Introduction
By certain estimates, gastric cancer is the fourth
most common type of cancer after lung, breast and colorectal, and
the second leading cause of cancer-related death (1). Although its prognosis has improved in
recent years, particularly in the West, gastric cancer remains a
key public health issue in China (2,3). In
contrast to the patterns of incidence in the West, more new cases
are diagnosed each year in China (4–7).
However, gastric cancer is often diagnosed at an advanced stage.
Increased evidence has indicated that gastric cancer results from
various genetic and epigenetic alterations of oncogenes,
tumor-suppressor genes, DNA repair genes, cell adhesion molecules
and cell cycle regulators (8–10).
Molecules involved in each step of the carcinogenesis process are
potential prognostic and therapeutic markers.
The RASSF family of proteins consists of 10 members
(RASSF1 to 10) with various isoforms, all of which share a region
of homology, the ras association domain. Members of this family
have been reported to suppress cell growth when expressed
exogenously in cultured cells (11). The RASSF2 gene has been shown to be
frequently inactivated by promoter methylation in a wide range of
tumor types, including colorectal, gastric, oral, nasopharyngeal,
breast and lung cancers (12–16).
Further studies have identified that RASSF2 has a putative nuclear
localization signal (NLS) and a nuclear export signal (NES) at the
N- and C-terminal regions, respectively (17). In addition, RASSF2 acts in a
complex manner that extends beyond simple protein-protein
association to play an important role in MST1 regulation as RASSF2
protein stability is significantly decreased in the absence of MST1
in vitro and in vivo (18). To date, all evidence indicates that
RASSF2 is a K-Ras-specific effector and potential tumor
suppressor.
The present study was carried out to investigate
alterations in the expression of RASSF2 in surgical specimens of
gastric cancer, to explore the possible correlation between RASSF2
expression and clinicopathological variables, and to correlate the
expression of RASSF2 with lymph node and distant metastasis. In
addition, we also analyzed the prognostic significance of RASSF2
expression and assessed the impact of expression of the studied
protein on patient survival.
Materials and methods
Patients and tissue samples
This study included a total of 276 Chinese patients
with primary gastric cancer. Gastric cancer tissues were obtained
from gastrectomy specimens at the Department of Surgery and
Pathology, The Second Affiliated Hospital of Kunming Medical
University, from July 2000 to May 2006. Sixty-five non-cancerous
human gastric tissues were obtained from gastrectomies of adjacent
gastric cancer margins >5 cm. None of the patients had received
chemotherapy or radiotherapy prior to surgery. Tissues were
formalin-fixed, paraffin-embedded, and clinically and
histopathologically diagnosed at the Departments of
Gastrointestinal Surgery and Pathology. All patients had follow-up
records for over 5 years. The follow-up deadline was March 2011.
The survival time was determined from the date of surgery to the
follow-up deadline or date of death, which was mostly caused by
recurrence or metastasis. Clinicopathological findings were
determined according to the TNM-7th edition 2009 (UICC/AJCC) and
Japanese Classification 2010 in Gastric Cancer (19,20).
There were 8 papillary adenocarcinomas, 187 tubular
adenocarcinomas, 47 mucinous adenocarcinomas, 34 signet ring cell
carcinomas and 17 highly differentiated adenocarcinomas; 90 were
classified as moderately differentiated adenocarcinomas, 165 as
poorly differentiated adenocarcinomas and 4 as undifferentiated
adenocarcinomas or others. There were 32 cases with distant
metastasis. Sixty cases were categorized as stage I, 97 were stage
II, 86 were stage III and 33 were stage IV.
Immunohistochemistry of RASSF2 in gastric
cancer and its evaluation
According to the protocol for immunohistochemistry,
on paraffin-embedded tissue sections, slides were baked at 60°C for
2 h followed by deparaffinization with xylene and rehydration. The
sections were submerged into EDTA antigenic retrieval buffer and
microwaved for antigenic retrieval, after which they were treated
with 3% hydrogen peroxide in methanol to block endogenous
peroxidase activity, followed by incubation with 1% bovine serum
albumin to block non-specific binding. Sections were incubated with
RASSF2 goat anti-human polyclonal antibody (LifeSpan Biosciences,
USA) overnight at 4°C. Normal goat serum was used as a negative
control. After rinsing 2 x 5 min with TBST, tissue sections were
treated with a secondary antibody in TBS for 1 h at room
temperature. Development with chromogen (DAB) at room temperature
was observed under a microscope. Subsequently, all tissue sections
were counterstained with hematoxylin, dehydrated and mounted. The
nucleus with RASSF2 was stained as buffy, whereas weak expression
was associated with the cytoplasm. Evaluation of
immunohistochemistry was independently carried out by two
investigators. In scoring the expression of RASSF2 protein, both
the extent and intensity of immunopositivity were considered. The
intensity of positivity was scored as follows: 0, negative; 1,
weak; 2, moderate; 3, strong. The extent of positivity was scored
according to the percentage of cells showing positive staining: 0,
<5%; 1, >5–25%; 2, >25–50%; 3, >50–75%; 4, >75% of
the cells in the respective lesions. The final score was determined
by multiplying the intensity of positivity and the extent of
positivity scores, yielding a range from 0 to 12. The expression
for RASSF2 was considered positive when the scores were ≥5.
Statistical analysis
Statistical analyses were performed with SPSS 16.0
software. The correlations among the expression of RASSF2 and
clinicopathological characteristics were calculated by the
Student's t-test and the Chi-square correlation test. The
Kaplan-Meier method was used to estimate survival as a function of
time, and survival differences were analyzed with the log-rank
test. A multivariable test was performed to determine the factor
correlated with survival length by Cox regression analysis. The
statistical significance level was defined as P<0.05.
Results
Expression of RASSF2 in gastric cancer
and non-cancerous mucosa
RASSF2 was detected in 42 (64.6%) of 65 human
non-tumor mucosa. Positive expression of RASSF2 protein was
detected in 31.2% (86/276) of 276 human gastric cancer cases, and
negative expression was detected in 190 (68.8%). RASSF2 staining
was detected in the majority of normal cells, particularly in the
nucleus and cytoplasm. We also found RASSF2-positive expression in
intestinal metaplasia. However, RASSF2 was apparently
down-expressed in the primary cancer. The differences in RASSF2
expression between gastric cancer and non-cancerous mucosa were
also statistically significant (χ2=21.115, P<0.0001;
Fig. 1).
RASSF2 expression and clinicopathological
features
Positive expression of RASSF2 was correlated with
patient age, histological differentiation, depth of tumor invasion,
regional lymph node and distant metastasis, and TNM stage (all
P<0.05). RASSF2 expression did not correlate with patient
gender, size of tumor and histological type (P>0.05; Table I). The factors with possible
prognostic effects in gastric carcinoma were analyzed by Cox
regression analysis. The study revealed that patient gender
(P=0.003), depth of tumor invasion (P=0.040), distant metastasis
(P<0.0001), TNM stage (P<0.0001) and the expression of RASSF2
(P<0.0001) were independent prognostic factors for patients with
gastric carcinoma. However, patient age, tumor size, histological
type, histological differentiation and regional lymph node
metastasis had no prognostic value (Table II).
 | Table I.Relationship of RASSF2 expression
with pathological parameters of gastric cancer. |
Table I.
Relationship of RASSF2 expression
with pathological parameters of gastric cancer.
| Clinical
parameters | n | RASSF2
|
χ2-test | P-value |
|---|
| Positive, n
(%) | Negative, n
(%) |
|---|
| Age (years) | | | | | |
| <50 | 100 | 40 (40.0) | 60 (60.0) | | |
| ≥50 | 176 | 46 (26.1) | 130 (73.9) | 5.714 | 0.0170 |
| Gender | | | | | |
| Male | 169 | 53 (31.4) | 116 (68.6) | | |
| Female | 107 | 33 (30.8) | 74 (69.2) | 0.008 | 0.9280 |
| Size (cm) | | | | | |
| <5 | 137 | 36 (26.3) | 101 (73.7) | | |
| ≥5 | 139 | 50 (36.0) | 89 (64.0) | 3.023 | 0.0820 |
| Histology | | | | | |
| Papillary
adenocarcinoma | 8 | 4 (50.0) | 4 (50.0) | | |
| Tubular
adenocarcinoma | 187 | 63 (33.7) | 124 (66.3) | | |
| Mucinous
adenocarcinoma | 47 | 11 (23.4) | 36 (76.6) | | |
| Signet ring cell
carcinoma | 34 | 8 (23.5) | 26 (76.5) | 4.123 | 0.2490 |
| Histological
differentiation | | | | | |
| Well | 17 | 7 (41.2) | 10 (58.8) | | |
| Moderate | 90 | 38 (42.2) | 52 (57.8) | | |
| Poor | 165 | 38 (23.0) | 127 (77.0) | | |
| Other | 4 | 1 (25.0) | 3 (75.0) | 9.680 | 0.0080 |
| T stage | | | | | |
| T1 | 21 | 19 (90.5) | 2 (9.5) | | |
| T2 | 54 | 27 (50.0) | 27 (50.0) | | |
| T3 | 163 | 38 (23.3) | 125 (76.7) | | |
| T4a | 30 | 2 (6.7) | 28 (93.3) | | |
| T4b | 8 | 2 (25.0) | 6 (75.0) | 59.941 | <0.0001 |
| N stage | | | | | |
| N0 | 145 | 81 (55.9) | 64 (44.1) | | |
| N1 | 35 | 2 (5.7) | 33 (94.3) | | |
| N2 | 50 | 2 (4.0) | 48 (94.0) | | |
| N3a | 22 | 3 (13.6) | 19 (86.4) | | |
| N3b | 24 | 1 (4.2) | 23 (95.8) | 87.028 | <0.0001 |
| M stage | | | | | |
| M0 | 244 | 84 (34.4) | 160 (65.6) | | |
| M1 | 32 | 2 (6.3) | 30 (93.7) | 10.470 | 0.0010 |
| TNM stage | | | | | |
| I | 60 | 44 (73.3) | 16 (26.7) | | |
| II | 97 | 36 (37.1) | 61 (62.9) | | |
| III | 86 | 3 (3.5) | 83 (96.5) | | |
| IV | 33 | 3 (9.1) | 30 (90.9) | 93.519 | <0.0001 |
 | Table II.Multivariate analysis with Cox
proportional hazards model for prognostic factors. |
Table II.
Multivariate analysis with Cox
proportional hazards model for prognostic factors.
| Factor | P-value | Hazard ratio | 95% CI |
|---|
| Gender | 0.0030 | 1.527 | 1.157–2.014 |
| Depth of
invasion | 0.0400 | 0.755 | 0.577–0.987 |
| Distant
metastasis | <0.0001 | 6.498 | 3.288–12.844 |
| TNM stage | <0.0001 | 2.150 | 1.575–2.934 |
| RASSF2 | <0.0001 | 0.129 | 0.082–0.202 |
Correlation between RASSF2 expression and
patient prognosis
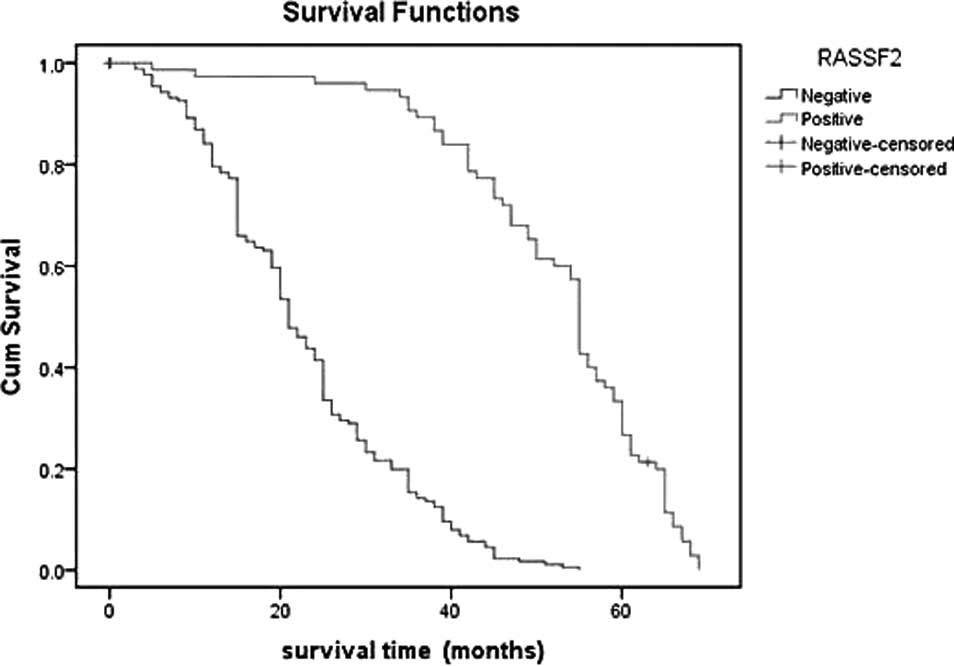
The Kaplan-Meier plot showed that the survival time
of patients with negative RASSF2 expression was significantly lower
than that in patients with positive RASSF2 expression. The survival
estimates showed a marked difference in median survival between
patients with positive and negative RASSF2 expression; the former
averaged 55 months (95% CI 52.24–55.76), whereas the latter
averaged 21 months (95% CI 19.15–22.86). For patients with negative
RASSF2 protein expression, the 1- and 3-year survival rate was 79.5
and 14.2%, respectively, significantly lower than in patients with
positive expression (97.3 and 89.3%, respectively;
χ2=156.874, P<0.0001). From this result, we concluded
that decreased expression of RASSF2 is a prognostic indicator of
poor survival for patients with gastric cancer (Table III and Fig. 2). Additionally, we further compared
the survival times between the patients who differed in terms of
RASSF2 expression, respectively, and who were in early TNM stage (I
and II) or late TNM stage (III and IV). The results showed that
RASSF2-negative expression had a much more significant effect on
the survival of those patients with early-stage tumors
(χ2=127.167, P<0.0001), highlighted by a >50.9%
reduction in 3-year survival compared to that of patients with
RASSF2-positive expression. In late stages, the difference was also
significant (χ2=6.246, P=0.019), with a 35.5% reduction
in 3-year survival. Notably, these data indicate that ectopic
expression of RASSF2 is an independent prognostic variable for
gastric cancer in early stage and late stage (Table III).
 | Table III.Survival estimates (and 95% CIs) for
disease-related deaths. |
Table III.
Survival estimates (and 95% CIs) for
disease-related deaths.
| Group | Median survival
(months) | 1-year survival
rate | 3-year survival
rate |
χ2-test | P-value |
|---|
| Positive | 55.0
(52.24–55.76) | 73 (97.3%) | 67 (89.3%) | | |
| Negative | 21.0
(19.15–22.86) | 140 (79.5%) | 25 (14.2%) | 156.874 | <0.0001 |
| Stage I–II | | | | | |
| Positive | 52.5
(25.00–59.00) | 85 (86.5%) | 81 (82.6%) | | |
| Negative | 29.6
(24.00–36.00) | 79 (95.1%) | 27 (31.7%) | 127.167 | <0.0001 |
| Stage III–IV | | | | | |
| Positive | 31.0
(2.50–39.50) | 7 (77.9%) | 5 (45.6%) | | |
| Negative | 16.0
(10.00–21.00) | 79 (67.8%) | 10 (10.1%) | 6.246 | 0.0190 |
Discussion
Epigenetic inactivation of tumor-suppressor genes is
a fundamental event in the development of many types of cancers
(21,22). Here, we demonstrated that reduced
expression of RASSF2 frequently occured in gastric cancer lesions.
Several previous studies have shown that down-regulation of RASSF2
by promoter hypermethylation occurs in different tumor cell lines
and primary tumors, including lung, breast, colorectal,
nasopharyngeal and thyroid cancer. RASSF2 inhibits the growth of
tumor cells, and its growth-inhibitory properties are enhanced by
activated K-Ras (23,24). RASSF2 promotes both cell cycle
arrest and apoptosis.
Akino et al (12) used RT-PCR and bisulfite PCR to
analyze the expression and methylation status of six RASSF family
genes in colorectal cancer cell lines and in primary colorectal
cancers and colorectal adenomas. They found that aberrant
methylation and histone deacetylation of RASSF2 were associated
with the gene silencing in colorectal cancer. Primary colorectal
cancers that showed K-ras/BRAF mutations also frequently showed
RASSF2 methylation, and inactivation of RASSF2 enhanced
K-ras-induced oncogenic transformation. Moreover, RASSF2
methylation was also frequently observed in colorectal adenomas.
Finally, they concluded that RASSF2 is a novel tumor-suppressor
gene that regulates Ras signaling and plays a critical role in the
early stages of colorectal tumorigenesis.
On the other hand, Endoh et al (25) carried out the first detailed
investigation and determined that frequent silencing of RASSF2 was
associated with promoter hypermethylation in gastric cancer. Their
study revealed that methylation frequencies of RASSF2 varied in the
regions upstream and downstream of the transcription start site.
They also observed that gastric cancers with methylation at U1 and
D1 exhibited significantly less frequent lymphatic permeation than
unmethylated gastric cancers. Afterwards, epigenetic inactivation
of RASSF2 was found in oral squamous cell carcinoma (16), head and neck squamous cell
carcinoma (26), hepatocellular
(27) and thyroid cancers
(28). Although a previous study
demonstrated that expression of the RASSF2 gene is silenced by
methylation in human gastric cancer (29), the authors did not clarify the
clinical impact of RASSF2 expression or the prognostic value for
patients with gastric cancer since the number of cases was too
small. Thus, our study is the first to determine the correlation
between RASSF2 expression and clinical and prognostic factors in
gastric cancer.
The present study demonstrated that RASSF2
expression was markedly down-regulated in gastric cancer tissues in
comparison to that in normal gastric tissues. RASSF2 was detected
in 42 (64.6%) of 65 human non-tumor mucosa. However, positive
expression of RASSF2 protein was detected in 31.2% (86/276) of 276
human gastric cancer cases, and negative expression was detected in
190 (68.8%). Moreover, our data revealed that positive expression
of RASSF2 was correlated with patient age, histological
differentiation, depth of tumor invasion, regional lymph node and
distant metastasis, and TNM stage. RASSF2 expression was not
correlated with patient gender, size of the tumor and histological
type. Further multivariate analysis revealed that patient gender,
depth of invasion, distant metastasis, TNM stage and the expression
of RASSF2 were independent prognostic factors for the disease.
Additionally, the Kaplan-Meier plot showed that survival time of
patients with negative RASSF2 expression was significantly lower
compared to patients with positive RASSF2 expression. For patients
with negative RASSF2 protein expression, the 1- and 3-year survival
rate was 79.5 and 14.2%, respectively, which was significantly
lower compared to patients with positive expression (97.3 and
89.3%, respectively).
In addition, the survival time between the patients
who differed in terms of RASSF2 expression and who were in early
TNM stage (I and II) or late TNM stage (III and IV) was compared.
The results showed that RASSF2-negative expression had a much more
significant effect on the survival of those patients with
early-stage tumors, highlighted by a >50.9% reduction in 3-year
survival compared to that of patients with RASSF2-positive
expression. In late stages, the difference was also significant,
with a 35.5% reduction in 3-year survival. Therefore, all evidence
suggests that RASSF2 expression is an independent prognostic
variable for gastric cancer in early and late stage.
To sum up, the potentially important consequence of
our study is that RASSF2 may be an attractive therapeutic candidate
for gastric cancer, as negative RASSF2 expression is predictive of
outcome in early-stage disease, and may also be a feasible target
for early intervention and treatment. In view of this, routine
detection of methylation of this gene in blood may have utility in
monitoring and detecting tumor recurrence in early-stage gastric
cancer after curative surgical resection. Thus, the studied protein
has provided a basis for the development of a potential biomarker
for diagnosis and a candidate for molecular-targeted therapeutics
of gastric cancer.
Acknowledgements
This study was supported by research
grants from the Health Technology Fund of Yunnan Province, China
(no. 2010NS066). Thanks to ProteinTech Group for the antibody, and
to Jiang Chang for the statistical work.
References
|
1.
|
A JemalR SiegelE WardCancer statistics,
2009CA Cancer J Clin59225249200910.3322/caac.20006
|
|
2.
|
YW YeRZ DongY ZhouPrognostic analysis of
familial gastric cancer in Chinese populationJ Surg
Oncol1047682201110.1002/jso.2189621400534
|
|
3.
|
M ThunA JemalC DesantisAn overview of the
cancer burden for primary care physiciansPrim
Care36439454200910.1016/j.pop.2009.04.00119616149
|
|
4.
|
JG ChenJ ZhuYH ZhangJH LuCancer survival
in Qidong, China, 1992–2000IARC Sci Publ43532011
|
|
5.
|
SC LawOW MangCancer survival in Hong Kong
SAR, China, 1996–2001IARC Sci Publ3341201121675404
|
|
6.
|
YB XiangF JinYT GaoCancer survival in
Shanghai, China, 1992–1995IARC Sci Publ55682011
|
|
7.
|
H XishanK ChenH MinCancer survival in
Tianjin, China, 1991–1999IARC Sci Publ69842011
|
|
8.
|
M Rodriguez-ParedesM EstellerCancer
epigenetics reaches mainstream oncologyNat
Med17330339201110.1038/nm.230521386836
|
|
9.
|
Y WatanabeM MaekawaMethylation of DNA in
cancerAdv Clin Chem52145167201010.1016/S0065-2423(10)52006-7
|
|
10.
|
M HatziapostolouD IliopoulosEpigenetic
aberrations during oncogenesisCell Mol Life
Sci6816811702201110.1007/s00018-010-0624-z21249513
|
|
11.
|
C LiuY PanX WangActivation of RASSF2A by
p300 induces late apoptosis through histone hyperacetylationCell
Biol Int3411331139201010.1042/CBI2009038820716062
|
|
12.
|
K AkinoM ToyotaH SuzukiThe Ras effector
RASSF2 is a novel tumor-suppressor gene in human colorectal
cancerGastroenterology129156169200510.1053/j.gastro.2005.03.05116012945
|
|
13.
|
K KairaN SunagaY TomizawaEpigenetic
inactivation of the RAS-effector gene RASSF2 in lung cancersInt J
Oncol31169173200717549418
|
|
14.
|
Z ZhangD SunN Van doInactivation of
RASSF2A by promoter methylation correlates with lymph node
metastasis in nasopharyngeal carcinomaInt J
Cancer1203238200710.1002/ijc.2218517013896
|
|
15.
|
WN CooperRE DickinsonA DallolEpigenetic
regulation of the ras effector/tumour suppressor RASSF2 in breast
and lung
cancerOncogene2718051811200810.1038/sj.onc.121080517891178
|
|
16.
|
T ImaiM ToyotaH SuzukiEpigenetic
inactivation of RASSF2 in oral squamous cell carcinomaCancer
Sci99958966200810.1111/j.1349-7006.2008.00769.x18294275
|
|
17.
|
G KumariPK SinghalMR RaoS
MahalingamNuclear transport of Ras-associated tumor suppressor
proteins: different transport receptor binding specificities for
arginine-rich nuclear targeting signalsJ Mol
Biol36712941311200710.1016/j.jmb.2007.01.026
|
|
18.
|
H SongS OhHJ OhDS LimRole of the tumor
suppressor RASSF2 in regulation of MST1 kinase activityBiochem
Biophys Res
Commun391969973201010.1016/j.bbrc.2009.11.17519962960
|
|
19.
|
K Washington7th edition of the AJCC cancer
staging manual: stomachAnn Surg
Oncol1730773079201010.1245/s10434-010-1362-z20882416
|
|
20.
|
JM SantiagoM SasakoJ OsorioTNM-7th edition
2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric
Cancer. Towards simplicity and standardisation in the management of
gastric cancerCir Esp892752812011(In Spanish).
|
|
21.
|
C ResendeA RistimakiJC MachadoGenetic and
epigenetic alteration in gastric carcinogenesisHelicobacter15Suppl
13439201010.1111/j.1523-5378.2010.00782.x
|
|
22.
|
S NobiliL BrunoI LandiniGenomic and
genetic alterations influence the progression of gastric
cancerWorld J
Gastroenterol17290299201110.3748/wjg.v17.i3.29021253387
|
|
23.
|
H DonningerL HessonM VosThe Ras effector
RASSF2 controls the PAR-4 tumor suppressorMol Cell
Biol3026082620201010.1128/MCB.00208-0920368356
|
|
24.
|
LB HessonR WilsonD MortonCpG island
promoter hypermethylation of a novel Ras-effector gene RASSF2A is
an early event in colon carcinogenesis and correlates inversely
with K-ras
mutationsOncogene2439873994200510.1038/sj.onc.120856615806169
|
|
25.
|
M EndohG TamuraT HondaRASSF2, a potential
tumour suppressor, is silenced by CpG island hypermethylation in
gastric cancerBr J
Cancer9313951399200510.1038/sj.bjc.660285416265349
|
|
26.
|
K SteinmannA SandnerU SchagdarsurenginRH
DammannFrequent promoter hypermethylation of tumor-related genes in
head and neck squamous cell carcinomaOncol
Rep2215191526200919885608
|
|
27.
|
J RenW HeR ZhangRASSF2A promoter
methylation in hepatitis B virus-related hepatocellular
carcinogenesis and its correlation with elevated serum
alpha-fetoprotein levelJ Huazhong Univ Sci Technolog Med
Sci29309312200910.1007/s11596-009-0309-8
|
|
28.
|
U SchagdarsurenginAM RichterJ
HornungFrequent epigenetic inactivation of RASSF2 in thyroid cancer
and functional consequencesMol
Cancer9264201010.1186/1476-4598-9-26420920251
|
|
29.
|
R MaruyamaK AkinoM ToyotaCytoplasmic
RASSF2A is a proapoptotic mediator whose expression is
epigenetically silenced in gastric
cancerCarcinogenesis2913121318200810.1093/carcin/bgn06018310659
|