Introduction
Glioblastoma, a brain tumor that develops from glial
cells, mostly in adults, is considered to be the most malignant
form of gliomas, which represent half of all primitive brain
tumors. Its poor prognosis is related to the high malignant status
and the absence of effective therapies, explained by their
intrinsic resistance to many cytotoxic agents (1,2). As
the development of new therapeutic strategies is of crucial
importance, the mechanisms underlying the resistance to apoptosis
induction need to be elucidated in order to define potential
strategies involving therapeutic association of molecules, such as
those related to Fas-mediated apoptosis. The Fas receptor, a member
of the tumor-necrosis factor receptor superfamily, mediates
apoptosis in certain sensitive cells when it is activated by Fas
ligand (FasL). Therefore, it has been implicated in tumor
regression, such as in glioma, especially when used in combination
with other therapeutic molecules, such as etoposide and
dexamethasone as reported in an experimental model of glioblastoma
in the nude rat (3). However,
potential residual tumor cells may reduce survival depending on
fine interactions that counterbalance the Fas-signaling pathway.
Accordingly, in another malignant cell line, a neuroblastoma line,
we reported that the Fas receptor interacts with the
p75NTR receptor inhibiting Fas-induced apoptosis
(4). This resistance to cell death
was effective through co-activation of p75NTR, another
cell death receptor for neurotrophins, and the Fas receptor, by
their natural ligands, BDNF and FasL, respectively, allowing that
the Fas and p75NTR pathways are interactive (4). This dual function of
p75NTR in apoptosis induction or cell growth and
survival is in part due to its transcriptional function resulting
from sequential α and γ-secretase actions that cleave
p75NTR in the 24-kDa C-terminal fragment and a 19-kDa
intracellular domain (ICD) fragment. ICD is translocated into the
nucleus (5), where it modulates
transcriptional events (6). Recent
studies have demonstrated that p75NTR is a critical
regulator of glioma invasion (7)
endogenously expressed in patient tumors (8). Its biological function in invasion
and tumor growth is related to its proteolytic processing by
γ-secretase, leading to invasiveness and cell migration and their
suppression by γ-secretase inhibitors (8). In addition, γ-secretase inhibitors
were found to increase radiosensitivity of glioblastoma, and induce
reduction in CD133+-related stem cells (9). As proteolysis through γ-secretase
activity is a highly conserved mechanism responsible for
intramembrane cleavage of several proteins, such as β-amyloid
precursor protein, ErbB4, CD44 and Notch receptor (10), it was speculated by Lin et
al that γ-secretase inhibitors inhibit CD133+
glioblastoma cell lines through inhibition of the Notch signaling
pathway (9).
Notch signaling plays a key role in the normal
development of many tissues and cell types, through effects on
differentiation, survival and/or proliferation that are highly
dependent on signal strength and cellular context (11). Perturbations in the regulation of
cellular homeostasis trigger malignant transformation. Moreover,
Notch1 signaling potentially contributes to cancer development in
several different pathways by activating mitogen-activated protein
kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating
N-cadherin, or by acting on Myc transcription (12).
Accumulating data indicate that deregulated Notch or
p75NTR activities may be involved in human cancer, such
as glioblastoma (8). In order to
improve glioblastoma therapy and patient care, diagnostic and
prognostic factors are required.
The goal of the present study was to determine the
respective role of p75NTR and Notch in the resistance to
Fas-induced apoptosis in the U-87 MG glioblastoma cell line. Using
the γ-secretase inhibitor, we searched for a modulation of
Fas-induced apoptosis dependent on p75NTR-Fas receptor
interaction.
We reported herein that the γ-secretase inhibitor
diminished apoptosis through Fas activation and we hypothesized
that the suppression of the proteolysis of p75NTR
interferes with the Fas-p75NTR recruitment of
apoptosis.
Materials and methods
Cell line culture and treatments
U-87 MG human glioblastoma cells (ATCC, Manassas,
VA, USA) were cultured in MEM with Earl’s salts (Invitrogen)
supplemented with 10% decomplemented foetal calf serum (FCS;
Seromed, Invitrogen), 1.5 g/l sodium bicarbonate, 1% non-essential
amino acids, sodium pyruvate (1 mM), penicillin (50 U/ml),
streptomycin (50 μg/ml), L-glutamine (2 mM) and fungizone
(0.1%) (Invitrogen). Cells were grown in 25-cm2 flasks
(Nunc, France) at 37°C in a humidified 5% CO2-95% air
incubator. At subconfluence, cells were always recovered with
versene (Invitrogen) and cultured in Lab-Tek chamber slides (Nunc)
at 104 cells/well or in 6-well plates (Nunc) at 105 cells/well for
48 h and were treated for 24 h with 7C11, an agonistic anti-Fas
monoclonal antibody (mAb) at 100 ng/ml (Beckman Coulter Inc.,
Fullerton, CA, USA), the γ-secretase inhibitor at 1 μM
(Calbiochem, France) or both molecules.
Flow cytometry
U-87 MG cells were cultured in 6-well plates
(105 cells/well) at basal state or stimulated by
agonistic anti-Fas mAb or a γ-secretase inhibitor or both molecules
during 24 h. U-87 MG cells were first released with versene
permeabilized or not using Triton X-100 (0.1%; Promega, France) and
resuspended in 10% FCS/90% versene with either rabbit anti-Notch1
(C-20 or H131, 1/100; Santa Cruz Biotechnology), anti-Notch2
(25–255, 1/100; Santa Cruz Biotechnology), anti-Delta1 (H-265,
1/100; Santa Cruz Biotechnology), anti-Delta4 (H-70, 1/100; Santa
Cruz Biotechnology), anti-Jagged1 (H-114, 1/100; Santa Cruz
Biotechnology) or anti-Jagged2 (H-143, 1/100; Santa Cruz
Biotechnology) polyclonal antibodies (Abs). Two anti-Fas mAb
phycoerythrin (PE)-conjugated recognizing the N- or C-terminus
domain (UB2, 1/5; Immunotech, Marseille, France; or B-10, 1/50;
Santa Cruz Biotechnology) were used. p75NTR expression
was studied with an anti-p75NTR polyclonal Abs (H-137,
1/20; Santa Cruz Biotechnology) directed against the extracellular
domain of p75NTR. Immature cell marker, an anti-vimentin
mAb (1/50; Santa Cruz Biotechnology) was also evaluated. Controls
were performed with rabbit irrelevant Ig (Santa Cruz Biotechnology)
or mouse irrelevant Ig as isotypic controls. After a 30-min
incubation on ice, non-conjugated-rabbit Abs were detected with a
swine anti-rabbit Ig fluorescein isothiocyanate (FITC)-conjugated
antibody (Dako-Cytomation, Trappes, France) diluted at 1/100, in
10% FCS in versene during 30 min on ice. Finally, cells were
resuspended in 10% FCS/90% versene. Each analysis was performed on
at least 10,000 cells and repeated three times. Relative
fluorescence intensity was measured by fluorescence-activated cell
sorter analysis (Cytomics FC200; Beckman Coulter and LSR Fortessa,
BD).
Assessment of in vitro apoptosis
induction
To determine the apoptotic effect of Fas activation,
cells were stimulated for 24 h with 7C11 mAb or a γ-secretase
inhibitor alone or with both molecules. Two methods were used: i)
Soluble cytoplasmic nucleosome detection. After a 24-h treatment of
cells as described above, the rate of apoptosis was measured using
cell death detection ELISA Plus (Roche Diagnostic, Meylan, France)
according to the manufacturer’s instructions. ii) Annexin-V
staining. After two washes in PBS, cells were incubated with
Annexin V-FITC (Beckman Coulter) solution (a marker of
phosphatidylserine exposure to the cell membrane during early
apoptotic process) containing propidium iodide (a DNA-intercalating
dye to detect necrotic cells) for 15 min on ice. Cells were washed
according to the manufacturer’s instructions and analysis was
directly performed by flow cytometry (Cytomics FC200; Beckman
Coulter).
Cell cycle analysis
After a 24-h stimulation with 7C11, cell cycle
analysis was performed using propidium iodide cell incorporation
during 15 min and flow cytometric analysis (Cytomics FC200).
Statistical analyses
Each assay was performed at least in triplicate. For
direct comparison of the apoptosis level according to the different
conditions of treatment (Basal, 7C11, γ-secretase inhibitor and
7C11 + γ-secretase inhibitor), statistical significance was
determined by one-way analysis of variance with Statview 5.0
software. A value of p<0.05 was considered to denote statistical
significance.
Results
Fas and p75NTR membrane
expression in the U-87 MG cell line
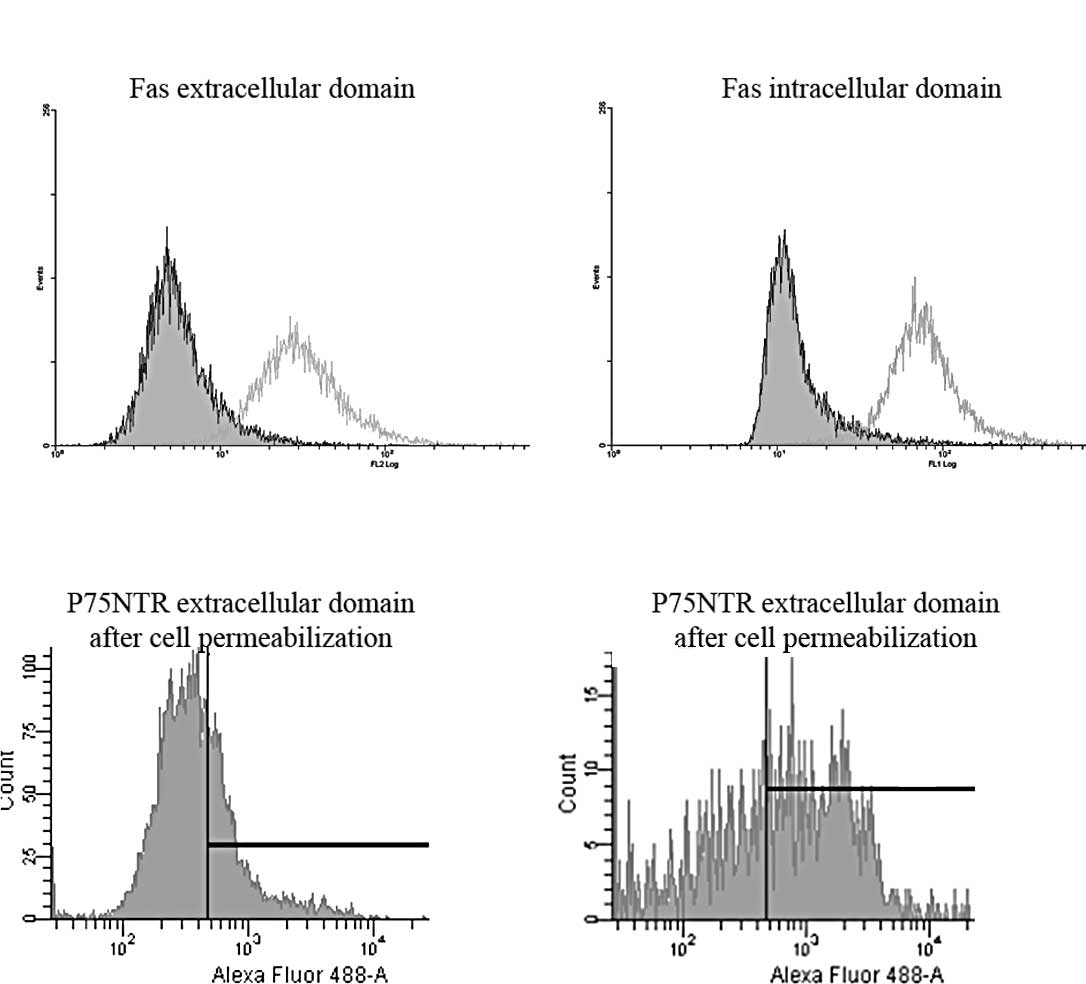
Flow cytometry was performed using anti-Fas and
p75NTR extracellular domain antibodies. As expected, Fas
was detected at the membrane level (3) with ∼100% of Fas-expressing cells
(Fig. 1A). To determine that the
Fas receptor was the full length receptor, potentially functional,
and not a truncated form of Fas, staining was performed using
specific mAb directed against anti-intracellular domain of Fas,
recognizing the death domain. Data showed similar patterns of
staining to those obtained with extracellular anti-Fas mAb,
assessing that Fas expressed in U-87 MG cells deals with the
full-length Fas protein containing the death domain (Fig. 1B).
In parallel, we searched for p75NTR
expression in U-87 MG cells. By contrast, by flow cytometry only
15% of cells expressed p75NTR at the membrane level,
while 70% was detected after cell permeabilization (Fig. 1C and D), as previously described
(13). In order to search for
potential interactions with the Notch pathway during Fas
activation, we studied Notch expression in U-87 MG cells.
Notch1, 2 and their ligands are
sequestered in the U-87 MG cells
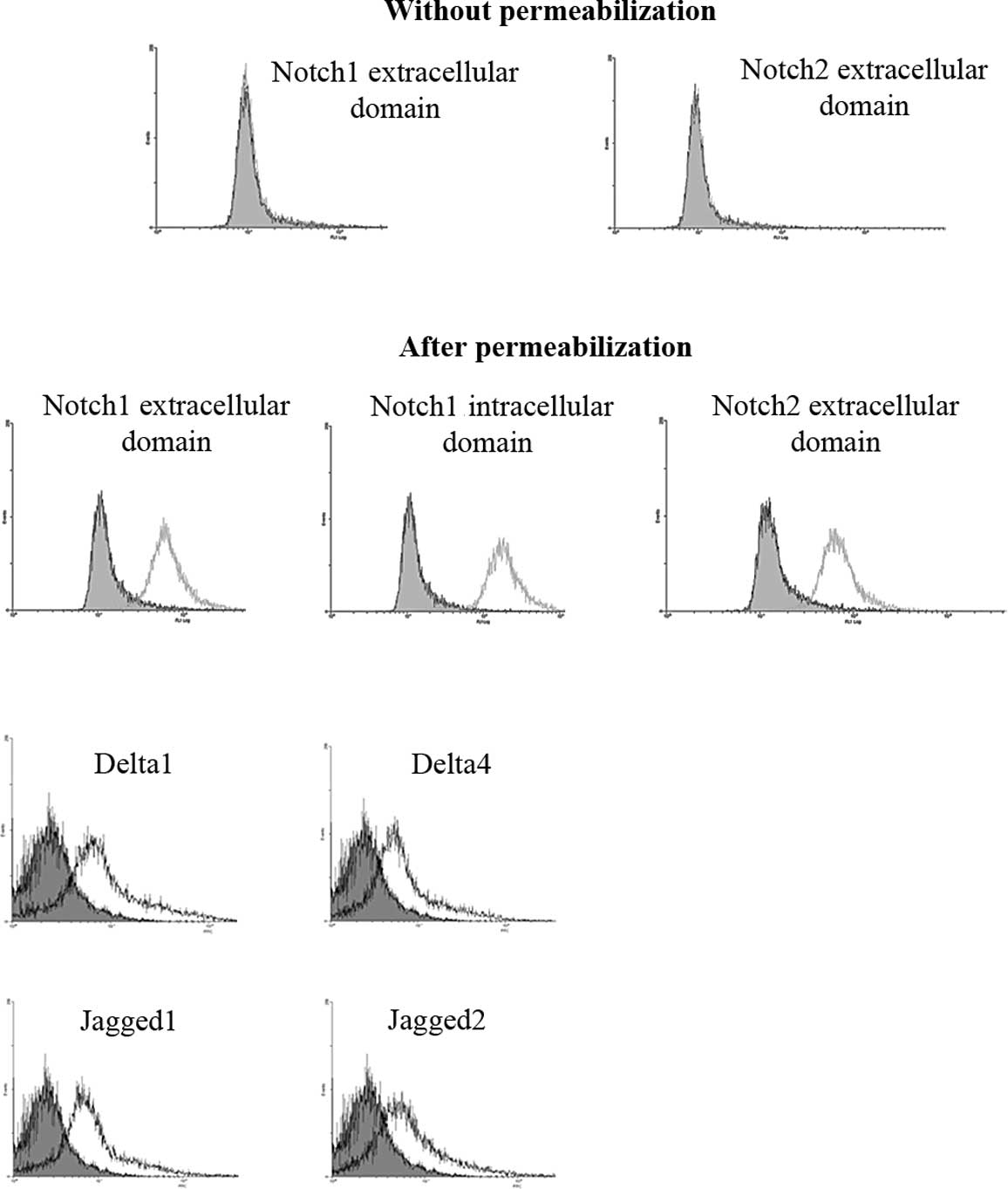
Using specific antibodies directed against
extracellular domains of Notch by flow cytometry (Fig. 2) or immunocytochemical (data not
shown) methods, we did not detect Notch1 as well as Notch2
expression on the cell membrane (Fig.
2A). By contrast, Notch1 and Notch2 proteins were only detected
after cell permeabilization in U-87 MG cells. Their detection with
extracellular- and intracellular-specific anti-Notch Abs in U-87 MG
permeabilized cells confirmed that their full-length proteins were
sequestered into the cytoplasm (Fig.
2B). In addition, we confirmed by flow cytometry that Notch
ligands Delta 1 and 4 and Jagged 1 and 2 were also expressed in
intra-cellular compartments of U-87 MG cells (Fig. 2C).
Fas activation induces apoptosis without
modifying cell cycle, vimentin or Notch expression in the U-87 MG
cell line
After a 24-h incubation with the agonistic anti-Fas
mAb 7C11 (100 ng/ml), only 15% of cells were detected as apoptotic
expressing cleaved caspase-3, assessing that U-87 MG cells are
relatively resistant to apoptosis (Fig. 3A). In addition, using the same
experimental conditions, no variation in cell cycle was observed
(Fig. 3B). Vimentin, an immature
cell marker, was detected in U-87 MG cells (14) by flow cytometry without change of
its expression after anti-Fas 7C11 treatment (Fig. 3C).
Furthermore, Notch1 expression, the most implicated
receptor of the Notch family in glioblastoma was studied in
Fas-activating conditions in U-87 MG cells treated with 7C11 (100
ng/ml) during 24 h. Fas activation did not modify Notch1 expression
that remained sequestered in the U-87 MG cells as under basal
conditions (Fig. 3D).
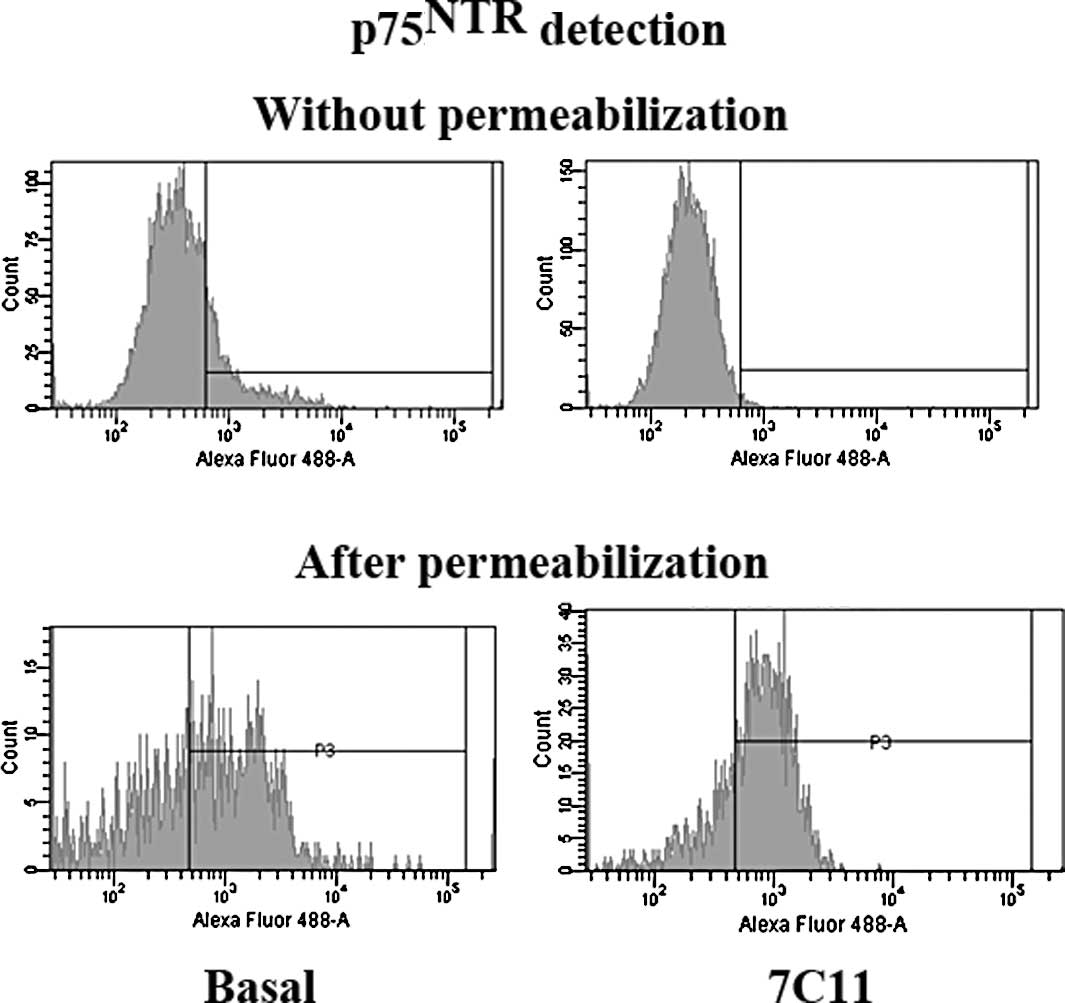
Fas activation tends to increase
p75NTR intracellular expression
While 20% of p75NTR was detected at the
cell surface under a basal condition, activation of the Fas
receptor by agonistic anti-Fas mAb 7C11, suppressed
p75NTR membranous expression in all U-87 MG cells.
Therefore, Fas-activation tended to increase (∼10%) the percentage
of cells expressing p75NTR in the intracellular
compartment, compared to that in basal conditions (a total of 80%
of cells expressed intracellular p75NTR vs. 70% under
basal conditions) (Fig. 4).
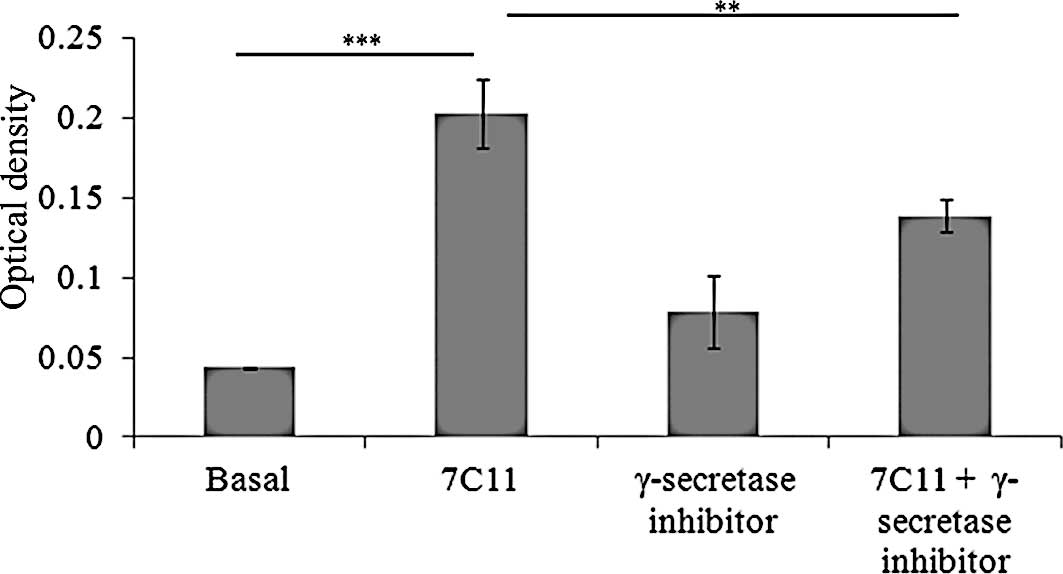
γ-secretase inhibitor modifies
Fas-induced apoptosis by p75NTR inhibiting pathway
To demonstrate that p75NTR interacts with
Fas-induced apoptosis, the γ-secretase inhibitor was incubated with
or without 7C11 (100 ng/ml), and the apoptotic rate was determined
by measurement of cytoplasmic soluble nucleosomes (Fig. 5) or membranous staining with
Annexin V (data not shown). Fas activation by a 24-h exposure to
agonistic anti-Fas mAb significantly induced apoptosis compared to
the basal apoptotic level (p<0.0001) (Fig. 5). The γ-secretase inhibitor did not
modify this apoptotic level in basal conditions. However,
concomitant treatment of U-87 MG cells with agonistic anti-Fas mAb
and the γ-secretase inhibitor significantly decreased cell
apoptosis compared to 7C11 alone (p=0.007) (Fig. 5).
Discussion
We demonstrated herein that γ-secretase inhibitors
decreased Fas-induced apoptosis in the human U-87 MG glioblastoma
cell line. These cells expressed the full-length receptor of the
Fas death receptor and its activation by an agonistic anti-Fas mAb
induced significant apoptosis. Another death receptor,
p75NTR, the low affinity receptor for neurotrophins, is
known to be linked to the Fas receptor through a common signaling
pathway as demonstrated in a neuroblastoma cell line. Indeed, when
stimulated alone, i.e., Fas by FasL and p75NTR by the
neurotrophin BDNF, each receptor induced cell death, whereas their
concomitant activation was found to suppress apoptotic cell death,
depending on simultaneous caspase-8 and sphingomyelinase activation
(4). However, BDNF-induced cell
death through p75NTR activation alone was found to be
inefficient in the U-87 MG glioblastoma cell line containing
p75NTR receptor sequestered in Golgi apparatus (13).
By contrast, p75NTR was found to be
involved as an activator of tumor invasion, mediated through
intra-membranous proteolysis by α and γ-secretase, releasing the
intracellular domain that binds the nucleus and activates signal
transduction (8,6). This tumor invasion was inhibited by
γ-secretase inhibitor in vitro and in experimental models in
a BDNF-dependent mechanism (7).
Nevertheless, this antitumor effect was not evaluated under cell
death activation through Fas activation in glioblastoma cells.
Indeed, the interaction of Fas and p75NTR (4) during cell-induced apoptosis may be
modified. On the other hand, γ-secretase is known to cleave other
proteins, including Notch receptors (10).
Notch signaling is well known to play a key role
during normal and neoplastic development (15,16).
Perturbation of the Notch signaling pathway may often lead to the
process of carcinogenesis, including glioblastoma (17). The Notch signaling pathway requires
γ-secretase cleavage resulting in Notch intracellular domain
translocation in the nucleus and activation of transcription. For
this reason, we investigated Notch1 and 2 expression in U-87 MG
cells. Indeed, intracellular expression of Notch1, 2 and their
ligands was detected in the U-87 MG cell line without membranous
location and was not modified by Fas-activation.
In a human neuroblastoma cell line,
p75NTR was demonstrated to induce cell death after BDNF
stimulation and to interact with the Fas receptor (4). Likewise, our results suggest a
potential interaction between Fas and p75NTR in the U-87
MG human glioblastoma cell line. Indeed, γ-secretase inhibitor
significantly decreased apoptosis induced through Fas activation.
These results suggest that γ-secretase is implicated in the cell
death induced by Fas activation through p75NTR cleavage
and signaling. Such p75NTR involvement in apoptosis was
described in primary neuronal cell cultures in which
p75NTR cleavage by γ-secretase resulted in nuclear
translocation of the intracellular domain and apoptotic cell death
(18).
The present results are in agreement with Fas and
p75NTR interactions in glioblastoma cells. Therefore we
hypothesized that the γ-secretase inhibitor diminishes Fas-induced
apoptosis depending on its interaction with p75NTR
through its intracellular domain. Such mechanisms interfering with
Fas activation will be of great importance in the search for cell
death-inducing treatment. Indeed, whereas glioblastoma cells are
spontaneously resistant to cell death induction, it was previously
demonstrated that a combination of Fas activation with concomitant
etoposide plus dexamethasone exhibits antiproliferative and
antitumor properties in experimental glioblastoma in the nude rat
(3). We hypothesize that Fas and
p75NTR activation are of major importance in apoptosis
induction of glioma cells, and that the γ-secretase inhibitor may
interfere with Fas-activating treatment.
Acknowledgements
This study was supported by grants
from the Conseil Régional du Limousin, Ligue Nationale Contre le
Cancer (comités de la Corrèze et de la Haute-Vienne).
References
|
1.
|
Weller M, Rieger J, Grimmel C, Van Meir
EG, De Tribolet N, Krajewski S, Reed JC, von Deimling A and
Dichgans J: Predicting chemoresistance in human malignant glioma
cells: the role of molecular genetic analyses. Int J Cancer.
79:640–644. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yount GL, Haas-Kogan DA, Levine KS, Aldape
KD and Israel MA: Ionizing radiation inhibits chemotherapy-induced
apoptosis in cultured glioma cells: implications for combined
modality therapy. Cancer Res. 58:3819–3825. 1998.
|
|
3.
|
Giraud S, Bessette B, Boda C, Lalloué F,
Petit D, Mathonnet M and Jauberteau MO: In vitro apoptotic
induction of human glioblastoma cells by Fas ligand plus etoposide
and in vivo anti-tumour activity of combined drugs in
xenografted nude rats. Int J Oncol. 30:273–281. 2007.
|
|
4.
|
Giraud S, Lautrette C, Bessette B, Decourt
C, Mathonnet M and Jauberteau MO: Modulation of Fas-induced
apoptosis by p75 neurotrophin receptor in a human neuroblastoma
cell line. Apoptosis. 10:1271–1283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jung KM, Tan S, Landman N, Petrova K,
Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao MV and Kim TW:
Regulated intramembrane proteolysis of the p75 neurotrophin
receptor modulates its association with the TrkA receptor. J Biol
Chem. 278:42161–42169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Parkhurst CN, Zampieri N and Chao MV:
Nuclear localization of the p75 neurotrophin receptor intracellular
domain. J Biol Chem. 285:5361–5368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Johnston AL, Lun X, Rahn JJ, Liacini A,
Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth
PA and Senger DL: The p75 neurotrophin receptor is a central
regulator of glioma invasion. PLoS Biol. 5:e2122007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wang L, Rahn JJ, Lun X, Sun B, Kelly JJ,
Weiss S, Robbins SM, Forsyth PA and Senger DL: Gamma-secretase
represents a therapeutic target for the treatment of invasive
glioma mediated by the p75 neurotrophin receptor. PLoS Biol.
6:e2892008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lin J, Zhang XM, Yang JC, Ye YB and Luo
SQ: γ-secretase inhibitor-I enhances radiosensitivity of
glioblastoma cell lines by depleting CD133+ tumor cells. Arch Med
Res. 41:519–529. 2010.
|
|
10.
|
Ebinu JO and Yankner BA: A RIP tide in
neuronal signal transduction. Neuron. 34:499–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Allenspach EJ, Maillard I, Aster JC and
Pear WS: Notch signaling in cancer. Cancer Biol Ther. 1:466–476.
2002. View Article : Google Scholar
|
|
12.
|
Callahan R and Raafat A: Notch signaling
in mammary gland tumorigenesis. J Mammary Gland Biol Neoplasia.
6:23–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Giraud S, Loum E, Bessette B, Mathonnet M
and Lalloué F: P75 neurotrophin receptor is sequestered in the
Golgi apparatus of the U-87 MG human glioblastoma cell line. Int J
Oncol. 38:391–399. 2011.PubMed/NCBI
|
|
14.
|
Bertrand J, Begaud-Grimaud G, Bessette B,
Verdier M, Battu S and Jauberteau MO: Cancer stem cells from a
human glioma cell line are resistant to Fas-induced apoptosis. Int
J Oncol. 34:717–727. 2009.PubMed/NCBI
|
|
15.
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jeffries S and Capobianco AJ: Neoplastic
transformation by Notch requires nuclear localization. Mol Cell
Biol. 20:3928–3941. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-Like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kenchappa RS, Zampieri N, Chao MV, Barker
PA, Teng HK, Hempstead BL and Carter BD: Ligand-dependent cleavage
of the P75 neurotrophin receptor is necessary for NRIF nuclear
translocation and apoptosis in sympathetic neurons. Neuron.
50:219–232. 2006. View Article : Google Scholar : PubMed/NCBI
|