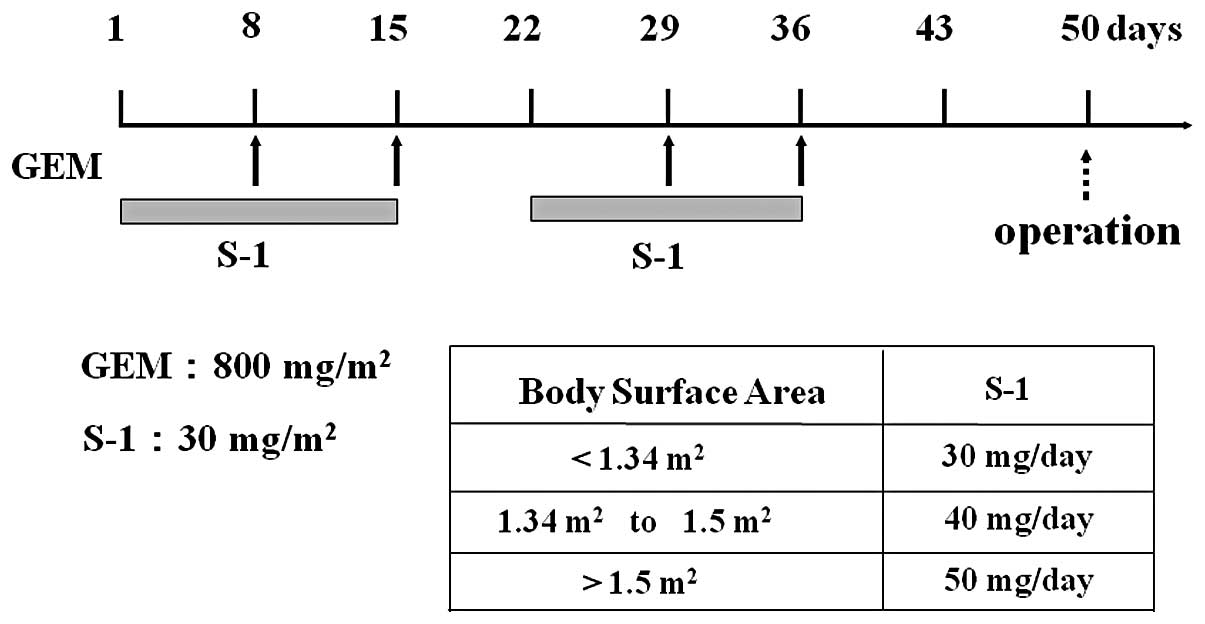
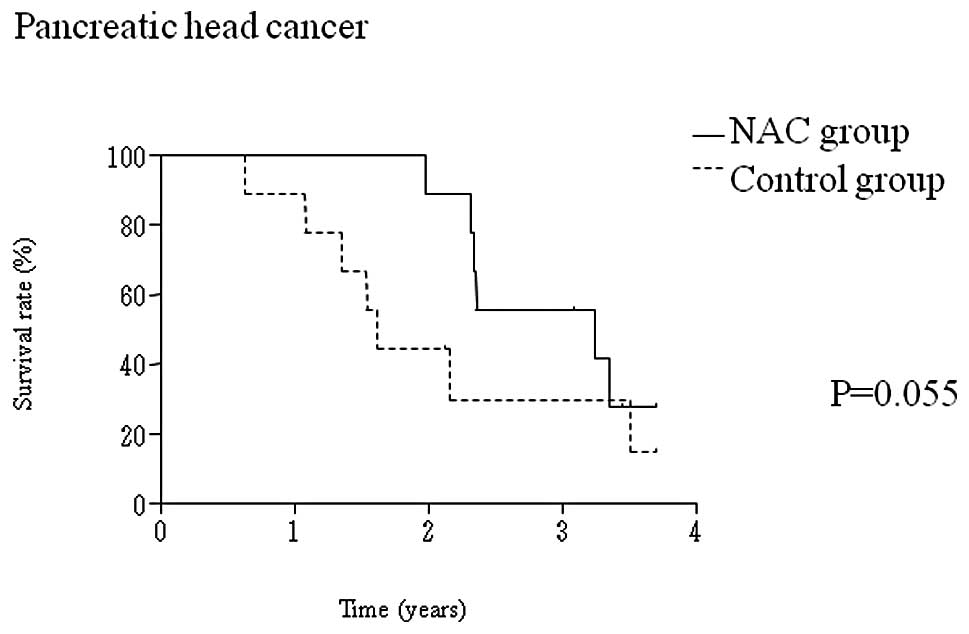
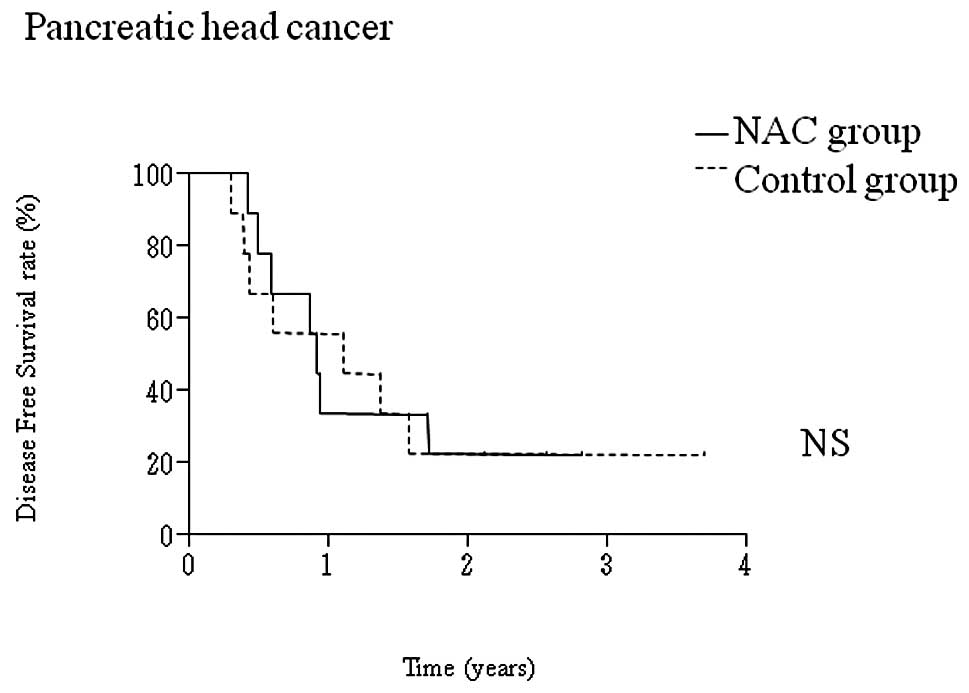
|
1.
|
Ministry of Health, Labour and Welfare:
The Dynamic Statistics of the Population in 2005. http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei05/hyo7/htmluri
(11 June 2009, data last accepted).
|
|
2.
|
Ishii H, Furuse J, Boku N, et al: Phase II
study of gemcitabine chemotherapy alone for locally advanced
pancreatic carcinoma: JCOG0506. Jpn J Clin Oncol. View Article : Google Scholar : 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nagakawa T, Kurachi M, Konishi K, et al:
Translateral retroperitoneal approach in radical surgery for
pancreatic carcinoma. Jpn J Surg. 12:229–233. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nagakawa T, Nagamori M, Futakami F, et al:
Result of extensive surgery for pancreatic carcinoma. Cancer.
77:640–645. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Miwa K, Ohta T, Shimizu K, et al:
Augmented regional pancreatoduodenectomy for pancreas head cancer:
combined resection of pancreas head and superior mesenteric artery
and vein. In: American College of Surgeons 90th Annual Clinical
Congress; Chicago. Isis 190. 2004
|
|
6.
|
Evans DB, Abbruzzese JL and Willett CG:
Cancer of the pancreas. Cancer: Principles and Practice of
Oncology. 6th edition. De Vita Hellman S and Rosenberg SA:
Lippincott, Williams and Wilkins; Philadelphia: pp. 1126–1161.
2001
|
|
7.
|
Oettle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar
|
|
8.
|
Klinlenbijl JH, Jeekel J, Sahmoud T, et
al: Adjuvant radiotherapy and 5-fluorouracil after curative
resection of cancer of the pancreas and periampullary region: phase
III trial of the EORTC Gastrointestinal Tract Cancer Cooperative
Group. Ann Surg. 230:776–782. 1999. View Article : Google Scholar
|
|
9.
|
Spitz FR, Abbruzzese JL, Lee JE, et al:
Preoperative and postoperative chemoradiation strategies in
patients treated with pancreaticoduodenectomy for adenocarcinoma of
the pancreas. J Clin Oncol. 15:928–937. 1997.PubMed/NCBI
|
|
10.
|
Yeo CJ, Abrams RA, Grochow LB, et al:
Pancreaticoduodenectomy for pancreatic adenocarcinoma:
postoperative adjuvant chemoradiation improves survival. A
prospective, single-institution experience. Ann Surg. 225:621–633.
1997. View Article : Google Scholar
|
|
11.
|
Shirasaka T, Shimamoto Y, Ohshimo H, et
al: Development of a novel form of an oral 5-fluorouracil
derivative (S-1) directed to the potentiation of the tumor
selective cytotoxicity of 5-fluorouracil by two biochemical
modulators. Anticancer Drugs. 7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tatsumi K, Fukushima M, Shirasaki T and
Fujii S: Inhibitory effects of pyrimidine, barbituric acid and
pyrimidine derivatives on 5-fluorouracil degradation in rat liver
extracts. Jpn J Cancer Res. 78:748–755. 1987.PubMed/NCBI
|
|
13.
|
Takechi T, Fujioka A, Matssushima E, et
al: Enhancement of the antitumor activity of 5-fluorouracil (5-FU)
by inhibiting dihydropyrimidine dehydrogenase activity (DPD) using
5-chloro-2,4-dihydroxypyridine (CDHP) in human tumor cells. Eur J
Cancer. 38:1271–1277. 2002. View Article : Google Scholar
|
|
14.
|
Peters GJ, van Groeningen CJ, Laurensse EJ
and Pinedo HM: A compression of 5-fluorouracil metabolism in human
colorectal cancer and colon mucosa. Cancer. 68:1903–1909. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Takechi T, Nakano K, Uchida J, et al:
Antitumor activity and low intestinal toxicity of S-1, a new
formulation of oral tegafur, in experimental tumor models in rats.
Cancer Chemother Pharmacol. 39:205–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nakamura K, Yamaguchi T, Ishihara T, et
al: Phase I trial of oral S-1 combined with gemcitabine in
metastatic pancreatic cancer. Br J Cancer. 92:2134–2139. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ueno H, Okusaka T, Ikeda M, et al: A phase
I study of combination chemotherapy with gemcitabine and oral S-1
for advanced pancreatic cancer. Oncology. 69:421–427. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nakamura K, Yamaguchi T, Ishihara T, et
al: Phase II trial of oral S-1 combined with gemcitabine in
metastatic pancreatic cancer. Br J Cancer. 1–5. 2006.PubMed/NCBI
|
|
19.
|
Vento P, Mustonen H, Joensuu T, et al:
Impact of preoperative chemoradiotherapy on survival in patients
with resectable pancreatic cancer. World J Gastroenterol.
21:2945–2951. 2007.PubMed/NCBI
|
|
20.
|
Palmer DH, Stocken DD, Hewitt H, et al: A
randomized phase 2 trial of neo-adjuvant chemotherapy in resectable
pancreatic cancer: gemcitabine alone versus gemcitabine combined
with cisplatin. Ann Surg Oncol. 14:2088–2096. 2007. View Article : Google Scholar
|
|
21.
|
Sato N, Kurashima K, Nagai H, et al: The
effect of adjuvant and neoadjuvant chemo (radio) therapy on
survival in 1,679 resected pancreatic carcinoma cases in Japan:
report of the national survey in the 34th annual meeting of
Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat
Surg. 16:485–492. 2009. View Article : Google Scholar
|
|
22.
|
Satoi S, Yanagimoto H, Toyokawa H, et al:
Surgical result after preoperative chemoradiation therapy for
patients with pancreatic cancer. Pancreas. 38:282–288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rauchwerger DR, Firby PS, Hedley DW and
Moore MJ: Equilibrative-sensitive nucleoside transporter and its
role in gemcitabine sensitivity. Cancer Res. 60:6075–6079.
2000.PubMed/NCBI
|
|
24.
|
Makino I, Kitagawa H, Ohta T, et al: Nerve
plexus invasion in pancreatic cancer. Spread patterns on
histopathologic and embryological analysis. Pancreas. 37:358–365.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Evans DB, Rich TA, Byrd DR, et al:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Esposito I, Kleeff J, Bergmann F, et al:
Most pancreatic cancer resections are R1 resections. Ann Surg
Oncol. 15:1651–1660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Nakahira S, Nakamori S, Tsujie M, et al:
Pretreatment with S-1, an oral derivative of 5-fluorouracil,
enhances gemcitabine effect in pancreatic cancer xenografts.
Anticancer Res. 28:179–186. 2008.PubMed/NCBI
|
|
28.
|
Takai S, Satoi S, Yanagimoto H, et al:
Neoadjuvant chemoradiation in patients with potentially resectable
pancreatic cancer. Pancreas. 36:26–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Golcher H, Brunner T, Grabenbauer G, et
al: Preoperative chemoradiation in adenocarcinoma of the pancreas.
A single centre experience advocating a new treatment strategy.
EJSO. 34:756–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Van Laethem JL, Hammel P, Mornex F, et al:
Adjuvant gemcitabine alone versus gemcitabine-based
chemoradiotherapy after curative resection for pancreatic cancer: A
randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J
Clin Oncol. 28:4450–4456. 2010.
|