Introduction
Gastric carcinoma (GC) is estimated to be the second
most common cause of cancer-related death in the world, although
both the incidence and mortality have declined in the past 50 years
(1). The prognosis of patients
with GC remains poor due to the high rate of tumor invasion into
underlying tissue and lymph node metastasis, which are major
prognostic indicators of neoplastic recurrence after treatment
(2). Thus, there is an urgent need
to identify cancer metastasis earlier and more accurately.
Accumulating evidence indicates that progression beyond the initial
stages of the malignant transformation in gastric adenocarcinoma is
associated with cellular immortality, which occurs in other
neoplasms (3).
The key factor responsible for cellular immortality
is telomerase, which is a specialized ribonucleoprotein complex
that adds telomeric DNA onto the ends of chromosomes. By
synthesizing the repetitive telomeric sequence using its RNA
template, telomerase prevents cellular senescence in somatic cells
(4). Moreover, human telomerase
reverse transcriptase (hTERT), as the rate-limiting step in the
activation of telomerase, is known to be an accurate measure of
telomerase activity (TA). The presence of hTERT is therefore
required for aberrant cell proliferation and carcinogenesis in most
cancer types (5). Thus, telomerase
is considered to be a potential marker of oncogenesis (6).
The prognostic role of high TA and overexpression of
hTERT has been reported by many authors (7,8).
Although several studies focusing on telomerase have referred to
clinicopathological variables, including tumor size, site,
histologic grade, depth of tumor invasion, lymph node metastasis,
distant metastasis and TNM stage, the relationship between
telomerase and tumor progression or metastasis in patients with GC
remains controversial. It is uncertain whether reported results
depend on the number of patients or ethnic heterogeneity present in
each trial. Therefore, it is appropriate to undertake a
meta-analysis of existing trials to achieve insight into the
metastatic value of TA and hTERT in GC.
In the present study, we enrolled
clinicopathological parameters (such as depth of tumor invasion and
lymph node metastasis) from case-control studies to predict the
clinical outcome of GC. The results demonstrated that telomerase
overexpression may play a key role in metastatic progression of
GC.
Materials and methods
Literature search
This meta-analysis followed the proposal set by the
Meta-analysis Of Observational Studies in Epidemiology (MOOSE)
group (9), and was performed by
searching PubMed, MEDLINE, EMBASE, Web of Science databases,
Cochrane Library and the Chinese Biomedical Literature database
(CBM) (last search updated in October 2011). The search strategy
included the following terms: (telomerase [MeSH] or Telomerase
Catalytic Subunit [TEXT WORD] or Telomerase Reverse Transcriptase
[TEXT WORD] or hTERT [TEXT WORD]) and (Stomach Neoplasms [MeSH] or
Gastric Cancer [TEXT WORD] or Gastric Neoplasms [TEXT WORD] or
Stomach Cancer [TEXT WORD]). Searches also included scanning
reference lists in relevant articles and conference proceedings as
well as correspondence with authors when additional data were
required. Two reviewers (Lü and Deng) independently screened titles
and abstracts of each identified reference, and categorized papers
based on the full text to evaluate their eligibility for
inclusion.
Inclusion criteria
The inclusion criteria for primary studies were as
follows: i) the data were from prospective or retrospective
case-control studies and included correlations of telomerase or
hTERT to GC; ii) each study presented a proven diagnosis of GC in
humans; iii) each study measured telomerase activity or hTERT
evaluation using immunohistochemistry (IHC), a telomeric repeat
amplification protocol assay (TRAP), a telomeric repeat
amplification protocol/enzyme-linked immunosorbent assay
(TRAP-ELISA), a membrane-array assay, a reverse
transcription-polymerase chain reaction (RT-PCR) or real-time
fluorescent quantitative PCR (qRT-PCR); iv) the papers had to
provide the sample size, ethnicity and other sample information; v)
if data were shared between multiple studies, only the most recent
or largest population was included (10), and vi) the publication was in
English.
Data extraction
The following items were collected from the reports:
first author, year of publication, sample size, ethnicity, TA or
hTERT assessment method, cutoff value of TA or hTERT positivity,
and telomerase or hTERT expression related to clinicopathological
parameters, including gender, age, tumor size, histologic grade,
depth of invasion, lymph node metastasis, distant metastasis and
TNM stage. Depth of tumor invasion was confirmed using histologic
examination, and infiltration into serosa indicated a poor
prognosis. The presence of lymph node metastasis in early GC was
not a good sign. Distant metastasis was a definite prognostic
marker of tumor recurrence. We required that each study
definitively reported at least two of the following criteria: the
depth of invasion, the presence of lymph node metastasis and the
presence of distant metastasis. Data extraction was performed
independently by two individuals (Lü and Deng), and any
disagreement was resolved by consensus or by consultation with
additional reviewers (Yang and Zhang).
Qualitative assessment
Quality assessment was performed with the
Newcastle-Ottawa quality assessment scale (NOS) for case-control
studies (Table I). A ‘star system’
has been used to judge data quality based on three broad
perspectives: the selection, comparability and outcome of interest
for cohort studies. Stars are added up to compare the study quality
in a quantitative fashion (11).
Based on these criteria, the content validity was evaluated by Lü
and Deng, and any disagreement was resolved via discussions between
Lü and Deng or with the other authors (Yang and Zhang) for
adjudication.
 | Table I.Newcastle-Ottawa quality assessment
scale. |
Table I.
Newcastle-Ottawa quality assessment
scale.
| Selection |
| 1) Is the case
definition adequate? |
| a) Yes, with
independent validation* |
| b) Yes (record
linkage or based on self reports) |
| c) No
description |
| 2)
Representativeness of the cases |
| a) Consecutive or
obviously representative series of cases* |
| b) Potential for
selection biases or not stated |
| 3) Selection of
controls |
| a) Community
controls* |
| b) Hospital
controls |
| c) No
description |
| 4) Definition of
controls |
| a) No history of
disease* |
| b) No description
of source |
| Comparability |
| 1) Comparability of
cases and controls on the basis of the design or analysis |
| a) Study controls
for metastasis* |
| b) Study controls
for any additional factor* (age, gender,
grade) |
| Exposure |
| 1) Ascertainment of
exposure |
| a) Secure record
(surgical records)* |
| b) Structured
interview blind to case/control status* |
| c) Interview not
blinded to case/control status |
| d) Written self
report or medical record only |
| e) No
description |
| 2) Same method of
ascertainment for cases and controls |
| a)
Yes* |
| b) No |
| 3) Non-response
rate |
| a) Same rate for
both groups* |
| b)
Non-respondents described |
| c) Rate different
and no designation |
Statistical analysis
Statistical analysis was performed using RevMan 5.0
according to the principles set out in the Cochrane Handbook for
Systematic Reviews of Interventions. The methodological quality of
each study was assessed with the QUADAS tool recommended by the
Cochrane Collaboration, and the kappa statistic (κ) for inter-rater
reliability was calculated. Agreement was assessed using the κ
statistic for evaluating methodological quality (12). For dichotomous outcomes, the
meta-analysis was performed using crude odds ratios (ORs) with 95%
confidence intervals (CIs) to assess the strength of association
between telomerase activity or hTERT and metastasis of GC. The data
were reported in a binary manner, elucidating the telomerase
activity or hTERT value as either ‘high’ or ‘low’. The pooled ORs
were conducted to assess the depth of invasion, lymph node
metastasis and distant metastasis. For analyzing clinical outcome,
well and moderate differentiation were merged, poor and
undifferentiated were merged, T1 and T2 were merged, T3 and T4 were
merged, stage I and stage II were merged, and stage III and stage
IV were merged. Assessment of heterogeneity was assessed by the
Chi-square test (χ2) and inconsistency index test (I2).
Heterogeneity was not considered statistically significant when
p>0.10 in the χ2-test, and acceptable heterogeneity
was defined as I2<50% in studies. For studies lacking
a measure of heterogeneity, a Mantel-Haenszel fixed effect model
was used for the primary meta-analysis (13); otherwise, a DerSimonian-Laird
random effects model was adopted (14). Assessment of publication bias for
each of the pooled study groups was tested using a funnel plot.
Results
Selection and characteristics of the
studies
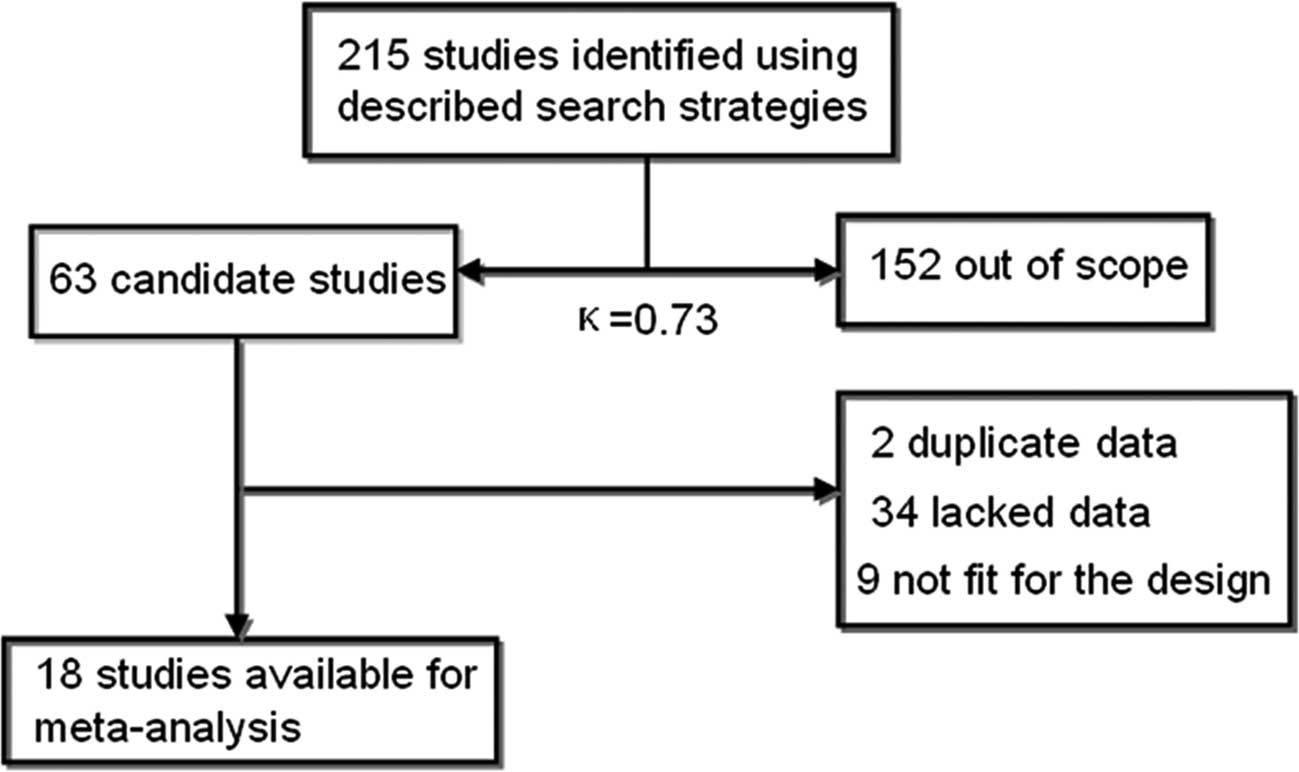
At the beginning, 215 records were examined
according to the search strategies. In total, 152 articles were
eliminated after scanning the titles or abstracts since they were
review articles, case reports, commentaries and letters or since
they were irrelevant to this analysis. After further review, an
additional 45 articles were excluded: first, 2 studies overlapped
with others. Second, 9 studies were experiments on cell cultures or
animals. Finally, 34 studies lacked usable data that correlated
telomerase or hTERT with lymph node status or TNM stage to create
2x2 tables. Thus, a total of 18 eligible studies related to GC
patients were finally identified in our meta-analysis with good
agreement between reviewers (κ=0.73) (15–32)
(Fig. 1).
In the remaining studies, all measurements were
performed using the primary tumor, and all of the patients had not
received chemotherapy or radiotherapy before enrollment. Although
research was conducted at tertiary referral centers, almost all
studies were performed in Asia and one in South America (22). Sample size varied from 20 to 95
participants, and the average age across all of the studies was
59.3 years, with a variation ranging from 32 to 89 years. The
number of GC patients with T3 and T4 invasion ranged from 11 to 78;
the number of GC patients with lymph node metastasis ranged from 7
to 75; the number of GC patients with distant metastasis ranged
from 2 to 32. Four studies used IHC, 9 studies used TRAP, 1 study
used TRAP-ELISA, 1 study used a membrane-array assay, 2 studies
used RT-PCR and 1 study used qRT-PCR. The quality assessment of
studies was performed using the NOS ranged from 5 to 7 (with a mean
star rating of 5.9), with a higher value indicating better
methodology. The scale is listed in Table II. The cutoff value of telomerase
or hTERT expression was determined using different methods in each
study. The basic feature description of the 18 studies is
summarized in Table II, and the
correlation between telomerase or hTERT expression and
clinicopathological factors is listed in Table III.
 | Table II.Main characteristics of the 18
studies included in the meta-analysis. |
Table II.
Main characteristics of the 18
studies included in the meta-analysis.
| Author/(Ref.) | Year of
publication | Language | Population | Study from
PubMed | No. of patients
(M/F) | Median age
(years) | TA/hTERT detection
method | Cutoff for TA
positivity (%) | Result | Study quality
points |
|---|
| Yang et al
(15) | 2001 | English | China | Yes | 29/13 | 52.9 | TRAP assay | >6-bp
ladder | All negative | 7/9 |
| Liu et al
(27) | 2008 | Chinese | China | Yes | 27/13 | 54.0 | RT-PCR | >0.6 | 3,4,6 positive | 6/9 |
| Okusa et al
(28) | 1998 | English | Japan | Yes | 22/14 | 62.3 | TRAP-ELISA | >5% | 5 positive | 6/9 |
| Hu et al
(16) | 2009 | English | China | Yes | 28/18 | 56.3 | TRAP assay | >0.2 units | All positive | 5/9 |
| Shin et al
(29) | 2002 | English | Korea | Yes | 35/30 | 55.4 | RT-PCR | NR | 2,4 positive | 6/9 |
| Mori et al
(30) | 2000 | English | Japan | Yes | 32/14 | 61.7 | TRAP assay | >6-bp
ladder | 3 positive | 5/9 |
| Wu et al
(17) | 2006 | English | China | Yes | 41/23 | 60.5 | Membrane-array
assay | ROC curve | All negative | 7/9 |
| Wang et al
(18) | 2004 | English | China | Yes | 30/11 | 57.2 | IHC | >5% | 1,2,4,6
positive | 6/9 |
| Yoo et al
(19) | 2003 | English | Korea | Yes | 38/13 | 61.3 | IHC | >10% | 2,3 positive | 6/9 |
| Yasui et al
(20) | 1998 | English | Japan | Yes | 10/10 | 68.9 | IHC | Focal or diffuse
staining | NR | 5/9 |
| Kameshima et
al (21) | 2000 | English | Japan | Yes | 19/8 | 66.9 | TRAP assay | >0.6 μg | All negative | 6/9 |
| Gigek et al
(22) | 2009 | English | Brasil | Yes | 36/19 | NR | IHC | No positive cells
were observed | All negative | 5/9 |
| Hu et al
(23) | 2004 | English | China | Yes | 25/10 | 55.2 | qRT-PCR | >5.39 | 2 positive | 6/9 |
| Ahn et al
(24) | 1997 | English | Korea | Yes | 57/38 | 54.3 | TRAP assay | >6-bp
ladder | All negative | 7/9 |
| Zhan et al
(31) | 1999 | English | China | Yes | 50/44 | 63.0 | TRAP assay | >6-bp
ladder | All negative | 6/9 |
| Gümüx-Akay et
al (32) | 2007 | English | Turkey | Yes | NR | NR | TRAP assay | NR | 2,3,4 negative | 5/9 |
| Hiyama et al
(25) | 1995 | English | Japan | Yes | 37/19 | 55.0 | TRAP assay | >0.6 μg | 1,4,6 positive | 7/9 |
| Tahara et al
(26) | 1995 | English | Japan | Yes | 13/7 | 64.0 | TRAP assay | >6-bp
ladder | 4,5 positive | 6/9 |
 | Table III.Main characteristics of 18 studies
relating TA expression to clinicopathological factors. |
Table III.
Main characteristics of 18 studies
relating TA expression to clinicopathological factors.
| Author/(Ref.) | Year of
publication | Language | Country | No. of positive/
(negative) | No. of patients
with TA-positivity
|
|---|
| Size >5 cm
(<5 cm) | Histo P/U
(W/M) | T T3/T4
(T1/T2) | N
positive/(negative) | M
positive/(negative) | TNM TIII/IV
(TI/TII) |
|---|
| Liu et al
(27) | 2008 | English | China | 26 (14) | - | - | 20 (6) | 22 (4) | 11 (15) | 24 (2) |
| Yang et al
(15) | 2001 | English | China | 40 (2) | 26 (14) | 30 (10) | 26 (14) | 20 (20) | - | 17 (23) |
| Wu et al
(17) | 2006 | English | China | 52 (12) | 23 (29) | 49 (3) | 40 (12) | 37 (15) | 14 (38) | 33 (19) |
| Hu et al
(23) | 2004 | English | China | 18 (17) | 10 (8) | 14 (4) | 7 (11) | 14 (4) | 10 (8) | - |
| Zhan et al
(31) | 1999 | English | China | 81 (13) | 46 (35) | 43 (38) | 68 (13) | 51 (30) | - | 60 (21) |
| Hu et al
(16) | 2009 | English | China | 41 (5) | 28 (13) | 33 (8) | 21 (20) | 5 (36) | 30 (11) | - |
| Wang et al
(18) | 2004 | English | China | 32 (9) | 19 (13) | 25 (7) | 13 (19) | 23 (9) | - | 27 (5) |
| Gümüx-Akay et
al (32) | 2007 | English | China | 42 (1) | - | 22 (20) | 31 (11) | 27 (15) | - | - |
| Ahn et al
(24) | 1997 | English | Korea | 85 (10) | - | 63 (22) | 60 (25) | 68 (17) | - | 61 (24) |
| Yoo et al
(19) | 2003 | English | Korea | 37 (14) | 18 (19) | 20 (17) | 11 (26) | 26 (11) | - | - |
| Shin et al
(29) | 2002 | English | Korea | 30 (35) | - | 20 (10) | 10 (20) | 22 (8) | 7 (23) | 13 (17) |
| Mori et al
(30) | 2000 | English | Japan | 19 (27) | - | 10 (9) | 16 (3) | 14 (5) | 6 (13) | 12 (7) |
| Tahara et al
(26) | 1995 | English | Japan | 17 (3) | - | 8 (9) | 14 (3) | 12 (5) | 2 (15) | 12 (5) |
| Hiyama et al
(25) | 1995 | English | Japan | 56 (10) | 28 (28) | 51 (5) | 42 (14) | 34 (22) | - | 24 (32) |
| Okusa et al
(28) | 1998 | English | Japan | 22 (14) | - | 10 (12) | 20 (2) | 18 (4) | 8 (14) | 16 (6) |
| Yasui et al
(20) | 1998 | English | Japan | 17 (3) | - | 9 (8) | 16 (1) | - | - | 11 (6) |
| Kameshima et
al (21) | 2000 | English | Japan | 19 (8) | 8 (11) | 7 (12) | 11 (8) | 8 (11) | - | 7 (12) |
| Gigek et al
(22) | 2009 | English | Brasil | 44 (11) | - | - | 23 (21) | 43 (1) | 18 (26) | 43 (1) |
Quantitative synthesis
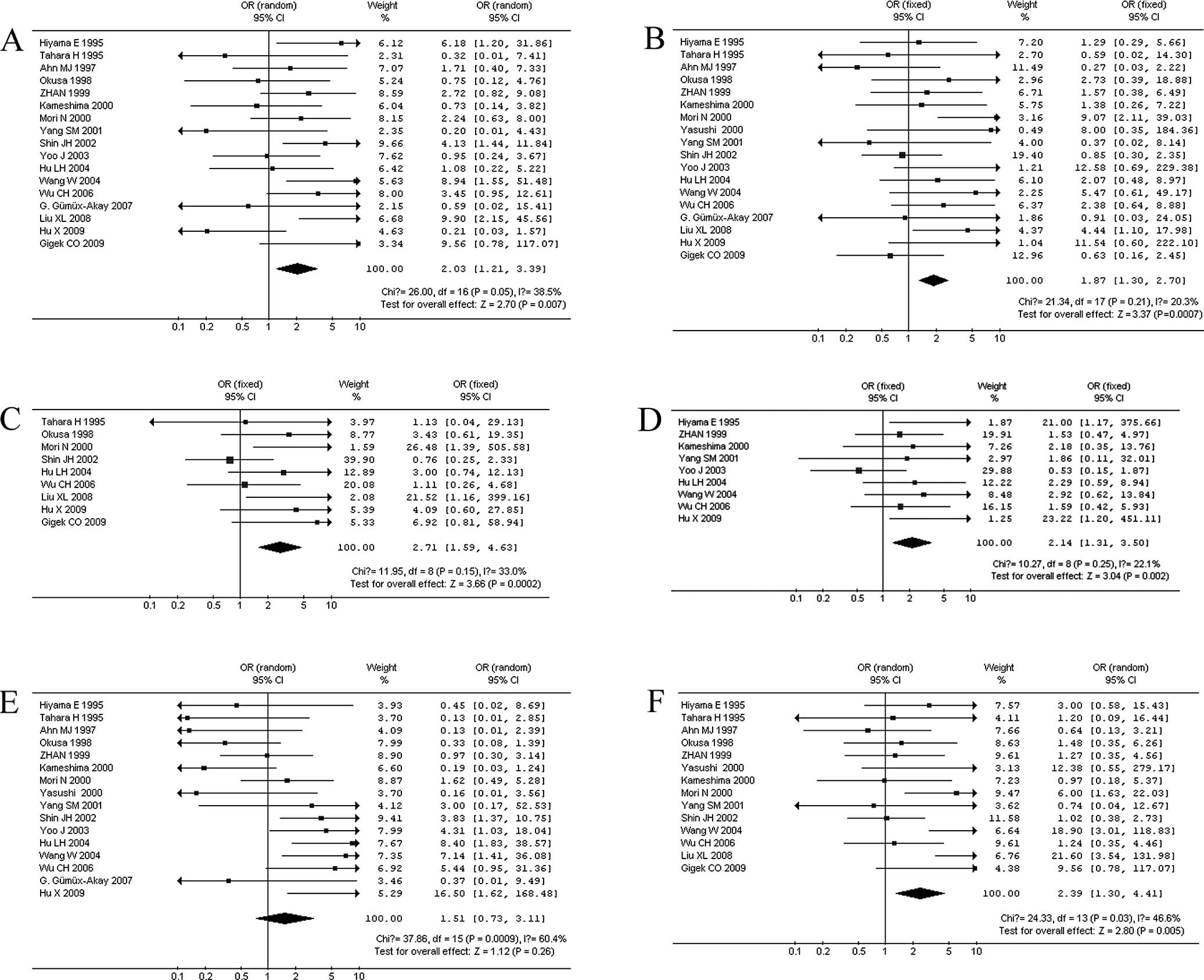
Correlation between TA and clinicopathological
characteristics. When stratifying variables by lymph node
metastasis, there was a slight heterogeneity in the data
(χ2=26, I2=38.5%, p=0.05). Patients with
lymph node metastasis in GC displayed a significantly higher TA
expression in 17 studies (866 patients) (OR=2.03, 95% CI 1.21–3.39,
p=0.007; Fig. 2A). When
stratifying for the depth of tumor invasion in GC, 18 studies (886
patients) were reported without significant heterogeneity
(χ2=21.34, I2=20.3%, p=0.21). We observed
that patients with T3 and T4 GC had a significantly higher TA
(OR=1.87, 95% CI 1.30–2.70, p=0.0007; Fig. 2B). When stratifying for distant
metastasis in GC, 9 studies (407 patients) were combined, and there
was no significant heterogeneity in the data (χ2=11.95,
I2=33%, p=0.15). Patients with distant metastasis had a
significantly higher TA in GC (OR=2.71, 95% CI 1.59–4.63, p=0.0002;
Fig. 2C). We also observed a
correlation between TA and other clinical characteristics,
including tumor size >5 cm in 9 studies (466 patients; OR=2.14,
95% CI 1.31–3.50, p=0.002; Fig.
2D), poor histologic differentiation in 16 studies (791
patients; OR=1.51, 95% CI 0.73–3.11, p=0.26; Fig. 2E), and a higher (III + IV) clinical
stage in 14 studies (711 patients; OR=2.39, 95% CI 1.30–4.41,
p=0.005; Fig. 2F). When
stratifying the variables by poor histologic differentiation of GC,
there was heterogeneity (I2=60.4%), then the
DerSimonian-Laird random effects model was used. There was no
significant correlation between TA and histologic differentiation
(p=0.26).
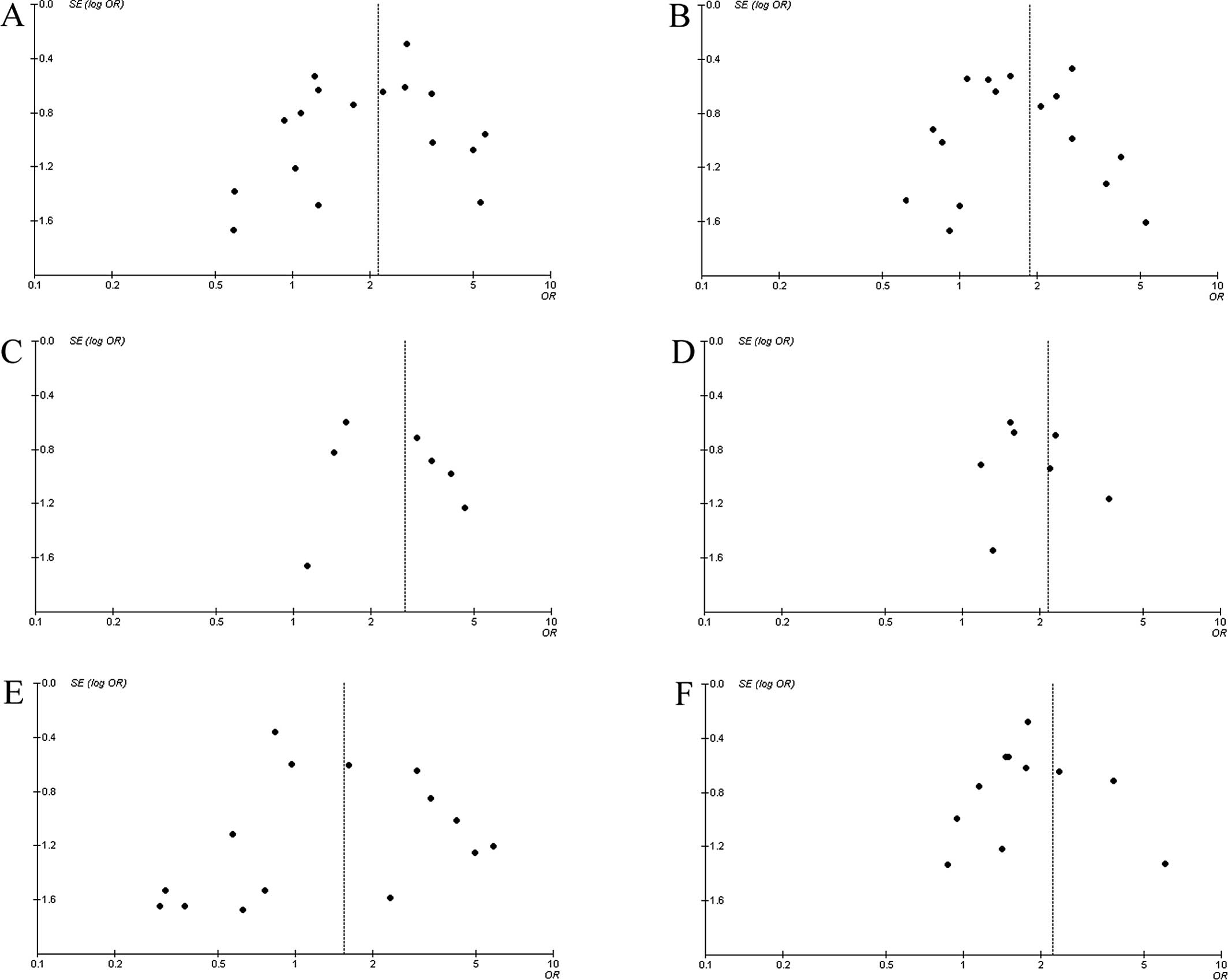
Publication bias. Funnel plots were used to
estimate the publication bias of the meta-analysis. As shown in
Fig. 3, the shape of the funnel
plot did not reveal obvious asymmetry.
Discussion
GC remains the second leading cause of
cancer-related mortality worldwide (33), in part because of its high rate of
metastasis and recurrence. It is critical to explore molecular
biomarkers to guide clinical decision-making with regard to the
treatment of GC. TA is thought to be a critical step in the
evolution of most tumor types (34–37),
including GC (38,39). Considerable clinical research has
been conducted with the aim of assessing the correlation between TA
expression and clinicopathological outcome in patients with GC, but
the results have been controversial. Several studies have shown
that the TA in GC tissues is related to depth of tumor invasion and
lymph node metastasis (25,29);
however, other studies found no association between clinical
outcome and TA (21,24).
Recently, many studies have indicated that hTERT is
the rate-limiting step in the activation of telomerase, and its
expression level is directly proportional to expression levels of
TA (40,41). In our analysis, TA was measured by
TRAP assay in 9 studies and TRAP-ELISA in 1 study, whereas hTERT
was measured by RT-PCR in 2 studies, qRT-PCR in 1 study, a
membrane-array assay in 1 study and IHC in 4 studies. TA expression
was detected using all of these methods. The cutoff value of TA
positivity obtained from different methods was recognized as a
standard to assess TA expression. The pooled statistical data
showed that the prognostic utility of TA was consistent with
clinical characteristics, including depth of tumor invasion
(p=0.0007), lymph node status (p=0.007), distant metastasis
(p=0.0002) and TNM stage (p=0.005). In addition, we identified and
evaluated the association of TA expression with tumor size and
tumor grade. Our findings also showed that there was a strong
association between high TA expression potential and tumor size
(p=0.002), but not tumor grade (p=0.26). Combining several
independent studies, our estimates supported the idea that TA and
hTERT overexpression were strongly related to gastric tumor
invasion and metastasis. Therefore, the role telomerase plays in
inducing tumor progression is not only based on its well-documented
effects on tumor proliferation rate (42).
Our findings were consistent with the reports on TA
expression in melanoma (43),
breast cancer (44),
hepatocellular carcinoma (45) and
giant-cell tumors of the bone (7),
in which TA contributed to the poor survival of patients. We
demonstrated at the cellular level that hTERT transfection in U2OS
(a hTERT-negative cell line) re-activated its telomerase activity
and further promoted its invasive and metastatic potential. The
mechanism that enhances these malignant phenotypes may be
correlated with the increasing adhesive ability of these tumor
cells to the extracellular matrix (46). High TA may activate the glycolytic
pathway to promote tumor growth and metastasis (43). TA suppression may render cells more
susceptible to anchorage-independent growth inhibition, and
unstable tumors require a higher TA level to prevent genomic
deterioration and to induce more aggressive cancer cells during
carcinogenesis (7). The mechanism
described above offered a possible interpretation of the observed
strong statistical association between TA overexpression and tumor
metastasis.
Caution must be taken to note the limitations of
this study. First, most of the patients with GC enrolled in our
meta-analysis came from Asia, which may be attributed to the
apparent decrease in the incidence and mortality rates for GC in
the past 50 years in Western countries compared to Japan and China
(47). Second, reports in
languages other than English were excluded. The risk of language
bias had to be considered, but may not result in any notable bias
in the assessment of interventional effectiveness (48). Third, data containing negative
results may be less likely published, although we took care to
access all available data. Fourth, the eligible data do not assess
whether TA may influence the prognosis of patients according to
distinct therapeutic schedules.
Based on the results of this analysis, we conclude
that telomerase overexpression is not only involved in the
carcinogenesis in the initiation of GC, but also promotes the
invasion and metastasis of GC. These results improve our
understanding of telomerase as a potentially important molecular
target in clinical diagnostics and therapeutics of gastric
cancer.
Abbreviations:
|
hTERT
|
human telomerase reverse
transcriptase
|
|
GC
|
gastric carcinoma
|
|
TA
|
telomerase activity
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
Acknowledgements
The authors would like to thank all of
the patients and clinical investigators who were involved in the
studies selected for this meta-analysis.
References
|
1.
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5(Suppl 1): 5–11. 2002. View Article : Google Scholar
|
|
2.
|
Yokota J: Tumor progression and
metastasis. Carcinogenesis. 21:497–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Greider CW and Blackburn EH: A telomeric
sequence in the RNA of Tetrahymena telomerase required for telomere
repeat synthesis. Nature. 337:331–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sealey DC, Zheng L, Taboski MA,
Cruickshank J, Ikura M and Harrington LA: The N-terminus of hTERT
contains a DNA-binding domain and is required for telomerase
activity and cellular immortalization. Nucleic Acids Res.
38:2019–2035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Catarino R, Araujo A, Coelho A, Gomes M,
Nogueira A, Lopes C and Medeiros RM: Prognostic significance of
telomerase polymorphism in non-small cell lung cancer. Clin Cancer
Res. 16:3706–3712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Poremba C, Heine B, Diallo R, Heinecke A,
Wai D, Schaefer KL, Braun Y, Schuck A, Lanvers C, Bankfalvi A, et
al: Telomerase as a prognostic marker in breast cancer:
high-throughput tissue microarray analysis of hTERT and hTR. J
Pathol. 198:181–189. 2002. View Article : Google Scholar
|
|
7.
|
Horvai AE, Kramer MJ, Garcia JJ and
O'Donnell RJ: Distribution and prognostic significance of human
telomerase reverse transcriptase (hTERT) expression in giant-cell
tumor of bone. Mod Pathol. 21:423–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: a
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar
|
|
9.
|
Little J, Bradley L, Bray MS, Clyne M,
Dorman J, Ellsworth DL, Hanson J, Khoury M, Lau J, O'Brien TR, et
al: Reporting, appraising, and integrating data on genotype
prevalence and gene-disease associations. Am J Epidemiol.
156:300–310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Whiting PF, Weswood ME, Rutjes AW, Reitsma
JB, Bossuyt PN and Kleijnen J: Evaluation of QUADAS, a tool for the
quality assessment of diagnostic accuracy studies. BMC Med Res
Methodol. 6:92006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cohen J: A coefficient of agreement for
nominal scales. Educ Psychol Meas. 20:37–46. 1960. View Article : Google Scholar
|
|
13.
|
Greenland S and Robins J: Estimation of a
common effect parameter from sparse follow-up data. Biometrics.
41:55–68. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang SM, Fang DC, Luo YH, Lu R, Battle PD
and Liu WW: Alterations of telomerase activity and terminal
restriction fragment in gastric cancer and its premalignant
lesions. J Gastroenterol Hepatol. 16:876–882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hu X, Wu H, Zhang S, Yuan H and Cao L:
Clinical significance of telomerase activity in gastric carcinoma
and peritoneal dissemination. J Int Med Res. 37:1127–1138. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wu CH, Lin SR, Yu FJ, Wu DC, Pan YS, Hsieh
JS, Huang SY and Wang JY: Development of a high-throughput
membrane-array method for molecular diagnosis of circulating tumor
cells in patients with gastric cancers. Int J Cancer. 119:373–379.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang W, Luo HS and Yu BP: Expression of
NF-kappaB and human telomerase reverse transcriptase in gastric
cancer and precancerous lesions. World J Gastroenterol. 10:177–181.
2004.PubMed/NCBI
|
|
19.
|
Yoo J, Park SY, Kang SJ, Kim BK, Shim SI
and Kang CS: Expression of telomerase activity, human telomerase
RNA, and telomerase reverse transcriptase in gastric
adenocarcinomas. Mod Pathol. 20:700–707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yasui W, Tahara H, Tahara E, Fujimoto J,
Nakayama J, Ishikawa F and Ide T: Expression of telomerase
catalytic component, telomerase reverse transcriptase, in human
gastric carcinomas. Jpn J Cancer Res. 89:1099–1103. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kameshima H, Yagihashi A, Yajima T,
Kobayashi D, Denno R, Hirata K and Watanabe N: Helicobacter
pylori infection: augmentation of telomerase activity in cancer
and noncancerous tissues. World J Surg. 24:1243–1249. 2000.
View Article : Google Scholar
|
|
22.
|
Gigek CO, Leal MF, Silva PN, Lisboa LC,
Lima EM, Calcagno DQ, Assumpcao PP, Burbano RR and Smith Mde A:
hTERT methylation and expression in gastric cancer. Biomarkers.
14:630–636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hu LH, Chen FH, Li YR and Wang L:
Real-time determination of human telomerase reverse transcriptase
mRNA in gastric cancer. World J Gastroenterol. 10:3514–3517.
2004.PubMed/NCBI
|
|
24.
|
Ahn MJ, Noh YH, Lee YS, Lee JH, Chung TJ,
Kim IS, Choi IY, Kim SH, Lee JS and Lee KH: Telomerase activity and
its clinicopathological significance in gastric cancer. Eur J
Cancer. 33:1309–1313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hiyama E, Yokoyama T, Tatsumoto N, Hiyama
K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW and
Matsuura Y: Telomerase activity in gastric cancer. Cancer Res.
55:3258–3262. 1995.PubMed/NCBI
|
|
26.
|
Tahara H, Kuniyasu H, Yokozaki H, Yasui W,
Shay JW, Ide T and Tahara E: Telomerase activity in preneoplastic
and neoplastic gastric and colorectal lesions. Clin Cancer Res.
1:1245–1251. 1995.PubMed/NCBI
|
|
27.
|
Liu Xl, Chen P and Wei JM: The
relationship between CK20 mRNA and hTERT mRNA expression in
peripheral blood of patients with gastric carcinoma and tumor
micrometastasis. Chin J Clin Oncol. 35:1286–1289. 2008.
|
|
28.
|
Okusa Y, Shinomiyo N, Ichikura T and
Mochizuki H: Correlation between telomerase activity and DNA ploidy
in gastric cancer. Oncology. 55:258–264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shin JH, Chung J, Kim HO, Kim YH, Hur YM,
Rhim JH, Chung HK, Park SC, Park JG and Yang HK: Detection of
cancer cells in peripheral blood of stomach cancer patients using
RT-PCR amplification of tumour-specific mRNAs. Aliment Pharmacol
Ther. 16(Suppl 2): 137–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mori N, Oka M, Hazama S, Iizuka N,
Yamamoto K, Yoshino S, Tangoku A, Noma T and Hirose K: Detection of
telomerase activity in peritoneal lavage fluid from patients with
gastric cancer using immunomagnetic beads. Br J Cancer.
83:1026–1032. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR,
Wang JP, Zheng ZQ and Wang L: Telomerase activity in gastric cancer
and its clinical implications. World J Gastroenterol. 5:316–319.
1999.PubMed/NCBI
|
|
32.
|
Gümüx-Akay G, Ünal AE, Bayar S, Karadayi
K, Elhan AH, Sunguroqlu A and Tükün A: Telomerase activity could be
used as a marker for neoplastic transformation in gastric
adenocarcinoma: but it does not have a prognostic significance.
Genet Mol Res. 6:41–49. 2007.(In Bulgarian).
|
|
33.
|
Bulanov D: Gastric Cancer – Current state
of the problem. Part I. Epidemiology. Pathology. Classification.
Staging. Khirurgiia (Sofia). 48–59. 2007.(In Bulgarian).
|
|
34.
|
Beisner J, Dong M, Taetz S, Nafee N,
Griese EU, Schaefer U, Lehr CM, Klotz U and Murdter TE:
Nanoparticle mediated delivery of 2′-O-methyl-RNA leads to
efficient telomerase inhibition and telomere shortening in human
lung cancer cells. Lung Cancer. 68:346–354. 2010.
|
|
35.
|
Lu L, Zhang C, Zhu G, Irwin M, Risch H,
Menato G, Mitidieri M, Katsaros D and Yu H: Telomerase expression
and telomere length in breast cancer and their associations with
adjuvant treatment and disease outcome. Breast Cancer Res.
13:R562011. View
Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Nakamura M, Saito H, Ebinuma H,
Wakabayashi K, Saito Y, Takagi T, Nakamoto N and Ishii H: Reduction
of telomerase activity in human liver cancer cells by a histone
deacetylase inhibitor. J Cell Physiol. 187:392–401. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Capezzone M, Cantara S, Marchisotta S,
Filetti S, De Santi MM, Rossi B, Ronga G, Durante C and Pacini F:
Short telomeres, telomerase reverse transcriptase gene
amplification, and increased telomerase activity in the blood of
familial papillary thyroid cancer patients. J Clin Endocrinol
Metab. 93:3950–3957. 2008. View Article : Google Scholar
|
|
38.
|
Miyachi K, Fujita M, Tanaka N, Sasaki K
and Sunagawa M: Correlation between telomerase activity and
telomeric-repeat binding factors in gastric cancer. J Exp Clin
Cancer Res. 21:269–275. 2002.PubMed/NCBI
|
|
39.
|
Gumus-Akay G, Elhan AH, Unal AE,
Demirkazik A, Sunguroglu A and Tukun A: Effects of genomic
imbalances on telomerase activity in gastric cancer: clues to
telomerase regulation. Oncol Res. 17:455–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Usselmann B, Newbold M, Morris AG and
Nwokolo CU: Telomerase activity and patient survival after surgery
for gastric and oesophageal cancer. Eur J Gastroenterol Hepatol.
13:903–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Poole JC, Andrews LG and Tollefsbol TO:
Activity, function, and gene regulation of the catalytic subunit of
telomerase (hTERT). Gene. 269:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Jin X, Beck S, Sohn YW, Kim JK, Kim SH,
Yin J, Pian X, Kim SC, Choi YJ and Kim H: Human telomerase
catalytic subunit (hTERT) suppresses p53-mediated anti-apoptotic
response via induction of basic fibroblast growth factor. Exp Mol
Med. 42:574–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Bagheri S, Nosrati M, Li S, Fong S,
Torabian S, Rangel J, Moore DH, Federman S, Laposa RR, Baehner FL,
et al: Genes and pathways downstream of telomerase in melanoma
metastasis. Proc Natl Acad Sci USA. 103:11306–11311. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Hochreiter AE, Xiao H, Goldblatt EM,
Gryaznov SM, Miller KD, Badve S, Sledge GW and Herbert BS:
Telomerase template antagonist GRN163L disrupts telomere
maintenance, tumor growth, and metastasis of breast cancer. Clin
Cancer Res. 12:3184–3192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Oh BK, Kim H, Park YN, Yoo JE, Choi J, Kim
KS, Lee JJ and Park C: High telomerase activity and long telomeres
in advanced hepatocellular carcinomas with poor prognosis. Lab
Invest. 88:144–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Yu ST, Chen L, Wang HJ, Tang XD, Fang DC
and Yang SM: hTERT promotes the invasion of telomerase-negative
tumor cells in vitro. Int J Oncol. 35:329–336.
2009.PubMed/NCBI
|
|
47.
|
Palli D: Epidemiology of gastric cancer.
Ann Ist Super Sanita. 32:85–99. 1996.
|
|
48.
|
Soler RE, Leeks KD, Razi S, Hopkins DP,
Griffith M, Aten A, Chattopadhyay SK, Smith SC, Habarta N, Goetzel
RZ, et al: A systematic review of selected interventions for
worksite health promotion. The assessment of health risks with
feedback. Am J Prev Med. 38:S237–S262. 2010. View Article : Google Scholar : PubMed/NCBI
|