Introduction
In Mexico, acute leukemia is considered a public
health issue; it represents the fourth leading cause of mortality
of all neoplastic malignancies in children under 15 years of age
(1). The mortality rate from 1996
to 2000 was 63.7 per 1 million children, one of the highest rates
reported in the world (2). In
2005, leukemia was the second cause of mortality in the State of
Guerrero in children less than 15 years of age, according to the
National Institute of Statistics, Geography and Computing (INEGI)
(3).
Methotrexate (MTX) is an antineoplastic agent used
in the treatment of patients with acute lymphoblastic leukemia
(ALL) and was introduced five decades ago to clinical oncology. It
is presently used in the treatment of other neoplastic diseases,
including osteosarcoma, breast cancer, head and neck cancer, and
non-Hodgkin's lymphoma (4,5). MTX is a folic acid antagonist, and
its efficacy as an antineoplastic treatment is largely attributed
to its high affinity for dihydrofolate reductase (DHFR) (EC
1.5.1.3), the enzyme which is responsible to catalyze the reduction
of dihydrofolate (DHF) to tetrahydrofolate (THF) (6). The major mechanism of MTX action
involves competitive inhibition of DHFR, leading to the impaired
regeneration of THF from DHF; essential for the biosynthesis of
purines and thymidylate, thus it also blocks the novo synthesis of
DNA (7,8). A subset of patients develop adverse
events of resistance to MTX; however, approximately 80% of ALL
children experience good clinical response (5,9,10).
The mechanisms that lead to clinical failure to MTX
are DHFR overexpression, impaired intracellular transport and
decreased levels of reduced folate carrier at the cell membrane
(11,12). Changes in the levels of DHFR
expression and consequently in the sensitivity to MTX can also be
due to single nucleotide polymorphisms (SNPs), particularly those
located in the regulatory elements. The C829T SNP is located at the
223 nucleotide downstream from the stop codon between the first and
second polyadenylation sites in the 3′UTR of the DHFR gene, which
leads to the stability of mRNA (13). A previous study reported that the
-A317G SNP in the DHFR promoter region results in higher
transcriptional activity (14).
The -A317G and C829T SNPs in the DHFR gene have not been studied as
a factor for the risk of relapse to ALL in Mexico. In the present
study, our objective was to evaluate the effect of the -A317G and
C829T polymorphisms in the DHFR gene on survival and risk of
relapse of ALL.
Materials and methods
Study population
A case control study was carried out in the
Pediatric Oncology Service of the State Cancer Institute (SCI) from
the South of Mexico (Acapulco, Guerrero), between September 1996
and May 2009. The cases consisted of 70 patients diagnosed with ALL
through bone marrow aspirate based on French-American-British
morphological criteria, cytochemical staining properties and
immunophenotyping of blast cells.
The diagnosis of ALL was further subclassified as
T-lineage (CD3+, CD7+ plus CD2+ or
CD5+, or both) or B-lineage (CD22+,
CD19+, HLA-DR+, CD10+). Patients
were treated with VDCPM (vincristine 1.4 mg/m2 at days
1, 8, 15 and 22, daunorubicin 45 mg/m2 at days 1–3,
cyclophosphamide 0.75 mg/m2 at days 1 and 15, prednisone
40 mg/m2 at days 1–28 and intrathecal methotrexate 8
mg/m2 for 1–2 years, 10 mg/m2 for 2–3 years,
12 mg/m2 for 3–8 years and 15 mg/m2 for >8
years) or VDLPM (vincristine 1.4 mg/m2 at days 1, 8, 15
and 22, daunorubicin 45 mg/m2 at days 1–3, asparaginase
6,000 U/m2 at days 19–28, prednisone 40 mg/m2
at days 1–28 and intrathecal methotrexate 8 mg/m2 for
1–2 years, 10 mg/m2 for 2–3 years, 12 mg/m2
for 3–8 years and 15 mg/m2 for >8 years) regimens
(15).
Complete remission was defined by <5% blast cells
in the bone marrow and normalization of peripheral blood counts at
4 weeks after starting induction therapy (16). Relapse was defined as the
reappearance of >20% blast cells in the marrow, or the presence
of localized leukemic infiltrates at any site after completion of
induction chemotherapy (16).
Worse outcome was defined as a lack of response to induction
therapy, a relapse after achieving complete remission or death due
to any cause (16). Risk
classification: standard risk: 1–9 years of age and presenting
white blood cell (WBC) count of <50,000/mm3; high
risk: <1 and >9 years of age and WBC count
>50,000/mm3 (17).
The controls were 100 healthy individuals (4–10×103
leukocytes/mm3) without a family history of leukemia.
Subjects in both groups in the study were 1–18 years of age,
included both genders and were residence in the State of Guerrero,
Mexico.
Specimen collection
A bone marrow and/or blood sample was taken from the
170 participants and placed in tubes with anticoagulant. Leukocytes
were purified from the whole blood sample by a selective osmotic
lysis of erythrocytes; the leukocyte genomic DNA and RNA total was
extracted by the phenol-chloroform technique (18).
Genotyping of the -A317G and C829T
polymorphisms in the DHFR
The -A317G polymorphism (dbSNP; rs408626) was
detected using previously reported PCR primers (14): forward primer
5′-GTAGGTTCTGTCTGGGACTGG-3′ and reverse primer
5′-GCAGCTTTCTTCTAGTCACCC-3′; and using previously established
protocols (19). The PCR products
(400 bp) were digested with 4 units of the HinfI enzyme
(Invitrogen Life Technologies, USA). Individuals with the A/A
genotype presented two fragments (266 and 134 bp), individuals with
the A/G genotype presented four fragments (266, 134, 83 and 51 bp)
and those with the G/G genotype presented three fragments (266, 83
and 51 bp).
The C829T polymorphism (ddsSNP; rs34764978) was
detected using previously reported PCR primers (20): forward primer
5′-CTTCTCCAAGACCCCAACTG-3′ and reverse primer
5′-CTTCCAGGTTGTTTTCAATTTTT-3′; and using previously established
protocols (19), The amplified
products (269 bp) were digested with 3 units of the TspRI
enzyme (New England Biolabs, Berverly, MA, USA). The C/C genotype
presented three fragments (203, 36 and 30 bp), the C/T genotype
presented four fragments (239, 203, 36 and 30 bp) and the T/T
genotype presented two fragments (239 and 30 bp).
Statistical analysis
Continuous data are presented as the means ±
standard deviation or median, 25th and 75th inter-quartiles.
Categorical data were compared by Chi-square or Fisher's exact
test. Univariate logistic regression analysis for the association
with the risk of relapse of ALL was tested first for -A317G and
C829T genetic polymorphisms, gender and other clinical
characteristics, and those factors were included into a second
multivariate logistic analysis. The log-rank test and Kaplan-Meier
curves were used to analyze the effect of the -A317G and C829T
genetic polymorphisms, gender and relapse of ALL on overall
survival (OS). OS was defined as the time between surgery and
either death or the time of the last follow-up. The Hardy-Weinberg
equilibrium (HWE) was used to determine the genetic equilibrium in
the healthy group. p<0.05 was considered statistically
significant. All statistical analyses were performed using SPSS
software, version 15.0 (SPSS Inc., Chicago, IL, USA) and STATA
software, version 9.2 (StataCorp, College Station, TX, USA).
Ethics statement
The bone marrow samples of the patients and blood
samples of healthy individuals used in this study were part of the
samples taken for clinical diagnostic tests in the hospital. Since
no extra amount of samples was collected from the study subjects,
informed consent was obtained from all the individuals or their
guardians, after a detailed briefing of the study purposes. The
study and the informed consent procedure were approved by the
Institutional Review Board of the Cancer Institute from Guerrero
State, Mexico.
Results
Characteristics of the study
population
The 70 ALL patients had ages ranging from 1.0 to 18
years (mean ± SD, 7.65±4.67 years). There were 45 (64.29%) males
and 25 (35.71%) females. Eighteen patients (25.71%) were in the age
group of 1–9 years (standard risk). Fifty-two patients (72.29%)
were <1 year and >9 years of age (high risk) at the time of
initial diagnosis. Relapse of ALL occurred in 68.57% of the
patients. WBC and characteristics of immunophenotype are depicted
in Table I.
 | Table I.General characteristics of the
population and clinical data of patients with childhood acute
lymphoblastic leukemia (ALL) and healthy individuals. |
Table I.
General characteristics of the
population and clinical data of patients with childhood acute
lymphoblastic leukemia (ALL) and healthy individuals.
| Variable | Patients with ALL
(n=70) | Healthy individuals
(n=100) |
|---|
| Age (years; mean ±
SD) | 7.65±4.67 | 9.99±5.49 |
| No. of
leukocytes/mm3 | 13,000
(5,400–39,000)a | 8,000
(7,000–9,000)a |
| Gender | | |
| Male | 45 (64.29) | 53 (53.00) |
| Female | 25 (35.71) | 47 (47.00) |
| Status of
participants | | |
| Alive | 30 (42.86) | 100 (100) |
| Deceased | 40 (57.14) | 0 |
|
Immunophenotype | | |
| B-lineage | 66 (94.28) | 0 |
| T-lineage | 4 (5.72) | 0 |
| Risk by age | | |
| Standard (1–9
years) | 18 (25.71) | 0 |
| High (<1 and
>9 years) | 52 (74.29) | 0 |
| Relapse during
treatment | | |
| No | 22 (31.43) | 0 |
| Yes | 48 (68.57) | 0 |
We also included 100 control subjets (controls). In
this group, the age range was 1–18 years (mean ± SD, 9.99±5.49
years), and the leukocyte count was normal (4–10×103
leucocytes/mm3; median 8,000). In this group, 53 healthy
individuals (53%) were male and 47 (47%) were female (Table I).
Associations of -A317G and C829T SNP of
DHFR with risk of ALL
The allele and genotype distributions for two SNPs
of DHFR in the cases and controls are summarized in Table II. The genotype frequencies of
these polymorphisms were in HWE (p>0.05) in the controls. When
the genotype frequencies were compared between cases and controls,
they showed a statistically significant association with the
disease. The distribution of -A317G and C829T SNP genotypes was
significant between the cases and controls (p=0.037 and p=0.016,
respectively), and the A/G and C/T genotypes were more prevalent
among the patients (47.14 and 75.71%, respectively). The homozygous
variant G/G [odds ratio (OR)=2.89, 95% CI 1.22–6.86] and
heterozygote variant C/T (OR=2.83, 95 %CI 1.03–4.88) were risk
factors for ALL (Table II).
 | Table II.Genotype and allele frequencies of
DHFR SNPs in the childhood acute lymphoblastic leukemia (ALL) cases
and controls, and association with risk of ALL. |
Table II.
Genotype and allele frequencies of
DHFR SNPs in the childhood acute lymphoblastic leukemia (ALL) cases
and controls, and association with risk of ALL.
| Modela | Genotype | ALL cases
(n=70) | Controls
(n=100) | p-valueb | OR | 95% CI | p-valuec | p-value HWE |
|---|
| -A317G
(rs408626) | | | | | | | | |
| Co | A/A | 14 (20.00) | 36 (35.00) | 0.037e | 1.00 | | | 0.152d |
| A/G | 33 (47.14) | 42 (43.57) | | 2.24 | 1.03–4.88 | 0.042e | |
| G/G | 23 (32.86) | 22 (21.43) | | 2.89 | 1.22–6.86 | 0.016e | |
| Do | A/A | 14 (20.00) | 36 (35.00) | | 1.00 | | | |
| A/G + G/G | 56 (80.00) | 64 (65.00) | | 2.47 | 1.19–5.11 | 0.015e | |
| Re | A/A + AG | 47 (67.14) | 78 (78.57) | | 1.00 | | | |
| G/G | 23 (32.86) | 22 (21.43) | | 1.74 | 0.87–3.45 | 0.116 | |
| Allele | | | | | | | |
| A | 61 (43.57) | 114 (57.00) | 0.011e | 1.00 | | | |
| G | 79 (56.43) | 86 (43.00) | | 1.73 | 1.12–2.68 | 0.014e | |
| C829T
(rs34764978) | | | | | | | | |
| Co | C/C | 10 (14.29) | 33 (33.00) | 0.016e | 1.00 | | | 0.077d |
| C/T | 53 (75.71) | 56 (56.00) | | 2.83 | 1.27–6.33 | 0.011e | |
| T/T | 7 (10.00) | 11 (11.00) | | 1.97 | 0.60–6.46 | 0.261 | |
| Do | C/C | 10 (14.29) | 33 (33.00) | | 1.00 | | | |
| C/T + T/T | 60 (85.71) | 67 (67.00) | | 2.69 | 1.22–5.95 | 0.014e | |
| Re | C/C + C/T | 63 (90.00) | 89 (89.00) | | 1.00 | | | |
| T/T | 7 (10.00) | 11 (11.00) | | 0.89 | 0.33–2.44 | 0.835 | |
| Allele | | | | | | | |
| C | 73 (52.14) | 122 (61.00) | 0.119 | 1.00 | | | |
| T | 67 (47.86) | 78 (39.00) | | 1.38 | 0.89–2.12 | 0.151 | |
Genotype distribution and allele
frequency of SNPs in individuals with and without relapse of
ALL
The A/A genotype was present in 10.42% of the
patients with relapse, compared to 40.91% of those who did not
relapse, whereas 50% of the patients with relapse were carriers of
the A/G genotype compared to 40.91% of those who did not relapse,
and 39.58% of the patients with relapse were carriers of the G/G
genotype, compared to 18.18% of those who did not relapse.
Regarding the distribution of the C829T polymorphism in patients
with relapse, 81.25% were carriers of the C/T genotype, 12.50% had
the T/T genotype and only 6.25% carried the C/C genotype. In
contrast to those who did not relapse, 31.82% were carriers of the
C/C genotype, 63.64% of the C/T genotype and 4.54% carriers of the
T/T genotype. Genotypic and allelic frequencies of both
polymorphisms were statistically significant between patients with
and without relapse of ALL (p<0.05) (Table III).
 | Table III.Genotype distribution and allele
frequency of the -A317G and C829T polymorphisms of DHFR in
individuals with and without relapse of childhood acute
lymphoblastic leukemia. |
Table III.
Genotype distribution and allele
frequency of the -A317G and C829T polymorphisms of DHFR in
individuals with and without relapse of childhood acute
lymphoblastic leukemia.
| Without relapse n
(%) | With relapse n
(%) | p-valuea |
|---|
| -A317G
(rs408626) | | | |
| Genotypes | | | |
| A/A | 9 (40.91) | 4 (8.33) | |
| A/G | 9 (40.91) | 25 (52.08) | |
| G/G | 4 (18.18) | 19 (39.58) | 0.014b |
| Allele | | | |
| A | 27 (61.36) | 34 (35.42) | |
| G | 17 (38.64) | 62 (64.58) | 0.004b |
| C829T
(rs34764978) | | | |
| Genotypes | | | |
| C/C | 7 (31.82) | 3 (6.25) | |
| C/T | 14 (63.64) | 39 (81.25) | |
| T/T | 1 (4.54) | 6 (12.50) | 0.015b |
| Allele | | | |
| C | 28 (63.64) | 45 (46.88) | |
| T | 16 (36.36) | 51 (53.12) | 0.048b |
Risk of relapse based on the -A317G and
C829T SNP genotypes and other risk factors in ALL
In a logistic regression analysis, an association
was found between the -A317G, C829T polymorphisms and the risk of
relapse of disease (p<0.05). Those patients carrying the G/G
genotype of the -A317G polymorphism, showed a significant increase
in the risk of relapse of ALL (OR=8.55, 95% CI 1.84–39.70) compared
to carriers of the A/A genotype (p=0.006) (Table IV). Carriers of the T/T genotype of
the C829T polymorphism had a 14.00 greater chance of a relapse of
the disease (OR=14.00, 95% CI 1.13–172.63) compared to carriers of
the C/C genotype (p=0.039) (Table
IV). Other variables, such as age, leukocytes, but not gender,
were associated with the risk of relapse of disease (p<0.05)
(Table IV).
 | Table IV.Association of the -A317G and C829T
polymorphisms in the DHFR gene and other clinical features with the
risk of relapse of childhood acute lymphoblastic leukemia. |
Table IV.
Association of the -A317G and C829T
polymorphisms in the DHFR gene and other clinical features with the
risk of relapse of childhood acute lymphoblastic leukemia.
| Modela | Genotype | No. (%) | OR | 95% CI | p-valueb |
|---|
| -A317G
(rs408626) | | | | | |
| Co | A/A | 14 (20.00) | 1.00 | | |
| A/G | 33 (47.14) | 4.80 | 1.26–18.24 | 0.021c |
| G/G | 23 (32.86) | 8.55 | 1.84–39.70 | 0.006c |
| Do | A/A | 14 (20.00) | 1.00 | | |
| A/G + G/G | 56 (80.00) | 7.62 | 2.01–28.81 | 0.003c |
| Re | A/A + AG | 47 (67.14) | 1.00 | | |
| G/G | 23 (32.86) | 2.94 | 0.86–10.06 | 0.084 |
| C829T
(rs34764978) | | | | | |
| Co | C/C | 10 (14.29) | 1.00 | | |
| C/T | 53 (75.71) | 6.50 | 1.47–28.67 | 0.013c |
| T/T | 7 (10.00) | 14.00 | 1.13–172.63 | 0.039c |
| Do | C/C | 10 (14.29) | 1.00 | | |
| C/T + T/T | 60 (85.71) | 7.00 | 1.60–30.54 | 0.010c |
| Re | C/C + C/T | 63 (90.00) | 1.00 | | |
| T/T | 7 (10.00) | 3.00 | 0.348–26.55 | 0.323 |
| Gender | | | | | |
| Female | | 25 (35.71) | 1.00 | | |
| Male | | 45 (64.29) | 1.38 | 0.49–3.92 | 0.540 |
| Risk by age | | | | | |
| 1–9 years (low
risk) | | 18 (25.71) | 1.00 | | |
| <1 and >9
years (high risk) | | 52 (74.29) | 4.54 | 1.14–18.09 | 0.032c |
| Leukocytes at
diagnosis | | | | | |
|
<50,000/mm3 | | 18 (25.71) | 1.00 | | |
|
>50,000/mm3 | | 52 (74.29) | 7.64 | 1.90–30.73 | 0.004c |
The following variables were included in a
multivariate analysis: leukocytes count at diagnosis, age, -A317G
and C829T polymorphism genotypes, to determine whether either or
both the SNPs predict the risk of relapse independently. It was
observed that A/G or G/G genotype carriers (p=0.041 and p=0.017,
respectively), together with the C/T or T/T genotype carriers
(p=0.015 and p=0.049, respectively), were two independent
prognostic markers for the risk of relapse of ALL compared to the
other variables (Table V).
 | Table V.Factors influencing the risk of
relapse of ALL in a multivariate regression analysis. |
Table V.
Factors influencing the risk of
relapse of ALL in a multivariate regression analysis.
| OR | 95% CI | p-valuea |
|---|
| -A317G
(rs408626) | | | |
| A/G | 4.06 | 0.01–0.58 | 0.041b |
| G/G | 6.75 | 0.07–0.67 | 0.017b |
| C829T
(rs34764978) | | | |
| C/T | 7.49 | 0.08–0.71 | 0.015b |
| T/T | 12.38 | 0.002–0.93 | 0.049b |
| Risk by age | | | |
| <1 and >9
years (high risk) | 0.86 | 0.25–2.96 | 0.814 |
| Leukocytes at
diagnosis | | | |
|
>50,000/mm3 | 0.52 | 0.11–2.36 | 0.395 |
Association between DHFR -A317G and C829T
SNP and overall survival in the ALL patients
Kaplan-Meier analysis of OS curves showed
significant results between the relapse of ALL and DHFR -A317G,
C829T polymorphisms. We found no significant associations between
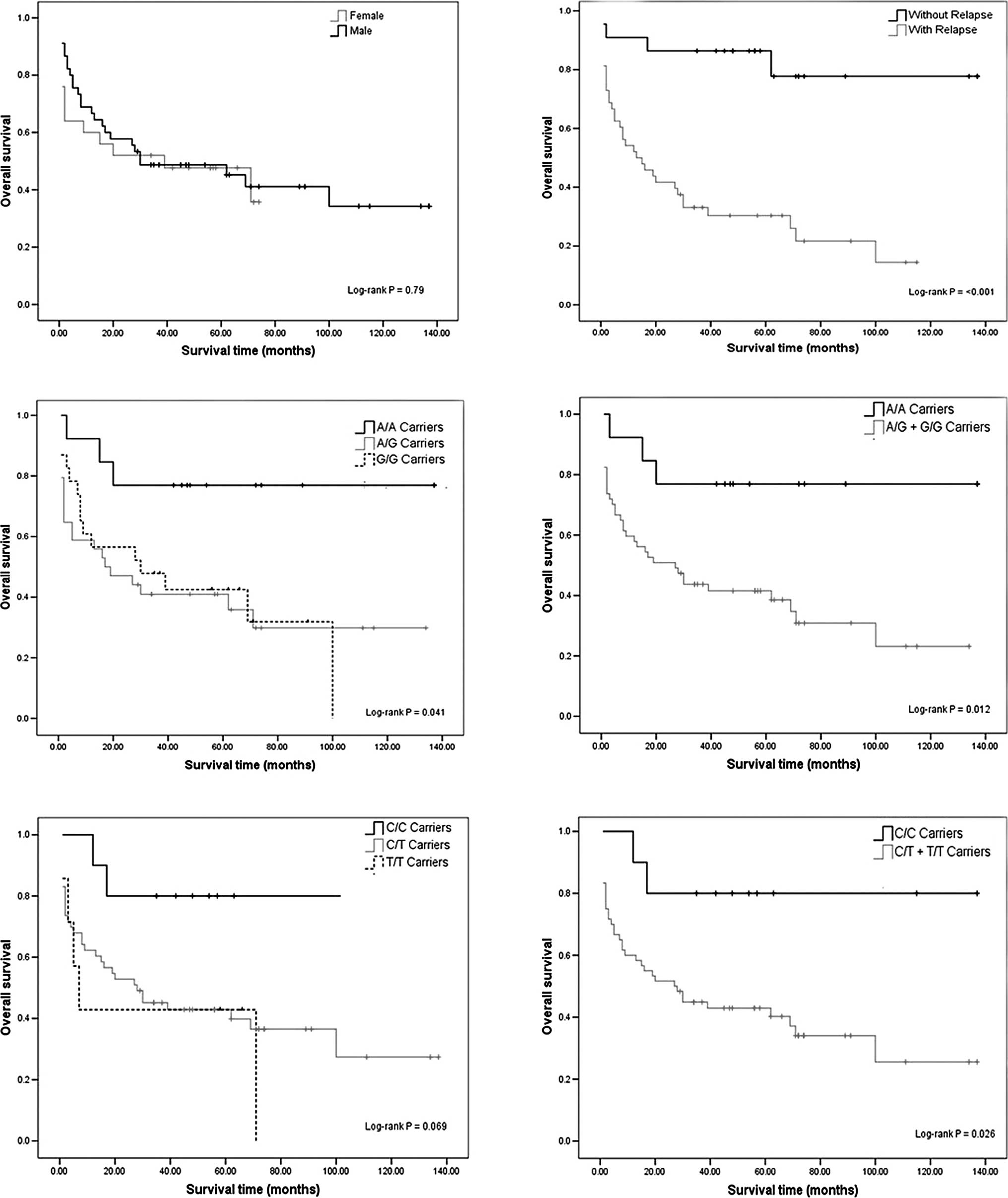
gender and OS (log-rank test; p=0.79) (Fig. 1A). A different rate of OS was
evident between the individuals with and without relapse of ALL
(log-rank test; p<0.001) (Fig.
1B). In fact, 81.82% of the patients without relapse were
alive, while only 25% of patients with relapse were alive (Table I). Those patients with relapse had
a 13.5 greater chance of death (OR=13.5, 95% CI 3.81–47.84)
compared to those without relapse (p<0.001) (data not
shown).
The relationship between OS and the polymorphisms
was calculated. Individuals with the G/G genotype had poorer OS
compared to the A/A genotype (log-rank test; p<0.05) (Fig. 1C and D). We found no significant
association for the DHFR C829T polymorphism, although we observed a
reduction in survival at 11 years of follow-up among T/T-carriers
with respect to that of patients with the wild-type genotype
(log-rank test; p=0.069) (Fig.
1E). However, we carried out log-rank test for combined
genotypes, C/T + T/T vs. C/C (Fig.
1F), showing significant genotype-dependent effects for OS
(log-rank test; p=0.026).
Discussion
There have been attempts to explain the mechanisms
by which patients show different response to the same drug used to
treat ALL. However, there are few studies addressing the
association of SNPs with response to treatment in patients with
ALL. There are no studies in Mexico regarding the -317A/G and
829C/T polymorphisms of DHFR; therefore, it is important to
determine their distribution in the Mexican population, its
association and impact on the risk of relapse and survival in
patients with ALL.
Patients with ALL predominantly showed the
heterozygous A/G genotype (47.14%) of the -A317G polymorphism
(Table II), a result similar to
that reported by Dulucq et al, in a Canadian population
where the A/G genotype was reported as the most frequent in
individuals with ALL (48.4%) (14). However, the genotypic frequencies
A/A (31.4%), A/G (48.4%) and G/G (20.2%) reported by Dulucq et
al are statistically significant to those found in this study
(p=0.040).
The genotypic frequencies of the C829T polymorphism
reported in this study differ (p<0.001) from those reported by
Goto et al, in a Japanese population with ALL, where the C/C
genotype (83.8%) was the most frequently reported, followed by the
C/T genotype (10.8%) and the T/T genotype (5.4%) (13). The C829T polymorphism has been
studied in other disorders that involve the metabolic pathway of
folate. However, it was not identified in non-Japanese American,
Caucasian or Israeli populations (21–23),
suggesting that the C829T polymorphism was found more frequently in
patients with ALL than in other disorders in which the activity
enzymatic of DHFR is involved.
In the present study, 93.75% of the patients with
relapse were carriers of the T allele (Table III); this agrees with previous
experimental studies, which identified the T allele as a risk for
relapse to ALL (28). This
suggests that the presence of the -A317G and C829T polymorphisms,
and the strong association with the risk of relapse (p<0.05)
(Table IV) may be a factor that
led to more than 50% of deaths in the patients with ALL included in
this study.
In many studies, age has been found to be a
prognostic factor in childhood ALL (24); this feature also retained its
significance in our study. Patients in the age group of 1–9 years
(low risk) had the best prognosis, whereas patients <1 and >9
years of age (high risk) showed the worst prognosis (OR=4.54, 95%
CI 1.14–18.09) (Table IV), which
agrees with other studies (25).
Regarding gender, it has been reported in various
studies that females have better survival than males of the same
age with ALL (24,26). However, we did not observe such a
gender-based outcome (p>0.05) (Table IV), similarly to Dulucq et
al, Kim et al and Dervieux et al, (14,27,28).
This could be due to the small sample size of the female group
(n=25/70) in our study. The WBC has also been reported as a
prognostic factor in many studies on pediatric ALL. We observed
that a WBC count more than 50×109
leukocytes/mm3 at diagnosis was associated with poor
outcome (OR=7.64, 95% CI 1.90–30.73) (Table IV), similar to other studies
(24,29). Immunophenotype has been considered
as the most important prognostic factor impacting the therapeutic
strategy. The pre-B ALL and the immature T-cell precursor are
generally associated with a poorer prognosis (30). Two studies have shown that the
Hispanic population has a high frequency of B-cell precursor
immunophenotype (31,32). Our findings are consistent with
these studies; we found that the 68.18% of cases with relapse of
leukemia were classified within this immunological lineage (data
not shown).
On separate analyses of the -A317G and C829T
polymorphisms in the DHFR gene, analyses of the G/G and T/T
genotypes alone appear to be worse prognostic markers than
leukocytes at diagnosis and age (OR=8.55, 95% CI 1.84–39.70;
OR=14.00, 95% CI 1.13–172.63; OR=7.64, 95% CI 1.90–30.73; OR=4.54,
95% CI 1.14–18.09, respectively). However, in a multivariate
analysis we observed that the -A317G and C829T polymorphisms were
the worst independent prognostic factors. The analysis showed that
G/G and T/T genotypes were independent prognostic markers,
excluding leukocytes at diagnosis and age (Table V). A second goal of this study was
to investigate the impact of the polymorphisms on survival. The OS
rate of G and T allele carriers of the -A317G and C829T
polymorphisms, respectively, was lower than that of patients
carrying A and C alleles. Our results are in line with those
reported by Dulucq et al (14), showing an association between the
G/G variant and reduced survival in ALL patients. We also evaluated
the relationship between OS and the C829T polymorphism. During
follow-up, we observed a reduction in OS among T-carriers and
patients with the wild-type genotype (Fig. 1).
In conclusion, the -A317G and C829T polymorphisms
are strongly associated with the risk of relapse to ALL, presenting
a higher risk of relapse in ALL carriers of G/G and T/T genotypes
than in carriers of the A/A and C/C genotypes, respectively. Our
data showed that the polymorphisms of DHFR C829T and -A317G had an
impact on survival of ALL Mexican patients. These data seem to
suggest a role for the DHFR polymorphisms in the relapse of ALL and
overall survival. This is the first study which evaluated the
effect of the C829T polymorphism on overall survival. However, this
analysis was based only on 70 patients and needs to be confirmed in
a larger population.
Acknowledgements
The authors would like to thank the
National Council of Science and Technology (CONACYT, Mexico) for a
fellowship awarded to Yazmín Gómez-Gómez (August 2007 to July
2009). They also thank the technicians of the Laboratorio de
Biomedicina Molecular for their laboratory assistance, and Dinorah
Leyva-Illades (Texas A&M Health Science Center) for revising
the English style of the manuscript.
References
|
1.
|
Coronel-Morán QR: Importancia del
laboratorio en el diagnóstico y pronóstico de leucemia aguda
linfoblástica de la infancia. Acta Pediátrica de México.
26:129–136. 2005.
|
|
2.
|
Mejia-Arangure JM, Bonilla M, Lorenzana R,
et al: Incidence of leukemias in children from El Salvador and
Mexico City between 1996 and 2000: population-based data. BMC
Cancer. 5:332005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Salud/INEGI, S.d., Fuente: base de datos
de defunciones INEGI/Secretaria de Salud, Dirección General de
Información en Salud, México. Información disponible en URL
https://www.inegi.org.mx/ (Accessed
April, 2008).
|
|
4.
|
Cheok MH and Evans WE: Acute lymphoblastic
leukaemia: a model for the pharmacogenomics of cancer therapy. Nat
Rev Cancer. 6:117–129. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hider SL, Bruce IN and Thomson W: The
pharmacogenetics of methotrexate. Rheumatology (Oxford).
46:1520–1524. 2007. View Article : Google Scholar
|
|
6.
|
Wang L, Goodey NM, Benkovic SJ and Kohen
A: Coordinated effects of distal mutations on environmentally
coupled tunneling in dihydrofolate reductase. Proc Natl Acad Sci
USA. 103:15753–15758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Volpato JP, Fossati E and Pelletier JN:
Increasing methotrexate resistance by combination of active-site
mutations in human dihydrofolate reductase. J Mol Biol.
373:599–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Allemann RK, Evans RM, Tey LH, et al:
Protein motions during catalysis by dihydrofolate reductases.
Philos Trans R Soc Lond B Biol Sci. 361:1317–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fotoohi AK: Resistance of human leukaemia
cells to the antimetabolites. Oncology-Pathology, Suecia. 1:1–84.
2007.
|
|
10.
|
Serra M, Reverter-Branchat G, Maurici D,
et al: Analysis of dihydrofolate reductase and reduced folate
carrier gene status in relation to methotrexate resistance in
osteosarcoma cells. Ann Oncol. 5:151–160. 2004. View Article : Google Scholar
|
|
11.
|
De Jonge R, Hooijberg JH, van Zelst BD, et
al: Effect of polymorphisms in folate-related genes on in vitro
methotrexate sensitivity in pediatric acute lymphoblastic leukemia.
Blood. 106:717–720. 2005.PubMed/NCBI
|
|
12.
|
Assaraf YG: Molecular basis of antifolate
resistance. Cancer Metastasis Rev. 26:153–181. 2007. View Article : Google Scholar
|
|
13.
|
Goto Y, Yue L, Yokoi A, et al: A novel
single-nucleotide polymorphism in the 3′-untranslated region of the
human dihydrofolate reductase gene with enhanced expression. Clin
Cancer Res. 7:1952–1956. 2001.
|
|
14.
|
Dulucq S, St-Onge G, Gagne V, et al: DNA
variants in the dihydrofolate reductase gene and outcome in
childhood ALL. Blood. 111:3692–3700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Seguro-popular: Secretaria de Salud/Seguro
Popular, Información disponible en URL http://www.seguro-popular.salud.gob.mx/uri
(Accessed May, 2009).
|
|
16.
|
Reiter A, Schrappe M, Ludwig WD, et al:
Chemotherapy in 998 unselected childhood acute lymphoblastic
leukemia patients. Results and conclusions of the multicenter trial
ALL-BFM 86. Blood. 84:3122–3133. 1994.PubMed/NCBI
|
|
17.
|
Smith M, Arthur D, Camitta B, et al:
Uniform approach to risk classification and treatment assignment
for children with acute lymphoblastic leukemia. J Clin Oncol.
14:18–24. 1996.PubMed/NCBI
|
|
18.
|
Merante F, Raha S, Reed JK and Proteau G:
The simultaneous isolation of RNA and DNA from tissues and cultured
cells. Methods Mol Biol. 58:3–9. 1996.PubMed/NCBI
|
|
19.
|
Organista-Nava J, Gómez-Gómez Y,
Saavedra-Herrera MV, et al: Polymorphisms of the γ-glutamyl
hydrolase gene and risk of relapse to acute lymphoblastic leukemia
in Mexico. Leukemia Res. 34:728–732. 2010.
|
|
20.
|
Kooloos WM, Wessels JAM, van der Straaten
T, Allaart CF, Huizinga TWJ and Guchelaar HJ: Functional
polymorphisms and methotrexate treatment outcome in recent-onset
rheumatoid arthritis. Pharmacogenomics. 11:163–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Gellekink H, Blom HJ, van der Linden IJ
and den Heijer M: Molecular genetic analysis of the human
dihydrofolate reductase gene: relation with plasma total
homocysteine, serum and red blood cell folate levels. Eur J Hum
Genet. 15:103–109. 2007. View Article : Google Scholar
|
|
22.
|
Mishra PJ, Longo GSA, Menon LG, Abali EE,
Humeniuk R, Cole PD, Kamen BA, Banerjee D and Bertino JR: The
829C-T single nucleotide polymorphism in the 30 UTR of the
dihydrofolate reductase gene results in methotrexate resistance and
is rare among non-Japanese American patients. Proc Amer Assoc
Cancer Res. 301:12742006.
|
|
23.
|
Parle-McDermott A, Pangilinan F, Mills JL,
et al: The 19-bp deletion polymorphism in intron-1 of dihydrofolate
reductase (DHFR) may decrease rather than increase risk for spina
bifida in the Irish population. Am J Med Genet Part A.
143:1174–1180. 2007. View Article : Google Scholar
|
|
24.
|
Ng SM, Lin HP, Ariffin WA, Zainab AK, Lam
SK and Chan LL: Age, sex, haemoglobin level, and white cell count
at diagnosis are important prognostic factors in children with
acute lymphoblastic leukemia treated with BFM-type protocol. J Trop
Pediatr. 46:338–343. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Frankel LS, Ochs J, Shuster JJ, et al:
Therapeutic trial for infant acute lymphoblastic leukemia: the
Pediatric Oncology Group experience (POG 8493). J Pediatr Hematol
Oncol. 19:351997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shuster JJ, Wacker P, Pullen J, et al:
Prognostic significance of sex in childhood B-precursor acute
lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin
Oncol. 16:2854–2863. 1998.PubMed/NCBI
|
|
27.
|
Kim K, Kang SB, Chung HH, Kim JW, Park NH
and Song YS: XRCC1 Arginine 194 Tryptophan and GGH-401
Cytosine/Thymine polymorphisms are associated with response to
platinum-based neoadjuvant chemotherapy in cervical cancer. Gynecol
Oncol. 111:509–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Dervieux T, Greenstein N and Kremer J:
Pharmacogenomic and metabolic biomarkers in the folate pathway and
their association with methotrexate effects during dosage
escalation in rheumatoid arthritis. Arthritis Rheum. 54:3095–3103.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chessells JM, Veys P, Kempski H, et al:
Long term follow up of relapsed childhood acute lymphoblastic
leukaemia. Br J Haematol. 123:396–405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chen SH, Yang CP, Hung IJ, Jaing TH, Shih
LY and Tsai MH: Clinical features, molecular diagnosis, and
treatment outcome of infants with leukemia in Taiwan. Pediatr Blood
Cancer. 55:1264–1271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Daniel-Cravioto A, Gonzalez-Bonilla CR,
Mejia-Arangure JM, et al: Genetic rearrangement MLL/AF4 is most
frequent in children with acute lymphoblastic leukemias in Mexico
City. Leuk Lymphoma. 50:1352–1360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Bernaldez-Rios R, Ortega-Alvarez MC,
Perez-Saldivar ML, Alatoma-Medina NE and del Campo-Martinez M: The
age incidence of childhood B-cell precursor acute lymphoblastic
leukemia in Mexico City. J Pediatr Hematol/Oncol. 30:199–203. 2008.
View Article : Google Scholar : PubMed/NCBI
|