Introduction
Exposure of the human skin to ultraviolet (UV)
irradiation can cause photoaging and photo-carcinogenesis. It is
well documented that UV irradiation causes oxidative attack and
damage to DNA primarily by formation of photoproducts, and even
initiates targeted gene mutation in cells (1,2).
Subsequently, the UV-damaged cell begins DNA repair or turns to
apoptosis. If the injury is too severe to be repaired completely
and correctly, the damaged cells may begin an initiative event of
mutant colony formation followed by cancer development.
Chemoprevention of cancer is a novel and more
effective means of cancer management. Natural agents are considered
less toxic and more effective in controlling various human
malignancies, including skin cancer (3–5). It
has been proven that epigallocatechin-3-gallate (EGCG) extracted
from green tea is characterized by effects of anti-oxidation,
anti-inflammation and immunoregulation both in vitro and
in vivo (6–8). Our previous studies proved that EGCG
protects human keratinocytes and Langerhans cells from UVB-induced
photo-damage (8,9). However, the effect of EGCG on mutant
colony formation of cultured human skin fibroblasts (HSFs) caused
by multiple ultraviolet A (UVA) irradiation and its underlying
mechanism remain unclear.
The X-chromosomal gene for hypoxanthine-guanine
phosphoribosyl transferase (HPRT), first recognized by its human
germinal mutations, quickly became a useful target for studies on
somatic mutations in vitro and in vivo in human
beings and animals. In this role, HPRT serves as a simple reporter
gene. The HPRT gene locus is sensitive to irradiation and can be an
index of irradiation dosage effect (10,11).
Previously, senescence and apoptosis have been considered to be a
crucial defensive mechanism preventing damaged or abnormal cells
from cancergenesis, which has been used in cancer treatment
(12–16). It has been confirmed that
senescence-associated β-galactosidase (SA-β-Gal) expression
increases in aging individuals and serves as an aging-related
biological marker (17). In this
study, we investigated the effect of EGCG on the frequency of
mutations of HSFs with multiple UVA irradiations for 2 weeks. We
also compared the effects of EGCG on apoptosis and SA-β-Gal
expression in HSFs between the intrinsic senescence group and the
multiple UVA irradiation-induced senescence group, aiming to
ascertain the underlying mechanism.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM; Gibco/BRL,
USA); epigallocatechin-3-gallate (Sigma, USA); MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide)
(Sigma); solar simulator (Sigma); dispase (Sigma); β-galactosidase
kit (Mirus Bio Co., USA); 6-thioguanine (Sigma); 0.2% methylene
blue (Sigma); propidium iodide (PI; Molecular Probe, USA); Vidas
Image and Analysis system (Zeiss, Germany) and a flow cytometer
(Epics, USA) were obtained.
Cell culture and subgroups
Human fibroblasts derived from the foreskin of young
donors (<5 years of age) were isolated and cultured in DMEM
(Gibco/BRL) supplemented with 2 mM glutamine (Gibco/BRL) and 10%
fetal bovine serum (HyClone) at 37°C in 5% CO2. In the
intrinsic senescence experiment, HSFs were cultured and passaged
for a total of 80 days. In the UVA irradiation-related experiment,
a serum-free version of the above medium was supplied. When growth
of HSFs reached the desired confluence, the cells were divided into
the following subgroups: control group, EGCG group, UVA irradiation
group and UVA+EGCG group. HSFs in different groups were used for
the determination of senescence, HPRT gene mutation and cellular
apoptosis.
Preparation and selection of EGCG
solution with optimal concentration
The EGCG solution was prepared with DMEM at the
concentration of 500 μg/ml, and stored at −20°C. A cell suspension
(100 μl) of HSFs (105 cells/ml) was seeded in a 96-well
plate. In the UVA+EGCG group, different concentrations (0, 25, 50
and 100 μg/ml) of EGCG solution were added. After incubation for 24
h, the cultures were irradiated with 10 J/cm2 UVA. HSFs
were incubated with the new medium containing EGCG for another 24 h
and then 20 μl MTT (5 mg/ml) was added. After another 4 h, the
supernatant was replaced by 100 μl DMSO, and the culture was
oscillated at room temperature for 15 min. The absorbance (A value)
at 490 nm was measured and the cell proliferation viability in each
group was determined. Since the experiments related to the SA-β-Gal
and HPRT gene mutation required a relatively longer culture time,
the optimal concentration of 25 µg/ml EGCG was chosen in the
following photo-protection study according to the preliminary cell
viability assay.
UV irradiation protocol
HSFs were pre-cultured with 25 μg/ ml EGCG solution
for 2 h, then irradiated with 10 J/cm2 UVA. The
accumulation course was designed for 2 weeks. After washing twice
with phosphate-buffered saline (PBS), cells were irradiated with a
thin cover of PBS to avoid drying and with a water bath at room
temperature to avoid overheating during irradiation. The intensity
of UVA (320–400 nm) emitted by a solar simulator (Sigma) was 4.4
mW/cm2. The irradiation distance between the cultured
cells and the UV source was 15 cm and the irradiation dosage was
controlled by a radiometer equipped with a UVA-sensor.
Sham-irradiated cultures were handled identically except that they
were shielded with aluminum foil during the irradiation. Each
treatment was conducted in triplicates and the experiments were
conducted three times.
HPRT mutagenesis assay
The HPRT-mutagenesis assay was used as described
previously to detect and to characterize UV-induced HPRT mutations.
Exponentially growing cells were irradiated with UVA or sham when
the HSF culture reached ∼50% confluence. After irradiation, cells
were propagated for 3.5–4 population doublings (expression period),
as verified by cell counts in parallel dishes, to allow the
expression of the mutated HPRT gene. After being cultured for 7–14
days (expression period), the cells were transferred to 10–20
tissue culture dishes with a selective medium containing 7 μg/ml
6-thioguanine (Sigma), at a density of 5x100 cells/cm2.
In this selective medium, only HPRT-mutated cells were able to
survive and form colonies, since they are unable to metabolize
6-thioguanine to a toxic agent. After a selection period of 4–6
weeks, HPRT-mutated, 6-thioguanine-resistant cell colonies were
stained with 0.2% methylene blue and counted to determine the
mutation frequency. The mutation frequency was calculated as the
number of mutants/the number of plated cell x the plating
efficiency. The latter was determined by plating 100 cells/dish
separately in non-selective medium at the end of the expression
period.
Histochemical method for β-galactosidase
detection
The semi-quantitative analysis of SA-β-Gal-positive
cells was performed when the confluence of the plated HSFs reached
50%. Cells were washed in PBS, fixed in 2% formaldehyde/0.2%
glutaraldehyde for 5 min (room temperature), washed again and then
incubated at 37°C (no CO2) with fresh SA-β-Gal stain
solution. The blue-co1ored cells were considered β-Gal-positive
cells. Randomly selected 500 cells within a field under the
microscope were counted. The percentage of positive cells, which
represented the aging rate of the HSF cultures, was calculated.
Senescence rate = the number of blue colored cells/the total cell
number × 100%.
Cellular apoptosis detection
The HSFs were treated as above by UVA with or
without EGCG. Conditioned cells were digested by 0.25% trypsin and
fixed by 80% alcohol solution. The cells were washed three times
and 200 μl PI (50 μg/ml) was added into every tube. Cells were then
resuspended into a single-cell suspension and left to set for 30
min at room temperature. Cellular apoptosis was detected by flow
cytometry.
Statistical analysis
SPSS 11.0 software was used to conduct the paired
t-test or AVONA test. Statistical significance was established at
p-values <0.05.
Results
Effects of different concentrations of
EGCG solution on UVA-irradiated HSF viability
Table I shows that
there was no significant difference in cellular viability between
the control and the EGCG-treated groups without UVA irradiation
(p>0.05), which meant that various concentrations of EGCG
exhibited no cytotoxicity to the cultured HSFs. The cellular
viability of the HSFs stably increased, while the EGCG
concentration ranged from 10 to 50 μg/ml under 10 J/ cm2
UVA irradiation (p<0.05). Since the experiments related to
SA-β-Gal and HPRT gene mutations require a relatively longer
culture time, such as 80 days, for spontaneous senescence study and
2 weeks for UVR-associated experiments, 25 µg/ml EGCG was thus
chosen for further study.
 | Table I.Effects of different concentrations of
EGCG on UVA-irradiated HSF viability. |
Table I.
Effects of different concentrations of
EGCG on UVA-irradiated HSF viability.
| A values | EGCG (μg/ml)
|
|---|
| 0 | 10 | 25 | 50 | 100 |
|---|
| 0 UVA
(J/cm2)a | 0.54±0.13 | 0.50±0.07 | 0.44±0.08 | 0.47±0.06 | 0.52±0.10 |
| 10 UVA
(J/cm2)b | 0.34±0.02 | 0.41±0.01 | 0.46±0.02 | 0.47±0.01 | 0.40±0.02 |
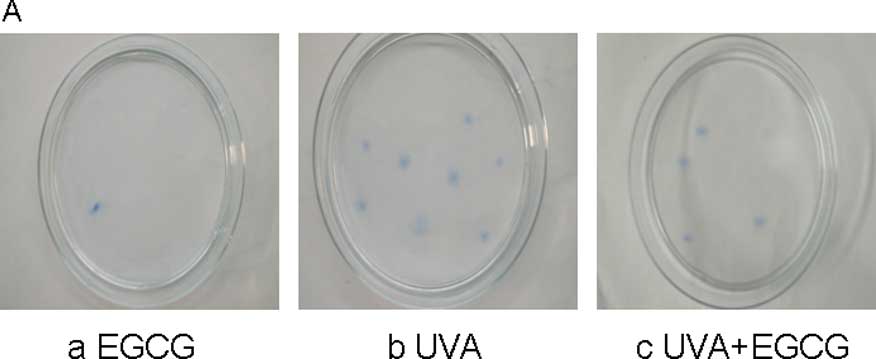
Effect of EGCG on mutation colony
frequency in irradiated HSFs
Different groups of HSF cells were co-cultured with
6-TG in the selective period and then stained, counted and
corrected. The intrinsic mutation frequency was very low in the
control group (1.8±1.6) and the EGCG group (2.8±1.6). The mutation
frequency in the UVA group was 433.8±40.6, almost 240 times higher
than that in the control group and the simple EGCG group,
respectively (p<0.001). Compared to simple UVA irradiation, the
mutation number in the EGCG+UVA group was much lower (293.4±27.2)
and was reduced by 47.85% (Fig.
1).
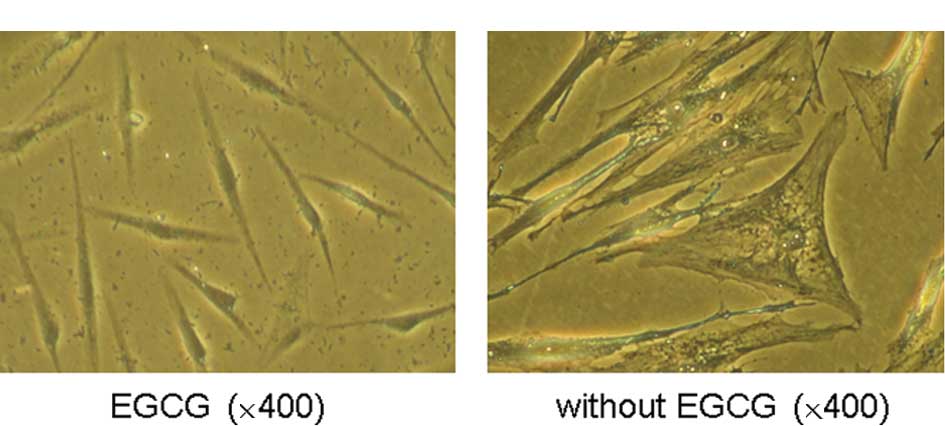
Effect of EGCG on the intrinsic
senescence in HSFs
Naturally, HSFs were passed for more than 20
passages during 80 culture days, and histochemistry was used to
detect SA-β-Gal-positive cells. Under microscopy, young fibroblasts
grew well, displaying a long shuttle or irregular shape (Fig. 2B). In contrast, with a prolonged
culture period, the aging cells arranged in a disordered pattern
were large and flat, with more cytoplasm and a higher ratio of
cellular cytoplasm/ cellular nucleus. Many granules and vacuoles
appeared in the cytoplasm, which was hardly observed in the HSFs
treated with 25 μg/ml EGCG, but obvious in the aging cells without
EGCG treatment (Fig. 2B), which
also implied some type of cell natural senescence and functional
decline. The number of SA-β-Gal-positive cells was quite different
between the two groups (82.30±7.21 in the control group vs.
43.12±6.48 in the EGCG-treated group) (Fig. 2C). The senescence rate of the HSFs
cultured with 25 μg/ml EGCG was reduced by 47.60%.
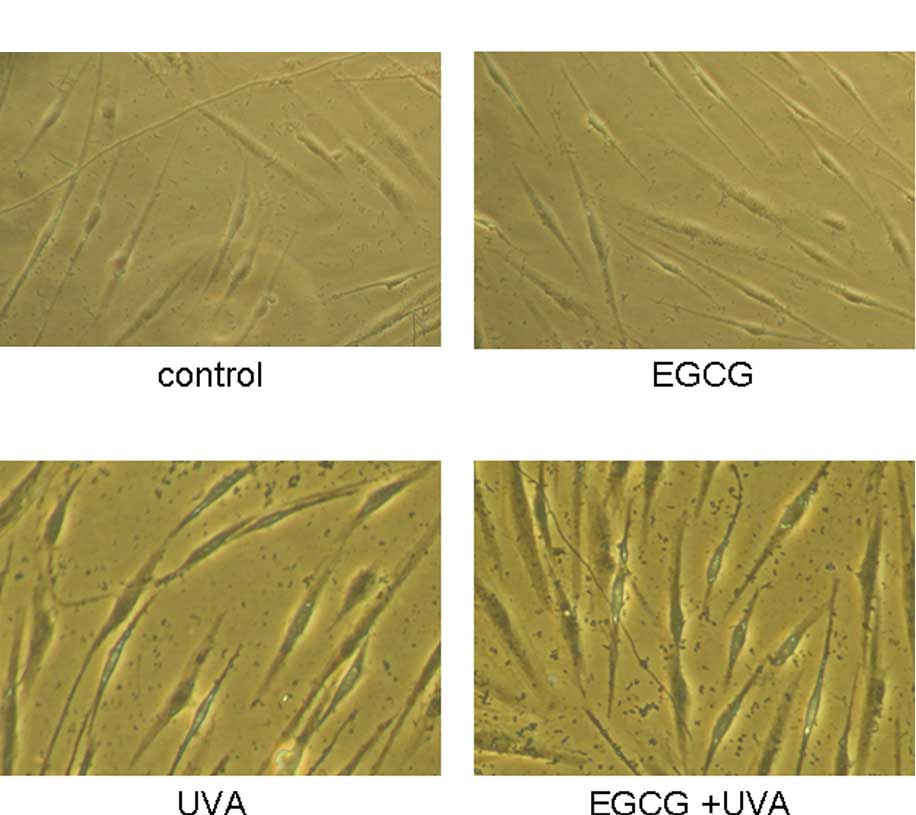
Effect of EGCG on UVA-irradiated cell
senescence in HSFs
After the HSFs were pre-cultured with 25 μg/ml EGCG
and/or UVA irradiation for 2 weeks, cell morphology and SA-β-Gal
positively-stained senescent cells were assessed in the cultures
(Fig. 3A–D). Images of the
UVA+EGCG group showed several large and flat cells. By
histochemical staining, less SA-β-Gal-positive cells were observed
in the control group (12.71±2.10) and the EGCG group (11.46±2.27).
The positive senescent cells in the multiple UVA group showed an
approximately three times higher value than the control and EGCG
groups (53.62±4.01), and the senescence rate was also increased by
38.92% in the EGCG-multiple UVA group compared to the UVA group
(74.49±5.03). Statistical analysis confirmed that there was
statistical significance between the UVA and EGCG+UVA groups
(Fig. 3E), which implied some
promotive effect of EGCG on cell senescence, while combined with
multiple UVA irradiation.
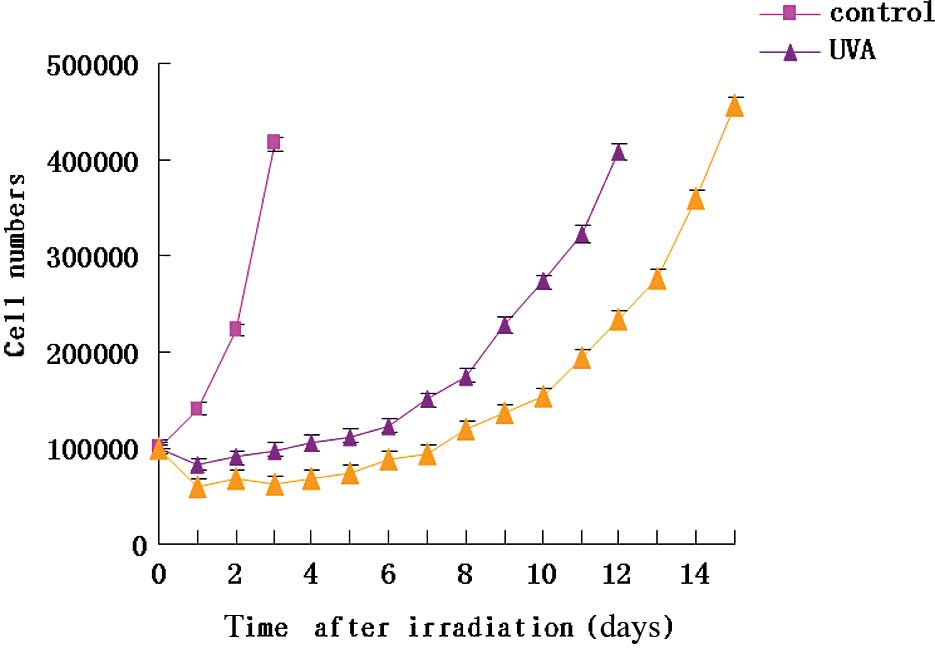
Effect of EGCG on UVA-induced cell growth
curve during the expression period
After 2 weeks of UVA irradiation, the HSFs required
several days to allow for the expression of the mutant phenotype
prior to replanting the mutant cells. Compared to the control
group, the expression period was longer in the multiple UV
irradiation group as the cells proliferated slowly and it required
1 week to enter into rapid growth period. Furthermore, the HSF
cells proliferated much more slowly after the cultures were
intervened by EGCG treatment. The period for the HSF cells in the
UVA+EGCG group to reach a growth peak was 14 days (Fig. 4).
Effect of EGCG on UVA-induced cell
apoptosis and cell cycle in HSFs
The apoptosis rates of the different groups of HSF
cells were also analyzed after 2 weeks of EGCG treatment with or
without UVA. There was no noticeable difference in the peak of
apoptosis in the control (0.15±0.06%) and the EGCG groups
(0.50±0.11%) (p>0.05). Compared to these two groups, the
apoptosis rate was apparently higher in the chronic UVA irradiation
group (20.06±1.14%), increasing by ∼200% (p<0.01). The cellular
apoptosis was further increased by ∼56.92% in the EGCG+UVA
(31.48±2.01) compared to the UVA only group (p<0.05), which
implied that EGCG prompts apotosis of UVA damaged HSFs. In
accordance with cell apoptosis, the percentage of cells in the cell
cycle displayed a similar change; the number of cells in arrested S
phase increased (64.15±3.98%) and was accompanied by a decrease in
cells in the G0/G1 (17.95±1.47%) and G2/M phase (17.9±1.21%).
Compared to the UVA group, co-culture with EGCG reduced the number
of cells in the S phase (26.78±1.10%, p<0.001), and an increase
in cells in the G0/G1 (2.7-fold) and G2/M phase (1.43-fold)
(p<0.05, Table II) was noted.
These results indicate that EGCG confers a photo-protective effect
on UVA-irradiated HSFs.
 | Table II.Effects of UV irradiation and EGCG
treatment on apoptosis and the cell cycle in HSF. |
Table II.
Effects of UV irradiation and EGCG
treatment on apoptosis and the cell cycle in HSF.
| Groups | Apoptosis (%) | S phase (%) | G0/G1 phase (%) | G2/M phase (%) |
|---|
| Control | 0.15±0.06 | 29.73±1.52 | 46.50±2.32 | 23.77±1.21 |
| EGCG | 0.50±0.11 | 30.36±1.83 | 48.79±2.42 | 20.85±1.05 |
| UVA | 20.06±1.14 | 64.15±3.98 | 17.95±1.47 | 17.90±1.21 |
| UVA+EGCG | 31.48±2.01a | 25.78±1.10b | 48.59±2.68 | 25.63±1.92 |
Discussion
Solar UV irradiation induces different hazardous
effects in the skin, including sunburn (18), photoaging (19) and cancer (20). Protection against solar-induced
damage is therefore a highly desirable goal. Several studies have
shown that EGCG has a photo-protective effect on human skin cells,
such as primary keratinocytes, fibroblasts, dendritic cells,
Langerhans cells and HaCaT cells (21–26).
These data suggest that EGCG may protect HSFs against UVA-induced
HPRT mutations in vitro.
It has been well known that DNA lesions are induced
by UV irradiation and the mutations can be formed in some critical
genes, which are believed to play an important role in
carcinogenesis (27,28). Previously, researchers have used
the alkaline comet assay to compare the DNA damage induced by UVR
in cultured human cells (lung fibroblasts, skin fibroblasts and
epidermal keratinocytes) with and without EGCG, and found that EGCG
protects human cellular DNA from UV and visible radiation-induced
damage (29). In the present
study, we confirmed that 2 weeks of UVA irradiation increased the
mutation frequency of the HPRT gene approximately 150–240 times
higher than that of the control and EGCG groups (intrinsic mutant
levels). Pre-treatment with EGCG inhibited the frequency of
mutation at the coding region of the HPRT gene by 47.85% in
UVA-irradiated HSFs. Therefore, we may infer that one important
photo-protective effect of EGCG on HSFs from multiple UVA
irradiation may be related to the depression of DNA mutation
formation.
Compared to freshly isolated HSF cells, the HSF
cells that were cultured for more than 20 passages, with their
special aging morphology, were positive for SA-β-Gal staining.
However, upon pre-treatment with EGCG, not only the aging
morphology was normalized, but also the number of SA-β-Gal-positive
cells was markedly reduced, which indicated that EGCG mitigated the
intrinsic senescence in cultured HSFs. However, after pre-treatment
with EGCG with irradiation of UVA for a continuous 2 weeks, the
positive number of senescent cells increased by 38.92% compared to
the UVA group. This indicated that treatment of HSFs with EGCG
prior to UVA exposure caused a further increase in the rate of
senescence. Similarly, UVA also caused an increase in cell growth
arrest and apoptosis rates, which were further increased by
treatment of EGCG prior to UVA exposure.
Several studies have discussed the relationship
between senescence and tumor genesis. Some researchers consider
senescence as an important defence mechanism by preventing damaged
or abnormal cells from cancer transformation (12–14,30).
On the one hand, by altering the tissue microenvironment, senescent
cells may contribute to the rise in cancer that occurs with age. On
the other hand, senescence is thought to be a powerful, albeit
imperfect, tumor-suppressive mechanism since one of the phenotypic
changes in senescent cells is irreversible growth arrest. Cellular
apoptosis is also an effective mechanism for eliminating senescent
or mutated cells to balance the intrinsic environment and interfere
with tumor growth. Certain studies have confirmed that UV-induced
cell apoptosis is also a protective mechanism in UV injury
(31–33). Treatment of HSFs with EGCG prior to
UVA exposure caused a further increase in the rates of senescence
and apoptosis, but a decrease in the cell proliferation rate and
mutant frequency. Thus, we may infer that a novel mechanism was
initiated, in which the mutant HSFs induced by multiple UVA
exposure underwent a state of arrested growth and entered
senescence and/or a course of apoptosis.
Previous studies have shown that the WRN helicase
may play a role in cancer development, by participating in both
oncogenic proliferation through the avoidance of senescence, as
well as protection of the genome from mutations that eventually
promote tumor establishment (34).
This is a paradigm that has been reported for telomerase as well.
Similarly, activation of telomerase promotes cell immortalization
and thus tumor genesis, while its absence protects cancer-prone
mice from tumor development (35,36).
However, the exacerbated genomic instability, occurring after
several generations in telomerase-negative mice, eventually
contributes to an increased incidence of cancers (37). Accordingly, future studies are also
required to assess the effect of EGCG on WRN and telomerase as an
adaptive response for its efficacy against UVA-caused HPRT
mutations in HSFs.
In summary, the results obtained in the present
study indicate that EGCG induces multiple UVA damaged cells to
proceed to either senescence and/or apoptosis. In this way, the
mutant cells cannot be replicated and inherited, and
photo-carcinogenesis can be finally reduced. However, the exact
molecular mechanisms remain unclear. Present and further studies
concerning EGCG may certainly provide new clues for new strategies
to avoid UV-induced damages.
Acknowledgements
This study was supported by grants
from the China National Natural Science Foundation (30771946,
81000700).
References
|
1.
|
Wang SQ, Setlow R, Benrick M, et al:
Ultraviolet A and melanoma: a review. J Am Acad Dermatol.
44:837–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kappes UP, Luo D, Potter M, et al: Short-
and long-wave UV light (UVB and UVA) induce similar mutations in
human skin cells. J Invest Dermatol. 126:667–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bickers DR and Athar M: Novel approaches
to chemoprevention of skin cancer. J Dermatol. 27:691–695. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bode AM and Dong Z: Signal transduction
pathways: targets for chemoprevention of skin cancer. Lancet Oncol.
1:181–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhou BR, Luo D, Lin XF, et al: Protective
effect of the Baicalin against DNA damage induced by ultraviolet B
irradiation to mouse epidermis. Photodermatol Photoimmunol
Photomed. 24:175–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Morley N, Clifford T, Salter L, et al: The
green tea polyphenol (-)-epigallocatechin gallate and green tea can
protect human cellular DNA from ultraviolet and visible
radiation-induced damage. Photodermatol Photoimmunol Photomed.
21:15–22. 2005. View Article : Google Scholar
|
|
7.
|
Katiyar SK: Skin photoprotection by green
tea: antioxidant and immunomodulatory effects. Curr Drug Targets
Immune Endocr Metabol Disord. 3:234–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Conney AH, Lou YR, Xie JG, et al: Some
perspectives on dietary inhibition of carcinogenesis: studies with
curcumin and tea. Proc Soc Exp Biol Med. 216:234–245. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Luo D, Min W, Lin XF, et al: Effect of
epigallocatechin-gallate on ultraviolet B-induced photo-damage in
keratinocyte cell line. Am J Chin Med. 34:911–922. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hirai Y, Kusunoki Y, Kyolzumi S, et al:
Mutant frequency at the HPRT locus in peripheral blood T
lymphocytes of atomic bomb survivors. Mutat Res. 329:183–196. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Albertini RJ: HPRT mutations in human:
biomarkers for mechanistic studies. Mutat Res. 489:1–16. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Campisi J: The role of cellular senescence
in skin aging. J Investig Dermatol Symp Proc. 3:1–5.
1998.PubMed/NCBI
|
|
13.
|
Shay JW and Roninson IB: Hallmarks of
senescence in carcinogenesis and cancer therapy. Oncogene.
23:2919–2933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Campisi J: Suppressing cancer: the
importance of being senescent. Science. 309:886–887. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Huang C, Ma WY, Goranson A, et al:
Resveratrol suppresses cell transformation and induces apoptosis
through p53-dependent pathway. Carcinogenesis. 20:237–242. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pendergrass WR, Lane MA, Bodkin NL, et al:
Cellular proliferation potential during aging and caloric
restriction in rhesus monkeys. J Cell Physiol. 180:123–130. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dimri GP, Lee X, Basile G, et al: A
biomarker that identifies senescent human cells in culture and in
aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Honigsmann H: Erythema and pigmentation.
Photodermatol Photoimmunol Photomed. 18:75–81. 2002. View Article : Google Scholar
|
|
19.
|
Fisher GJ, Kang S, Varani J, et al:
Mechanisms of photoaging and chronological skin aging. Arch
Dermatol. 138:1462–1470. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Gruijl FR, Kranen HJ and Mullenders LH: UV
induced DNA damage, repair, mutations and oncogenic pathways in
skin cancer. J Photochem Photobiol B. 63:19–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ji X, Luo D, Lin XF, et al: The
phosphorylated P53 protein expressions in epidermal Langerhans
cells irradiated by UVB. J Clin Dermatol. 36:134–136. 2007.
|
|
22.
|
Ji X, Luo D, Miao X, et al: Inhibitory
effect of EGCG on apoptosis of Langerhans cells after UVB
irradiation. Chin J Dermatol. 39:344–346. 2006.
|
|
23.
|
Luo D, Xu J, Zhou BR, et al: Influence of
EGCG in different formulations on the apoptosis of epidermis cells
of Balb/C mice irradiated by UVB. J Chin Pharmaceut Univ.
38:535–538. 2007.
|
|
24.
|
Luo D, Zhou BR and Ji X: Influence of
epigallocatechin gallate on the immune function of dendritic cells
after ultraviolet B irradiation. J Microbiol Immunol. 5:90–98.
2007.
|
|
25.
|
Luo D, Lin XF, Xu J, et al: Effects and
regulatory mechanisms of three traditional Chinese medicines on
HACaT cells irradiated by UVB. Chin Pharmacol Bull. 23:750–755.
2007.
|
|
26.
|
Li M, Luo D and Lin XF: Photoprotection of
EGCG on HaCaT cells against oxidative damage and apoptosis from UVA
irradiation. J Clin Derm. 37:80–83. 2008.
|
|
27.
|
Wang YC, Maher VM, Mitchell DL, et al:
Evidence from mutation spectra that the UV hypermutability of
xeroderma pigmentosum variant cells reflects abnormal, error-prone
replication on a template containing photoproducts. Mol Cell Biol.
13:4276–4283. 1993.
|
|
28.
|
Xu G, Snellman E, Bykov VJ, et al: Effect
of age on the formation and repair of UV photoproducts in human
skin in situ. Mutat Res. 459:195–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Salter L, Clifford T, Morley N, et al: The
use of comet assay data with a simple reaction mechanism to
evaluate the relative effectiveness of free radical scavenging by
quercetin, epigallocatechin gallate and N-acetylcysteine in
UV-irradiated MRC5 lung fibroblasts. J Photochem Photobiol B.
75:57–61. 2004. View Article : Google Scholar
|
|
30.
|
Beausejour CM, Krtolica A, Galimi F, et
al: Reversal of human cellular senescence: roles of the p53 and P16
pathways. EMBO J. 22:4212–4222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Neades L, Cox J and Pelling O: S-phase
arrest in mouse keratinocytes exposed to multiple doses of
ultraviolet B/A radiation. Mol Carcinog. 23:159–167. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Li G and Ho VC: p53-dependent DNA repair
and apoptosis respond differently to high- and low-dose ultraviolet
radiation. Br J Dermatol. 139:3–10. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chow J and Tron VA: Molecular aspects of
ultraviolet radiation-induced apoptosis in the skin. J Cutan Med
Surg. 9:289–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Grandori C, Wu KJ, Fernandez P, et al:
Werner syndrome protein limits MYC-induced cellular senescence.
Genes Dev. 17:1569–1574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Greenberg RA, Chin L, Femino A, et al:
Short dysfunctional telomeres impair tumorigenesis in the
INK4a(delta2/3) cancer-prone mouse. Cell. 97:515–525. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Blasco MA: Telomeres in cancer and aging:
lessons from the mouse. Cancer Lett. 194:183–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Rudolph KL, Chang S, Lee HW, et al:
Longevity, stress response, and cancer in aging
telomerase-deficient mice. Cell. 96:701–712. 1999. View Article : Google Scholar : PubMed/NCBI
|