Introduction
The larynx is a mucosal organ positioned at the
divergence of the respiratory and digestive tracts. Laryngeal
cancer (LC) is a very frequent malignant neoplasm of the head and
neck region. It is responsible for approximately 159,000 new cases
and 90,000 mortalities every year (1). Recurrent respiratory papillomatosis
(RRP) is the most frequent benign laryngeal neoplasm, and is mainly
caused by human papillomavirus types 6 and 11. Patients with RRP
have exophytic airway lesions that may progress to respiratory
insufficiency. It is estimated that between 1,500 and 2,500 new
cases of RRP occur in the United States each year, and 3,870 cases
are expected in the Mexican infantile population (2,3).
Smoking is considered to be the main trigger factor of LC, and in
association with alcohol consumption the risk of cancer increases
(4). LC is characterized by high
proliferative potential mediated by an increase in the expressions
of cyclin D1 and Ki67 (5).
However, the mechanisms underlying the proliferation of this form
of cancer are not yet fully understood. Alterations in the
expression of prolactin (PRL) and certain signaling intermediate
levels can contribute to the development and progression of certain
types of cancer, particularly in hormone-dependent organs (e.g.,
breast, endometrium, prostate), but most likely at other primary
sites as well (6–8). The diverse activities of PRL are
mediated by its receptor (PRLR) and it involves the activation of a
number of signaling pathways including Jak2-STAT (9), PI3K (10) and MAPK (11,12).
There are multiple isoforms of the PRLR in humans, the long form of
70–90 kDa (LF), intermediate form of 40–50 kDa (IF), and two short
forms of 42–56 kDa (SF1a) and 32–42 kDa (SF1b) which are produced
by alternative splicing (13). To
date, there have been no studies focusing on the analysis of PRL or
PRLR expression in laryngeal tumor lesions. The objective of this
study was to determine the PRLR expression and its association in
RRP and LC in order to identify the possible participation of PRLR
in laryngeal tumors.
Materials and methods
Patients and tissue samples
We evaluated 48 tissue samples from male patients
obtained from the Pathology Department at the Civil Hospital of
Guadalajara ‘Fray Antonio Alcalde’, and the Departments of Oncology
and Pathology at the Mexican Social Security Institute,
Guadalajara, Mexico. Tissues were fixed in 4% formalin and embedded
in paraffin. All samples were evaluated and characterized by an
experienced pathologist. A total of 30 LC samples from patients
aged 44–81 years (mean age, 60.1 years) and 18 samples of RRP from
patients aged 3–58 years (mean age, 8.2 years) were included for
analysis by immunohistochemistry. A total of 20 samples were
included in the PRL/PRLR Western blot analysis and quantitative
real-time PCR assays. All samples were obtained in accordance with
the Guidelines of the Mexican Official Standard (Norma Oficial
Mexicana, NOM) and the World Medical Association Declaration of
Helsinki.
Immunohistochemical staining
Serial sections from the formalin-fixed
paraffin-embedded blocks were used for the detection of PRLR by
immunohistochemistry. Sections were deparaffinized by successive
immersions in 100% xylene, 100% ethanol, 96% ethanol and 70%
ethanol for 10, 10, 5 and 5 min, respectively. Endogenous
peroxidase activity was inactivated with peroxidase blocking
reagent (S2001; Dako, Glostrup, Denmark) for 10 min. Antigen
retrieval was achieved by exposure to 10 mM citrate buffer (pH 6.0)
and autoclaving at 121°C for 15 min. Following blockade with 50 μl
of 1% BSA (Sigma, USA) in TBST buffer (50 mM Tris-HCl, 300 mM NaCl,
0.1% Tween-20) for 5 min at room temperature, the sections were
incubated overnight with 40 μl of anti-PRLR primary antibody (clone
H-300; Santa Cruz Biotechnology, Santa Cruz, CA, USA) prediluted
1:100 in TBST at 4°C in a humidified chamber. The sections were
then washed with TBST and incubated with one drop of secondary
antibody conjugated with HRP (K4061; Dako) for 60 min at room
temperature. Following washing, the sections were incubated with
one drop of chromogenic 3,3′-diaminobenzidine (DAB) substrate
(K3468; Dako) for 15 min at room temperature. Sections were
counterstained with Mayer’s hematoxylin and mounted on a
hydrosoluble medium (VectaMount AQ). Additionally, all sections
were developed in parallel with a negative control reaction
omitting the primary antibody. No signal was observed.
Western blot analysis
Proteins were extracted from tissue samples with 300
μl of RIPA buffer [50 mM Tris, 150 mM NaCl, 1% NP40, 0.5% sodium
deoxycholate and 0.1% sodium dodecyl sulfate (SDS)], protease
inhibitors (pestatin, leupeptin, aprotinin, quimostatin, antipain
and PMSF) and phosphatase inhibitors (Na3VO4,
and NAF) were added, and were clarified by centrifugation at 4°C
for 20 min. Protein concentration was determined by the
bicinchoninic acid method (BCA Protein Assay Reagent; Pierce,
Rockford, IL, USA). Total protein (40 μg) was mixed with loading
buffer, electrophoresed on 7.5–10% SDS-polyacylamide gels and
transferred to a polyvinylidene difluoride membrane (Bio-Rad, CA,
USA). Non-specific binding was blocked with 5% milk and 1% bovine
serum albumin solution. Subsequently, the membranes were incubated
with 2 μg/ml polyclonal PRL or PRLR antibody (clone H-300; Santa
Cruz Biotechnology) at 4°C overnight. HRP-conjugated anti-rabbit
secondary antibody was used to reveal the immune detection and
blots were developed with a chemiluminescence system (Millipore,
Billerica, MA. USA). As an internal control to confirm that similar
amounts of protein were loaded for each lane, actin levels were
determined using a monoclonal anti-actin IgG (Chemicon
International, Temecula, CA, USA) at 1:10,000 and revealed with
anti-mouse IgG peroxidase (Santa Cruz Biotechnology).
Microscopic analysis
The immunostained slides were evaluated
independently by two of the authors. When disagreements occurred
between the two observers they were resolved using a double-headed
microscope. The staining intensity was evaluated semiquantitatively
as follows: negative (no immunolabeling), low, moderate and
intense. Negative controls included omission of the primary
antibody.
Quantitative real-time PCR
RNA was isolated from laryngeal tissues from various
groups of patients with TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). Retrotranscription using 2 μg of total RNA was achieved using
M-MLV reverse trans criptase (Invitrogen). Then, 2 μl of cDNAs were
subjected to real-time PCR using a Rotor-Gene Thermocycler under
the following conditions: 2 min at 50°C, 10 min at 95°C, and 45
cycles of 15 sec at 95°C and 1 min at 60°C. The reaction mixture
included a 200 nM final concentration of both forward
(5′agaccatggatactggagta-3′) and reverse (5′ggaaagatgcaggtca
ccat-3′) PRLR-specific primers, and a 100 nM final concen tration
of the PRLR-specific probe (5′-tctgctgtcatctgtttgatta-3′) labeled
with FAM reporter fluorescent dye designed for amplification of all
three isoforms of PRLR (14). Gene
amplification was normalized against 18S expression with the
specific probe, human Hs03928985_g1 (Applied Biosystems Hammonton,
NJ, USA), labeled with FAM. Relative quantification using the
2−ΔΔCT method was then carried out using the comparison
to the control groups as an internal calibrator (15,16).
Statistical analysis
Data were either analyzed by the Student’s t-test or
one-way ANOVA to determine statistical differences between the
groups using Microsoft Excel and SPSS version 18.0 software (SPSS
Inc., Chicago, IL, USA). Results were considered to be
statistically significant at P<0.05.
Results
Detection and localization of PRLR in the
laryngeal tumors by immunohistochemistry and Western blot
analysis
PRLR expression was analyzed in 48 laryngeal tissues
from males by the immunoperoxidase method. Immunolabeling of PRLR
was observed in all malignant tumors; in 23 (76.7%) cases the
immunoreactivity was moderate to intense and low in 7 (23.3%)
cases. The staining pattern was confined to the tumor cells, and
was mostly cytoplasmic. Cells adjacent to the tumor showed very low
PRLR expression. The PRLR staining level was significantly higher
in the LC than RRP tissues (P<0.005); in 7 (38.9%) RRP cases the
staining was moderate and low in 11 (61.1%) cases. We did not
observe intense expression of PRLR in any RRP samples. A
representative example of each group is shown in Fig. 1. Table
I presents a semiquantitative estimation of the immunolabeling
studies of laryngeal tissues with anti-PRLR antibodies.
 | Table I.Prolactin receptor expression in
laryngeal cancer and recurrent respiratory papillomatosis by
immunohistochemistry. |
Table I.
Prolactin receptor expression in
laryngeal cancer and recurrent respiratory papillomatosis by
immunohistochemistry.
| PRLR
immunohistochemistry | Laryngeal
cancera n (%) | RRPa n (%) |
|---|
| Low | 7 (23.3) | 11 (61.1) |
| Moderate | 17 (56.7) | 7 (38.9) |
| Intense | 6 (20) | 0 (0) |
| Total | 30 (100) | 18 (100) |
To evaluate whether there are different PRLR forms
in RRP and LC samples, we determined the PRLR expression by Western
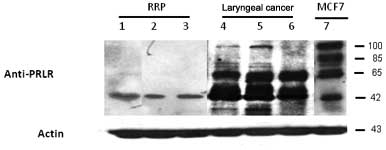
blot analysis in ten samples from each group.
In order to demonstrate the reactivity of the
anti-PRLR antibody by Western blot analysis, we analyzed total
proteins extracted from the MFC7 breast cancer cell line, which is
known to express a high amount of PRLR. The presence of four
different PRLR isoforms in the MCF7 breast cancer cell-line of
approximately 90–110, 65 and 42–45 kDa associated with long,
intermediate, and short isoforms were observed. The expression
levels of the PRLR isoforms were different for each sample group
(LC and RRP) analyzed. In RRP, one PRLR isoform of 42 kDa was
moderately expressed. However, the LC samples showed more prominent
bands of 42–45 kDa than the RRP samples. Also, strong bands of 65
kDa, as well as weak bands of approximately 100 kDa, were detected
in all cancer samples (Fig.
2).
PRLR mRNA expression by quantitative
real-time reverse transcriptase PCR (qRT-PCR) in laryngeal
tissues
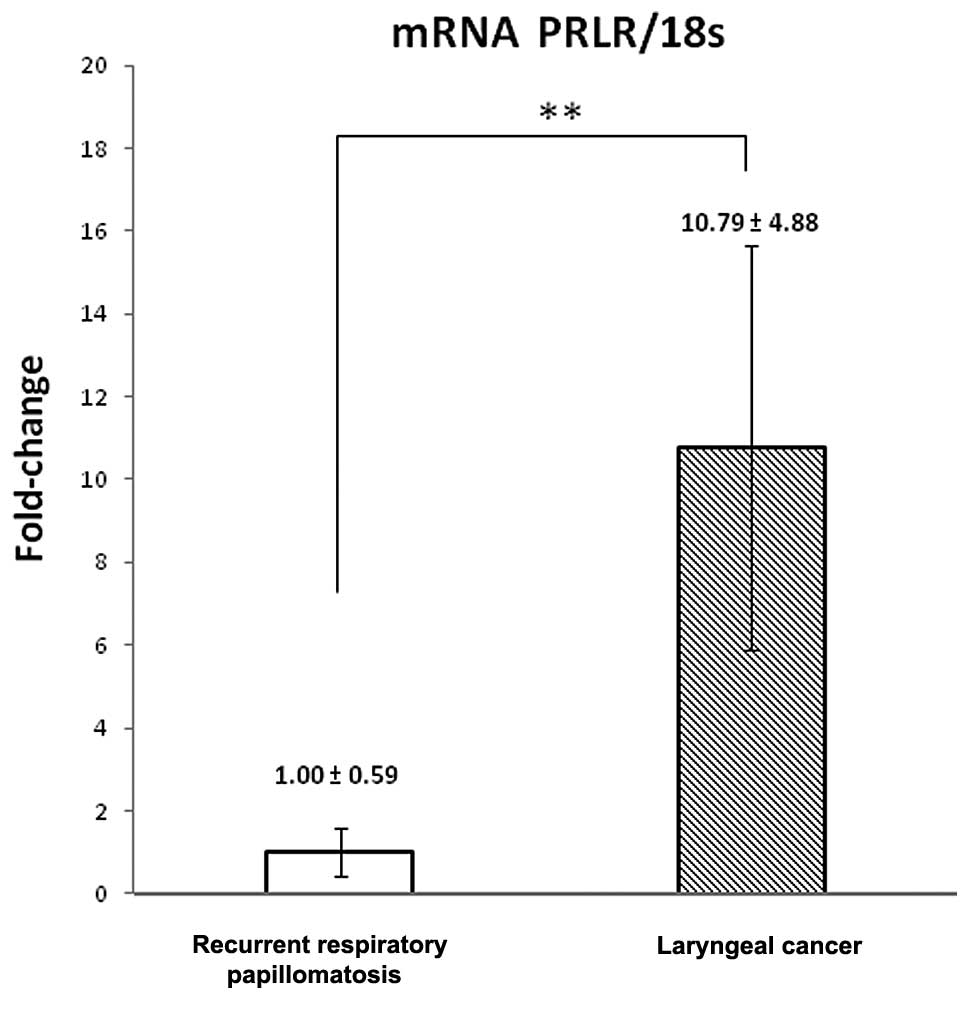
qRT-PCR was performed in ten laryngeal tissue
samples (five LC and five RRP samples). Gene amplification was
normalized against 18S expression with the specific probe. We
labeled the expression of PRLR mRNA in all these samples. The
levels of PRLR mRNA in the LC samples were higher than those in the
RRP samples (Fig. 3). LC results
showed a ten-fold (10.79±1.27) increase with respect to RRP with a
significance value of P<0.005.
Discussion
To date it is known that the amount of PRLR on the
cell surface directs both the intensity and length of PRL signals
in cells and therefore cellular response to PRL. Thus, alterations
in PRLR levels may lead to aberrant downstream signaling resulting
in the disruption of cellular homeostasis.
The tumorigenic potency of PRL based on circulating
PRL levels in various types of human tumors has been controversial.
For example, epidemiological studies performed during the 80’s and
90’s were unable to reach unified conclusions regarding any
correlations between circulating PRL levels and breast cancer
(17). With regard to other types
of cancer, the data are much sparser than for breast cancer. For
example, studies on prostate cancer and head and neck cancer
concluded that there was no correlation between PRL levels and
cancer risk (18,19). However, Yurkovetsky et al
showed that PRL was the strongest discriminative biomarker for
endometrial cancer (20).
Over the last decade, a number of experimental and
clinical studies have shown that PRLR is widely overexpressed in
tumors at a local level, including breast, colorectal, prostate and
head and neck cancer (7,21–23).
However, to date, the role of PRL/PRLR in laryngeal tumors remains
largely unknown. Our study is the first to show the expression of
PRLR in RRP and LC at RNA and protein levels. Notably, a strong
immunoreactivity was significantly associated only with malignant
laryngeal tumors, whereas only 38.9% of RRP samples showed a
moderate labeling. Moreover, immunohistochemistry results of PRLR
expression were consistent with the finding obtained by Western
blot analysis and real-time PCR. In a recent assay, Bauernhofer
et al reported that PRLR is widely expressed in squamous
cell cancer of the head and neck (SCCHN) tissues; however, they did
not explain which specific organs were involved (22). SCCHN includes cancer of the oral
cavity, pharynx and larynx. It is crucial to note that all samples
included in our study were obtained from male patients, in whom
there is no change in PRL levels in pre- and post-reproductive
stages. However, the molecular mechanism by which PRL/PRLR is
involved in laryngeal tumors remains unknown. It has been reported
in other tissues that this peptide hormone up-regulates the
expression of a set of genes involved in cell proliferation or
differentiation (24). The
proteolytic degradation of PRLR is usually induced through
ubiquitination (25). However, in
this study, in agreement with others, the PRLR showed a high
correlation with the malignant phenotype of different tissues.
Recently, it has been demonstrated that the stabilization of PRLR
in breast cancer by decreasing the activity of GSK3b, is a result
of the constitutive activation of a Ras-dependent oncogenic pathway
(26). The accumulation of PRLR in
the cytoplasm and its consequent translocation to the nucleus may
explain our observations. Additionally, other malignant processes
directed by non-ubiquitinated proteins may be operating (27).
Currently, it is known that PRL carries out its
activity by at least six recognized PRLR isoforms. These various
PRLR isoforms exhibit different signaling properties. The long PRLR
isoform is capable of activating virtually all of the signaling
pathways. By contrast, since the short PRLR isoform is not tyrosine
phosphorylated (which prevents its interaction directly with
SH2-containing proteins), Stat factors are not activated through
this isoform (28). In this study,
we found a number of isoforms of PRLR expressed in laryngeal
tissues, whereas in RRP, only a weak short band of approximately 42
kDa was expressed. Markedly, the LC samples strongly expressed
three PRLR isoforms of 42, 45 and 65 kDa and also expressed a weak
band of 100 kDa, suggesting that a signaling pathway may be
up-regulated. In this regard, the abundant isoform expression of
PRLR may indicate the progression toward cancer. To confirm this
hypothesis, further experiments are necessary to verify the
different isoforms expressed in our samples. These should include
other methodologies using specific antibodies or primers for PRLR,
as was recently carried out in a study on breast cancer (29).
In conclusion, the overexpression of PRLR suggests
that it may be a tumor biomarker, particularly in malignant
laryngeal tumors.
Acknowledgements
This study was financed by grants from
SEP-CONACYT (79709) and PROMEP UDG-PTC-605 both to A.L.P.-S.
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Derkay CS: Recurrent respiratory
papillomatosis. Laryngoscope. 111:57–69. 2001. View Article : Google Scholar
|
|
3.
|
Penaloza-Plascencia M, Montoya-Fuentes H,
Flores-Martinez SE, Fierro-Velasco FJ, Penaloza-Gonzalez JM and
Sanchez-Corona J: Molecular identification of 7 human
papillomavirus types in recurrent respiratory papillomatosis. Arch
Otolaryngol Head Neck Surg. 126:1119–1123. 2000. View Article : Google Scholar
|
|
4.
|
Hashibe M, Boffetta P, Zaridze D, et al:
Contribution of tobacco and alcohol to the high rates of squamous
cell carcinoma of the supraglottis and glottis in Central Europe.
Am J Epidemiol. 165:814–820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Cordes C, Munzel AK, Rudolph P, Hoffmann
M, Leuschner I and Gottschlich S: Immunohistochemical staining of
Ki-67 using the monoclonal antibody Ki-s11 is a prognostic
indicator for laryngeal squamous cell carcinoma. Anticancer Res.
29:1459–1465. 2009.PubMed/NCBI
|
|
6.
|
McHale K, Tomaszewski JE, Puthiyaveettil
R, Livolsi VA and Clevenger CV: Altered expression of prolactin
receptor-associated signaling proteins in human breast carcinoma.
Mod Pathol. 21:565–571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Leav I, Merk FB, Lee KF, et al: Prolactin
receptor expression in the developing human prostate and in
hyperplastic, dysplastic, and neoplastic lesions. Am J Pathol.
154:863–870. 1999. View Article : Google Scholar
|
|
8.
|
Levina VV, Nolen B, Su Y, et al:
Biological significance of prolactin in gynecologic cancers. Cancer
Res. 69:5226–5233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rui H, Kirken RA and Farrar WL: Activation
of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol
Chem. 269:5364–5368. 1994.PubMed/NCBI
|
|
10.
|
Al-Sakkaf KA, Dobson PR and Brown BL:
Prolactin induced tyrosine phosphorylation of p59fyn may mediate
phosphatidylinositol 3-kinase activation in Nb2 cells. J Mol
Endocrinol. 19:347–350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Das R and Vonderhaar BK: Activation of
raf-1, MEK, and MAP kinase in prolactin responsive mammary cells.
Breast Cancer Res Treat. 40:141–149. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nohara A, Ohmichi M, Koike K, et al:
Prolactin stimulates mitogen-activated protein kinase in human
leiomyoma cells. Biochem Biophys Res Commun. 238:473–477. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ben-Jonathan N, Lapensee CR and Lapensee
EW: What can we learn from rodents about prolactin in humans?
Endocr Rev. 29:1–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Peirce SK and Chen WY: Quantification of
prolactin receptor mRNA in multiple human tissues and cancer cell
lines by real time RT-PCR. J Endocrinol. 171:R1–R4. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yuan JS, Reed A, Chen F and Stewart CN Jr:
Statistical analysis of real-time PCR data. BMC Bioinformatics.
7:852006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tworoger SS and Hankinson SE: Prolactin
and breast cancer risk. Cancer Lett. 243:160–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Stattin P, Rinaldi S, Stenman UH, et al:
Plasma prolactin and prostate cancer risk: A prospective study. Int
J Cancer. 92:463–465. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Meyer F, Samson E, Douville P, Duchesne T,
Liu G and Bairati I: Serum prognostic markers in head and neck
cancer. Clin Cancer Res. 16:1008–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yurkovetsky Z, Ta’asan S, Skates S, et al:
Development of multi-marker panel for early detection of
endometrial cancer. High diagnostic power of prolactin. Gynecol
Oncol. 107:58–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Harbaum L, Pollheimer MJ, Bauernhofer T,
et al: Clinicopathological significance of prolactin receptor
expression in colorectal carcinoma and corresponding metastases.
Mod Pathol. 23:961–971. 2010. View Article : Google Scholar
|
|
22.
|
Bauernhofer T, Pichler M, Wieckowski E, et
al: Prolactin receptor is a negative prognostic factor in patients
with squamous cell carcinoma of the head and neck. Br J Cancer.
104:1641–1648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gill S, Peston D, Vonderhaar BK and
Shousha S: Expression of prolactin receptors in normal, benign, and
malignant breast tissue: an immunohistological study. J Clin
Pathol. 54:956–960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Brockman JL, Schroeder MD and Schuler LA:
PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol
Endocrinol. 16:774–784. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Varghese B, Barriere H, Carbone CJ, et al:
Polyubiquitination of prolactin receptor stimulates its
internalization, postinternalization sorting, and degradation via
the lysosomal pathway. Mol Cell Biol. 28:5275–5287. 2008.
View Article : Google Scholar
|
|
26.
|
Plotnikov A, Li Y, Tran TH, et al:
Oncogene-mediated inhibition of glycogen synthase kinase 3 beta
impairs degradation of prolactin receptor. Cancer Res.
68:1354–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Li Y, Clevenger CV, Minkovsky N, et al:
Stabilization of prolactin receptor in breast cancer cells.
Oncogene. 25:1896–1902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Binart N, Bachelot A and Bouilly J: Impact
of prolactin receptor isoforms on reproduction. Trends Endocrinol
Metab. 21:362–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ginsburg E, Alexander S, Lieber S, et al:
Characterization of ductal and lobular breast carcinomas using
novel prolactin receptor isoform specific antibodies. BMC Cancer.
10:6782010. View Article : Google Scholar
|