94311592097
|
|
5.
|
S BraunK PantelP
MullerCytokeratin-positive cells in the bone marrow and survival of
patients with stage I, II, or III breast cancerN Engl J
Med342525533200010.1056/NEJM20000224342080110684910
|
|
6.
|
S BraunFD VoglB NaumeA pooled analysis of
bone marrow micrometastasis in breast cancerN Engl J
Med353793802200510.1056/NEJMoa05043416120859
|
|
7.
|
YJ LiuFY WuXD WuDetection of
micrometastasis in bone marrow of patients with breast cancer and
its clinical significanceJ Clin Surg125275292004(In Chinese)
|
|
8.
|
A FabisiewiczJ KulikP KoberE BrewczyńskaT
PieńkowskiJA SiedleckiDetection of circulating breast cancer cells
in peripheral blood by a two-marker RT-PCR assayActa Biochim
Pol51747755200415448736
|
|
9.
|
A StathopoulouI VlachonikolisD
MavroudisMolecular detection of cytokeratin-19-positive cells in
the peripheral blood of patients with operable breast cancer:
evaluation of their prognostic significanceJ Clin
Oncol2034043412200210.1200/JCO.2002.08.135
|
|
10.
|
M OokaY TamakiI SakitaBone marrow
micrometastases detected by RT-PCR for mammaglobin can be an
alternative prognostic factor of breast cancerBreast Cancer Res
Treat67169175200110.1023/A:1010651632354
|
|
11.
|
FY WuYJ LiuXL WangHC YangYJ WangThe
expression of cathepsin-D in breast cancer tissue and the
correlation with other related targetsJ Tumor Treat
Prev121901922005(In Chinese)
|