Introduction
New targeted agents, i.e., targeted antibodies and
tyrosine kinase inhibitors, have been developed and used clinically
against various cancers (colorectal, lung, kidney and breast
cancer, etc.). As the antitumor activity of these agents originates
from anti-angiogenesis or the inhibition of growth signals, and
thus differs from that of traditional chemotherapeutic agents,
these targeted agents reportedly enhance antitumor activity when
used in combination with chemotherapy by means of their different
mechanisms (1–5).
Tegafur-gimeracil-oteracil (S-1), an oral
fluoropyrimidine, is composed of 1 M tegafur [a masked form of
5-fluorouracil (5-FU)], 0.4 M 5-chloro-2, 4-dihydroxypyrimidine
[gimeracil, a potent inhibitor of the 5-FU degradation enzyme
dihydropyrimidine dehydrogenase (DPD) in the liver and tumor
tissues] and 1 M potassium oxonate (oteracil, which mainly inhibits
the phosphorylation of 5-FU in the gastrointestinal tract)
(7). S-1 has been clinically shown
to be effective against various human cancers (8) and to have a potent antitumor
efficacy, with a low gastrointestinal toxicity, against various
types of cancers (9–11). Several combination chemotherapies
using S-1 and cytotoxic agents, including irinotecan (12), CDDP (13) and taxanes (14,15),
have been reported to be clinically effective. In addition, when
used in combination with the targeted agent gefitinib (16), the expression of the thymidylate
synthase (TS) protein and mRNA was decreased in a time-dependent
manner in the presence of 5 μM 5-FU in 3 human non-small cell lung
cancer (NSCLC) cell lines (Ma-1, Ma-53 and NII-H460) and the
antitumor activity of S-1 was potentiated by the combined therapy.
Furthermore, the exposure of the human epidermal growth factor
receptor 2 (HER2) amplification-positive human gastric cancer cell
lines (NCI-N87 and 4-1ST) to S-1 and epidermal HER2 inhibitors
(lapatinib and trastuzumab) resulted in the downregulation of TS
expression in a concentration-dependent manner. The antitumor
activity of S-1 was increased significantly in the combined therapy
in vivo (17).
In this study, we evaluated the effects of S-1 used
in combination with several targeted agents (three kinase
inhibitors and two antibodies) in vivo. This was achieved by
determining the tumor growth inhibition (TGI) ratio and the growth
delay period (GDP) and examining the correlation between antitumor
activity and the tumoral expression of DPD.
Materials and methods
Agents
Tegafur, gimeracil, oteracil and sunitinib malate
(sunitinib) were synthesized in our laboratory. Sorafenib tosilate
(sorafenib) and cetuximab were purchased from Kemprotec Ltd.
(Middlesbrough, UK) and Merck Serono Co., Ltd. (Zug, Switzerland),
respectively. Bevacizumab and erlotinib hydrochloride (erlotinib)
were purchased from Roche Ltd. (Basel, Switzerland).
Cremophor® was purchased from Nacalai Tesque, Inc.
(Kyoto, Japan). All other agents were commercially available
products of the highest grade.
Tumor xenografts
The human large cell lung cancer cell line Lu-99 was
purchased from the Health Science Research Resources Bank (Tokyo,
Japan) and the human lung differentiated adenocarcinoma cell line
PC-9 was provided by Showa University (Tokyo, Japan). The human
large cell lung cancer cell line NCI-H460 was purchased from the
American Type Culture Collection (Rockville, MD, USA). The human
colorectal cancer cell line DLD-1 was purchased from DS-Pharma
Biomedical Co. Ltd. (Osaka, Japan). The 5-FU-resistant human
colorectal cancer cell line, KM12C/5-FU, was established in our
laboratory, as described previously (18). The human breast cancer cell line
MX-1, the colorectal cancer cell line Col-1 and the large cell lung
cancer cell line LC-11 were obtained from the Central Institute for
Experimental Animals (Kawasaki, Japan). No KRAS mutations in Lu-99,
KM12C and DLD-1 and no EGFR mutation in Lu-99 were observed, but
NCI-H460 carried a KRAS mutation (19).
Antitumor activity in vivo
Male nude mice were purchased from CLEA Japan Inc.
(Tokyo, Japan) or Charles River Japan Inc. (Yokohama, Japan) and
were housed under specific pathogen-free conditions, with food and
water provided ad libitum. Following a 1-week quarantine
period, the animals were implanted subcutaneously with a solid
human tumor, the volume of which was ∼8 mm3. In order to
evaluate the antitumor activity, the mice were randomized according
to the tumor volume once the mean tumor volume reached ∼150–200
mm3 (day 0). Each group consisted of 6–8 nude mice.
S-1 was prepared by mixing tegafur, gimeracil and
oteracil at a molar ratio of 1:0.4:1 in 0.5% hydroxypropyl
methylcellulose (HPMC) and was administered orally once daily on
days 1–14. S-1 (6.9–8.3 mg) was administered at the reported
effective dose in mice (7).
Erlotinib, suspended in 0.5% HPMC, was administered
orally on days 1, 4, 8 and 11 at 100 mg/kg in mice carrying an
Lu-99 xenograft, according to the reported effective dose (20). For PC-9, which carries an EGFR
mutation that is supposedly more sensitive to erlotinib (21), erlotinib was administered at a dose
of 12.5 mg/kg. Sorafenib, dissolved with 8.3% cremophor and 8.3%
ethanol, or sunitinib, dissolved in 20 mM citrate buffer at pH 3.5,
was administered orally once daily on days 1–14 at a dose of 15 and
20 mg/kg, respectively (18,19).
Cetuximab and bevacizumab were diluted with saline and administered
intraperitoneally on days 1, 4, 8 and 11 at 40 and 5 mg/kg,
respectively (22,23).
The tumor diameters were measured twice a week and
the tumor volume was estimated as 0.5 × length × width2.
The relative tumor volume (RTV) was calculated using the following
formula: RTV = (tumor volume on measured day)/(tumor volume on day
0). On day 15, the TGI ratio was calculated using the formula: TGI
= [1 − (mean tumor volume of treated group)/(mean tumor volume of
control group)] × 100. The anti-tumor activity was evaluated based
on the GDP (24), which is the
difference in the time taken for the tumors to reach ∼25% of the
size of the control (the RTV value used to estimate the GDP was
designated as 2 for Lu-99; 5 for MX-1, NCI-H460 and KM12C/5-FU; and
3 for Col-1, KM20C and DLD-1). However, as the growth of PC-9 was
slower than that of the other tumors, the GDP was evaluated when
the RTV reached 2. The period during which the RTV of the treated
group reached 25% of the control on day 15 was calculated using
linear regression, as reported previously (24). The expected GDP of the combined
group was calculated using the formula: expected GDP of combined
group = (GDP of S-1 group) + (GDP of combined reagent). If the
observed GDP of the combined group was superior to the expected
value, the combination was designated as more than additive. The
relative body weight change (BWC) was calculated using the
following formula: BWC (%) = [(body weight on day 15) − (body
weight on day 0)]/(body weight on day 0) × 100. Cases in which the
BWC was <−20% were regarded as having received toxic
regimens.
The animal studies were performed according to the
guidelines and with the approval of the Institutional Animal Care
and Use Committee of Taiho Pharmaceutical Co., Ltd.
Real-time reverse transcription
(RT)-polymerase chain reaction (PCR) for DPD in tumor tissues
Total RNA was isolated from the residual section of
the tumor tissue and first-strand cDNA was synthesized from 500 ng
of total RNA using the High-Capacity cDNA Archive kit, as described
by the manufacturer, using i-cycler. Real-time RT-PCR was performed
using the QuantiTect Probe PCR kit and ABI PRISM 7900HT Sequence
Detection system, according to the manufacturer’s instructions.
Briefly, 2 ng of cDNA was added to a reaction mixture containing
12.5 μl of 2X QuantiTect Probe PCR master mix and 1.25
μl of 20X TaqMan gene expression assays mix in a final
volume of 25 μl. The conditions for the real-time RT-PCR
were: 1 cycle of 50°C for 2 min and 95°C for 15 min; 40 cycles of
94°C for 15 sec and 60°C for 1 min. Gene expression profiling was
achieved using the comparative cycle threshold method of relative
quantification [the calibrator samples were untreated cells, with
β-actin (ACTB) used as the endogenous control]. The Gene Assay IDs
of the TaqMan gene expression assay were Hs00559278_m1 for DPD
(DPYD) and Hs99999903_m1 for ACTB. The relative gene expression
levels of DPD were calculated using the Δ threshold cycle (Ct)
method according to the formula shown below. The expression levels
of estrogen receptor-α (ER-α) were expressed as 2−ΔCt ×
100 for the ease of calculation, where ΔCt = (Ct of DPD) − (Ct of
ACTB).
Statistical analysis
The significance of the differences in the mean RTV
between the treated and control groups on day 15 was analyzed using
the Aspin-Welch two-tailed t-test. The combinational effect of
targeted agents on the antitumor activity was analyzed according to
a closed testing procedure using the Aspin-Welch two-tailed t-test
(25) and EXSAS, ver. 7.11 (Arm
Systex Co., Ltd., Osaka, Japan).
Results
Increased antitumor activity of S-1in
combination with EGFR kinase inhibitor against human NSCLC in
vivo
The antitumor activities of S-1 and the EGFR kinase
inhibitor erlotinib, which have been applied against NSCLC
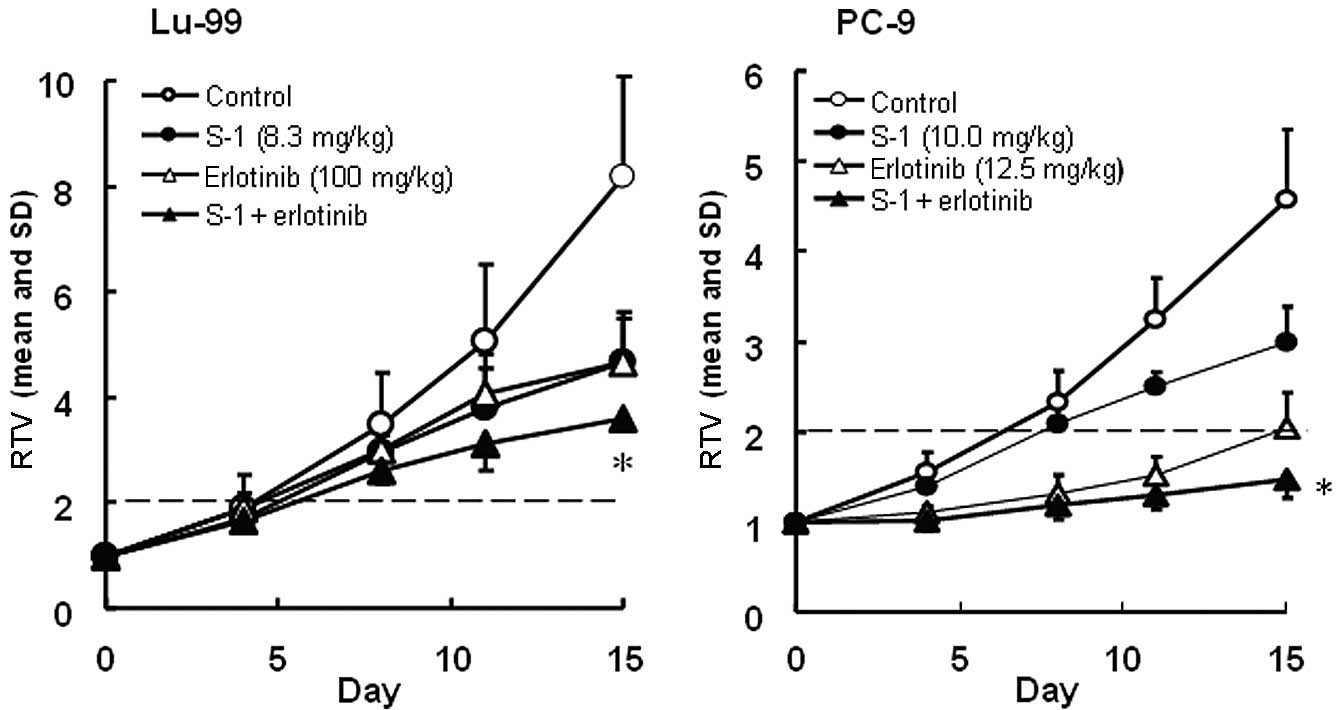
clinically, were evaluated. The RTV changes in the Lu-99 and PC-9
cancer cell lines are shown in Fig.
1.
The antitumor activity of the combination group on
day 15 was significantly higher than that of either monotherapy
group for Lu-99 (P<0.05) and PC-9 (P<0.05). The GDP values
were lengthened in the combination group compared with the
mono-therapy groups. As the observed GDP values for the combined
therapy (7.0 and 10.9 days for Lu-99 and PC-9, respectively) were
not less than the expected values (6.1 and 10.4 days,
respectively), the combination of S-1 with erlotinib was regarded
as being additive (Table I). As
the BWC was not <−20% in any of the groups and no significant
difference was observed between the combination therapy and either
of the monotherapy groups, these combinations appeared to be
tolerable.
 | Table I.Antitumor activity and body weight
changes in mice implanted with human non-small cell lung cancer
tumors from the cell lines, Lu-99 or PC-9, following treatment with
S-1 and the EGFR kinase inhibitor, erlotinib. |
Table I.
Antitumor activity and body weight
changes in mice implanted with human non-small cell lung cancer
tumors from the cell lines, Lu-99 or PC-9, following treatment with
S-1 and the EGFR kinase inhibitor, erlotinib.
| Body weight
changed
|
|---|
| Tumor | Drug (mg/kg) | Treatment | Tumor
volumea
(mm3, mean ± SD) | TGIb (%) | GDPc (days) | (g, mean ± SD) | (%) |
|---|
| Lu-99 | Control | - | 1257±196 | - | 0.0 | 2.5±0.5 | 10.1 |
| S-1 (8.3) | days 1–14 | 728±94 | 42.1 | 3.3 | 1.5±1.4 | 6.0 |
| Erlotinib
(100) | days 1–14 | 747±177 | 40.6 | 2.8 | 0.5±1.2 | 2.3 |
| S-1 +
erlotinib | | 563±60e | 55.3 | 7.0 | −3.0±2.5 NS | −13.0 |
| PC-9 | Control | - | 661±95 | - | 0.0 | 0.9±0.5 | 3.0 |
| S-1 (10.0) | days 1–14 | 427±20 | 35.4 | 1.6 | −0.09±0.8 | −0.3 |
| Erlotinib
(12.5) | days 1–14 | 289±55 | 56.4 | 8.8 | 0.4±0.9 | 1.6 |
| S-1 +
erlotinib | | 218±39e | 67.0 | 10.9 | −0.2±0.6 NS | −0.5 |
Increased antitumor activity of S-1 in
combination with tyro-sine kinase inhibitors in vivo
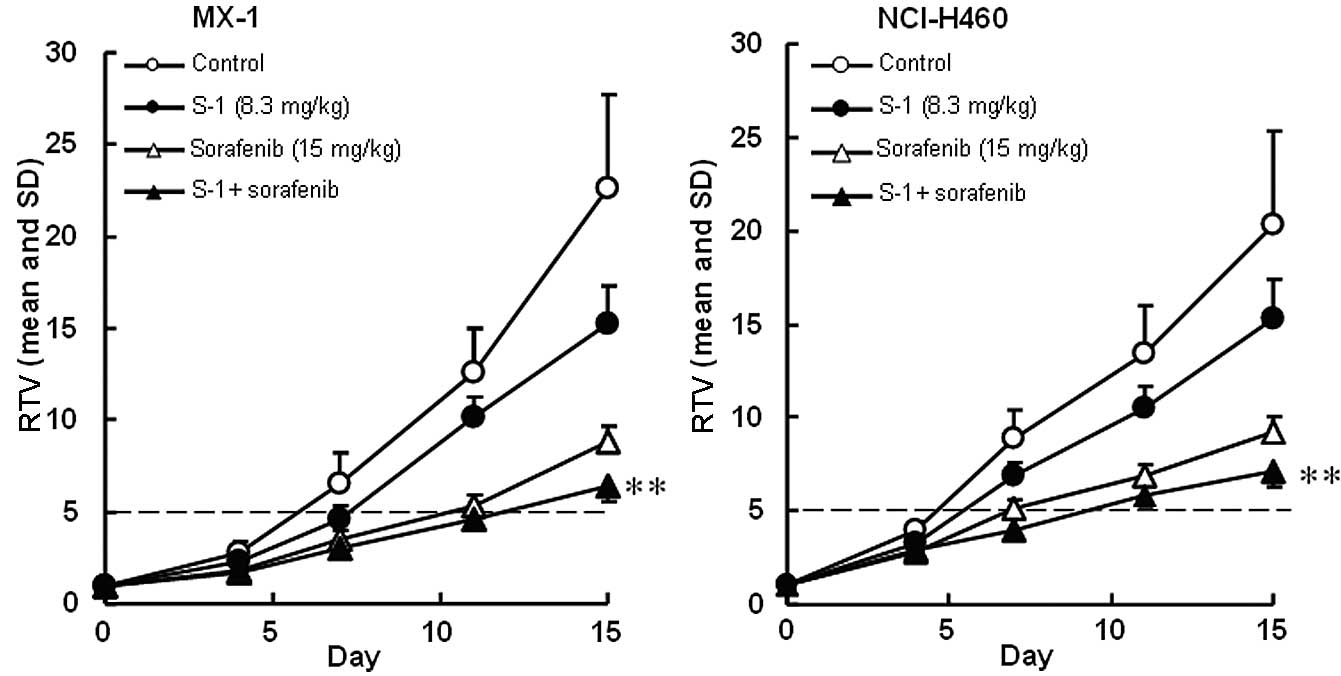
The antitumor activities of S-1 and the multi-kinase
inhibitors, sorafenib and sunitinib, were evaluated. The RTV
changes of the MX-1 and NCI-H460 cancer cell lines treated with
sorafenib are shown in Fig. 2. The
RTV of the combination group on day 15 was significantly lower than
that of either monotherapy group for the MX-1 and NCI-H460 cancer
cell lines (P<0.01). As the GDP values for the combined therapy
(6.0 and 5.4 days for the MX-1 and NCI-H460 cancer cell lines,
respectively) were almost equivalent to the expected values (6.5
and 4.9 days, respectively), the combination of S-1 with erlotinib
was regarded as being not competitive (Table II).
 | Table II.Antitumor activity and body weight
changes in mice implanted with tumors from human breast cancer MX-1
cells or human non-small cell lung cancer NCI-H460 cells, following
treatment with S-1 and the multi-kinase inhibitor, sorafenib. |
Table II.
Antitumor activity and body weight
changes in mice implanted with tumors from human breast cancer MX-1
cells or human non-small cell lung cancer NCI-H460 cells, following
treatment with S-1 and the multi-kinase inhibitor, sorafenib.
| Body weight
changed
|
|---|
| Tumor | Drug (mg/kg) | Treatment | Tumor
volumea
(mm3, mean ± SD) | TGIb (%) | GDPc (days) | (g, mean ± SD) | (%) |
|---|
| MX-1 | Control | - | 2747±497 | - | 0.0 | 4.2±0.9 | 19.0 |
| S-1 (8.3) | days 1–14 | 1832±112 | 33.3 | 1.6 | 3.7±0.6 | 16.7 |
| Sorafenib (15) | days 1–14 | 1076±214 | 60.8 | 4.9 | 2.8±0.6 | 12.8 |
| S-1 +
sorafenib | | 776±87e | 71.7 | 6.0 | 1.1±0.4 NS | 4.8 |
| NCI-H460 | Control | - | 2937±709 | - | 0.0 | 2.6±1.3 | 10.0 |
| S-1 (8.3) | days 1–14 | 2206±279 | 24.9 | 1.5 | 1.3±1.8 | 4.9 |
| Sorafenib (15) | days 1–14 | 1327±173 | 54.8 | 3.4 | −0.1±0.7 | −0.4 |
| S-1 +
sorafenib | | 1036±183e | 64.7 | 5.4 | −1.1±1.2 NS | −4.2 |
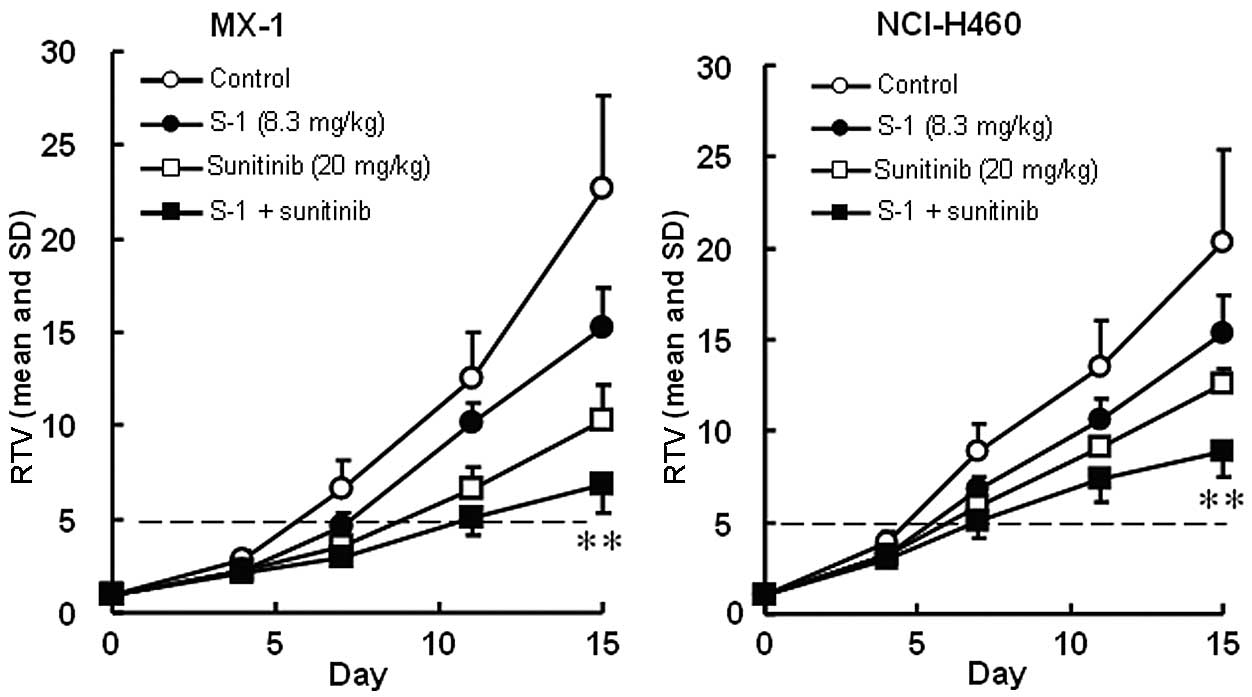
The RTV changes of MX-1 and NCI-H460 cancer cell
lines treated with sunitinib are shown in Fig. 3. The RTV of the combination group
on day 15 was significantly lower than that of either monotherapy
group for the MX-1 and NCI-H460 cancer cell lines (P<0.01). As
the GDP values for the combined therapy (5.4 and 3.0 days for the
MX-1 and NCI-H460 cancer cell lines, respectively) were almost
equivalent to the expected values (4.7 and 3.5 days, respectively),
the combination of S-1 with sunitinib was regarded as being not
competitive (Table III). As the
BWC was not <−20% in any group and no significant difference was
observed between the combination therapy and either of the
monotherapy groups, these combinations appeared to be
tolerable.
 | Table III.Antitumor activity and body weight
changes in mice implanted with tumors from human breast cancer MX-1
cells or human non-small cell lung cancer NCI-H460 cells, following
treatment with S-1 and the multi-kinase inhibitor, sunitinib. |
Table III.
Antitumor activity and body weight
changes in mice implanted with tumors from human breast cancer MX-1
cells or human non-small cell lung cancer NCI-H460 cells, following
treatment with S-1 and the multi-kinase inhibitor, sunitinib.
| Body weight
changed
|
|---|
| Tumor | Drug (mg/kg) | Treatment | Tumor
volumea
(mm3, mean ± SD) | TGIb (%) | GDPc (days) | (g, mean ± SD) | (%) |
|---|
| MX-1 | Control | - | 2747±497 | - | 0.0 | 4.2±0.9 | 19.0 |
| S-1 (8.3) | days 1–14 | 1832±112 | 33.3 | 1.6 | 3.7±0.6 | 16.7 |
| Sunitinib (20) | days 1–14 | 1231±222 | 55.2 | 3.1 | 3.4±1.1 | 15.2 |
| S-1 +
sunitinib | | 811±148e | 70.5 | 5.4 | 2.4±1.0 NS | 10.4 |
| NCI-H460 | Control | - | 2937±709 | - | 0.0 | 2.6±1.3 | 10.0 |
| S-1 (8.3) | days 1–14 | 2206±279 | 24.9 | 1.5 | 1.3±1.8 | 4.9 |
| Sunitinib (20) | days 1–14 | 1826±232 | 37.9 | 2.0 | 2.5±1.0 | 9.9 |
| S-1 +
sunitinib | | 1282±120e | 56.4 | 3.0 | 1.5±0.7 NS | 5.8 |
Increased antitumor activity of S-1 in
combination with targeted antibodies in vivo
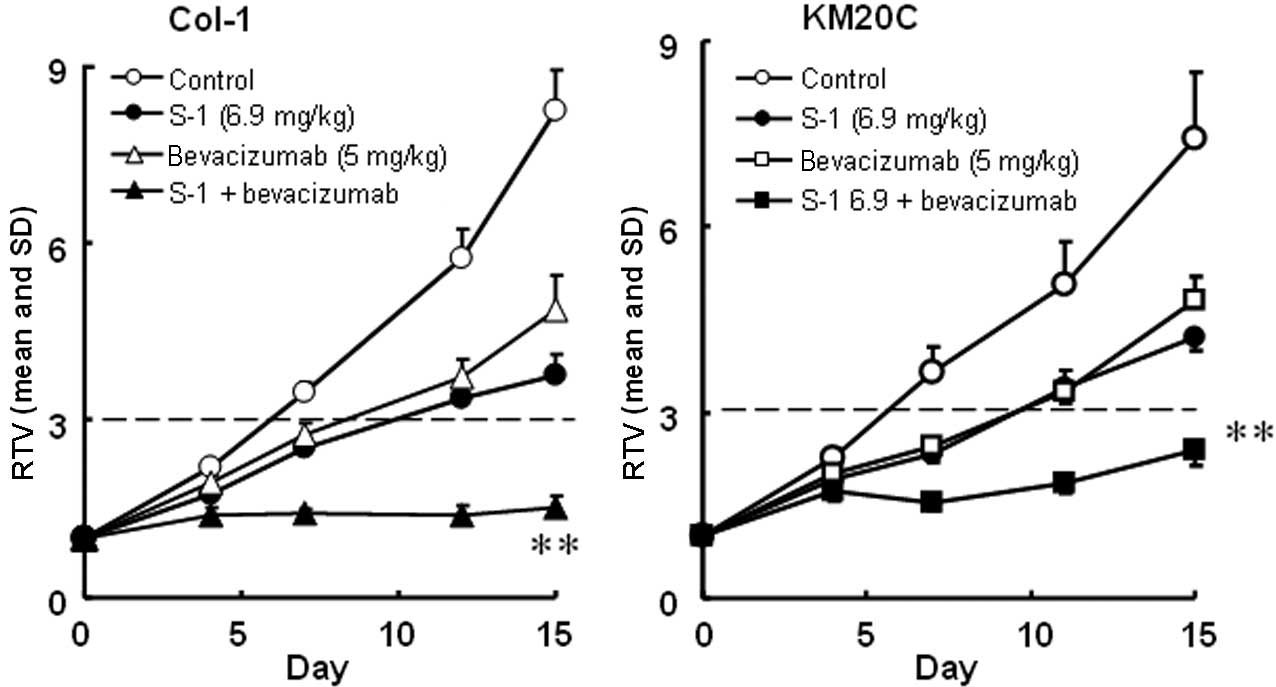
The antitumor activities of S-1 and targeted
antibodies (cetuximab and bevacizumab) against human colorectal
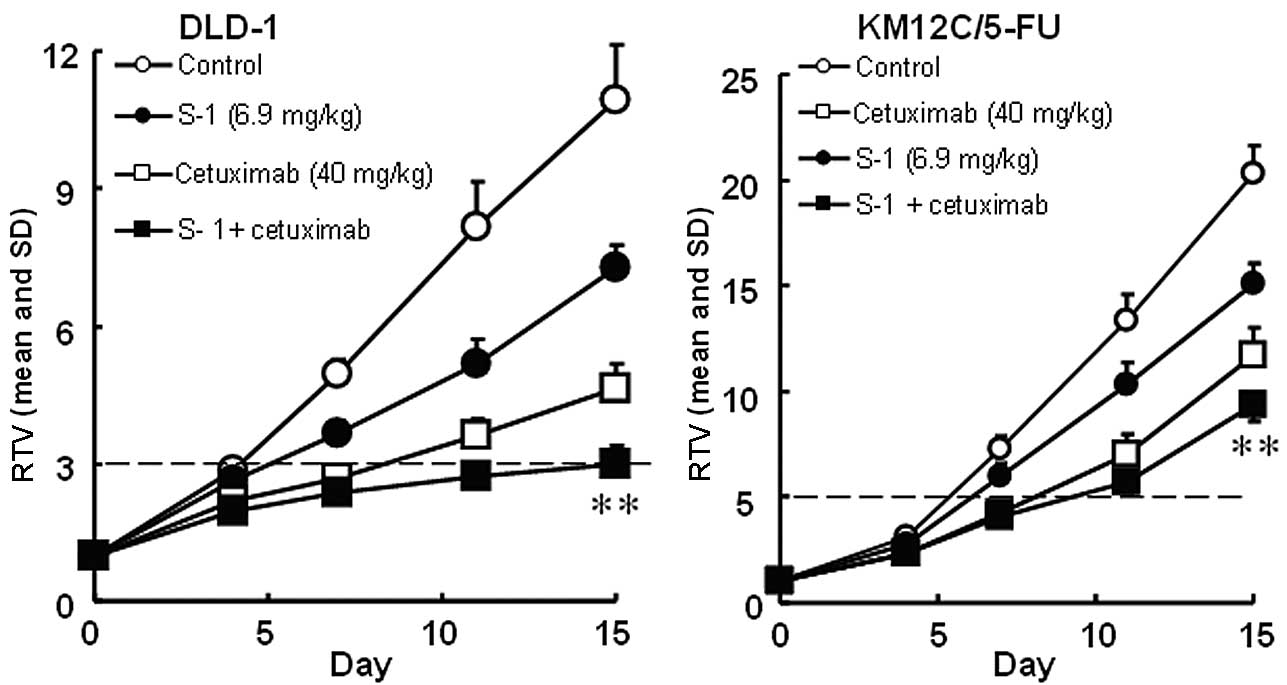
cancers were evaluated. The RTV changes of the Col-1, KM20C, DLD-1
and KM12C/5-FU cancer cell lines are shown in Figs. 4 and 5. The tumor volume on day 15, the GDP
value (observed or expected) and the BWC between days 0 and 15 are
listed in Tables IV and V.
 | Table IV.Antitumor activity and body weight
changes in mice implanted with human colorectal cancer tumors from
the cell lines, Col-1 or KM20C, following treatment with S-1 and
the targeted antibody, bevacizumab. |
Table IV.
Antitumor activity and body weight
changes in mice implanted with human colorectal cancer tumors from
the cell lines, Col-1 or KM20C, following treatment with S-1 and
the targeted antibody, bevacizumab.
| Body weight
changed
|
|---|
| Tumor | Drug (mg/kg) | Treatment | Tumor
volumea
(mm3, mean ± SD) | TGIb (%) | GDPc (days) | (g, mean ± SD) | (%) |
|---|
| Col-1 | Control | - | 924±80 | - | 0.0 | −1.7±1.5 | −7.0 |
| S-1 (6.9) | days 1–14 | 423±43 | 54.2 | 4.1 | −2.8±1.3 | −11.0 |
| Bevacizumab
(5) | days 1, 4, 8,
11 | 546±82 | 40.9 | 2.6 | −2.8±0.8 | −11.4 |
| S-1 +
bevacizumab | | 172 ± 21e | 81.3 | >9.1 | −3.0±1.5 NS | −12.3 |
| KM20C | Control | - | 1464±221 | - | 0.0 | 0.4±1.2 | 1.6 |
| S-1 (6.9) | days 1–14 | 826±58 | 43.6 | 3.9 | −1.7±2.3 | −6.5 |
| Bevacizumab
(5) | days 1, 4, 8,
11 | 943±96 | 35.6 | 3.1 | 0.5±1.1 | 2.1 |
| S-1 +
bevacizumab | | 470±32e | 67.9 | >9.5 | −0.1±0.7 NS | −0.5 |
 | Table V.Antitumor activity and body weight
changes in mice implanted with human colorectal cancer tumors from
the cell lines, DLD-1 and 5-FU-resistant KM12C/5-FU, following
treatment with S-1 and the targeted antibody, cetuximab. |
Table V.
Antitumor activity and body weight
changes in mice implanted with human colorectal cancer tumors from
the cell lines, DLD-1 and 5-FU-resistant KM12C/5-FU, following
treatment with S-1 and the targeted antibody, cetuximab.
| Body weight
changed
|
|---|
| Tumor | Drug (mg/kg) | Treatment | Tumor
volumea
(mm3, mean ± SD) | TGIb (%) | GDPc (days) | (g, mean ± SD) | (%) |
|---|
| DLD-1 | Control | - | 1252±108 | - | 0.0 | −1.9±1.9 | −8.0 |
| S-1 (6.9) | days 1–14 | 826±33 | 34.1 | 1.3 | −2.0±1.1 | −8.8 |
| Cetuximab (40) | days 1, 4, 8,
11 | 533±71 | 57.4 | 4.4 | 2.2±1.0 | 9.6 |
| S-1 +
cetuximab | | 344±66e | 72.7 | 11.0 | 1.4±1.2 NS | 6.5 |
| KM12C/5-FU | Control | - | 2276±243 | - | 0.0 | −1.1±0.5 | −4.6 |
| S-1 (6.9) | days 1–14 | 1680±53 | 26.2 | 1.5 | −1.8±1.3 | −7.1 |
| Cetuximab (40) | days 1, 4, 8,
11 | 1310±165 | 42.5 | 2.3 | −1.8 ± 1.4 | −3.7 |
| S-1 +
cetuximab | | 1036±94e | 54.5 | 2.4 | −1.6 ± 1.1 NS | −8.0 |
Using a closed testing procedure and the Aspin-Welch
t-test, the RTV of the combination groups on day 15 was
significantly lower (P<0.01) than that of either monotherapy
group for the 4 examined colorectal cancer cell lines, including
the KM12C/5-FU cancer cell lines. Furthermore, the GDP value was
increased in the combination groups compared with the monotherapy
groups and the observed GDP values for the Col-1 and DLD-1 cancer
cell lines were almost twice those expected. As the BWC was not
<−20% in any of the groups, these combinations appeared to be
feasible.
Gene expression levels of DPD
The gene expression level of DPD was measured in 5
xenografts (Table VI). Although
the DPD expression level relative to that of ACTB ranged from 0.031
to 78.3 for the 5 examined xenografts, the antitumor activity of
S-1 was potentiated in all the examined cancer cell lines.
 | Table VI.DPD mRNA expression levels normalized
by β-actin. |
Table VI.
DPD mRNA expression levels normalized
by β-actin.
| Cell line | DPD mRNA
expression |
|---|
| Lu-99 | 78.3 |
| MX-1 | 0.41 |
| Col-1 | 0.031 |
| KM20C | 11.1 |
| KM12C/5-FU | 0.54 |
Discussion
We examined combination therapies of S-1 with a
number of targeted agents in vivo. The antitumor effect of
S-1 was significantly potentiated when used in combination with the
examined tyrosine kinase inhibitors (erlotinib, sorafenib and
sunitinib) and antibodies (bevacizumab and cetuximab). During these
experiments, neither toxic mortality nor a BWC <−20% was
observed; therefore, these combinations are thought to be
feasible.
The cytotoxic activity of 5-FU is mainly dependent
on the inhibition of TS, which is the rate-limiting enzyme of
deoxythymidine monophosphate synthesis (26). The antitumor effects of 5-FU
derivatives are inversely correlated with the activity of TS
(27) and resistance against 5-FU
is reportedly overcome by the inhibition of TS (28). Tanizaki et al reported that
when used in combination with a targeted agent (lapatinib) or
antibody (trastuzumab), the antitumor activity of S-1 was
potentiated against HER2-overexpressing human gastric tumor cell
lines (NCI-N87 and 4-1ST) due to the downregulation of TS via a
reduction in E2F1 (17). The
agents, erlotinib, sorafenib, sunitinib and cetuximab, have been
reported to reduce the level of TS expression (29–33),
possibly via the same mechanism, the decrease in TS expression
could be the mechanism responsible for the potentiation of the
antitumor effect of S-1.
The expression of DPD is inversely correlated with
the antitumor activity of 5-FU, but in the present study, the
antitumor activity of S-1 was potentiated for all the tumors
examined, irrespective of DPD expression. In contrast to other
fluoropyrimidines, the antitumor activity of S-1 was not associated
with tumoral DPD activity in 30 xenografts (6 gastric, 6
colorectal, 6 breast, 7 lung and 5 pancreatic tumors), unlike the
antitumor activity of capecitabine (34). In a large-scale population analysis
using 12,783 solid tumors, the DPD protein expression level was
shown to be high in NSCLC or pancreatic cancer (35), similar to the results of the
present study. From this viewpoint, S-1 combination therapy might
be active against tumors with high levels of DPD expression, unlike
therapy with other fluoropyrimidines.
Furthermore, the antitumor activity of bevacizumab
depends on the inhibition of angiogenesis via the vascular
endothelial growth factor (VEGF). In node-positive colon cancer,
the reduction of VEGF and the S-phase status reportedly suppress
recurrence (36). Unlike other
fluoropyrimidines, metabolites of tegafur (γ-hydroxybutyric and
γ-butyrolactone) reportedly inhibit cancer-induced angiogenesis via
a VEGF-related pathway (37);
thus, the combination of S-1 with bevacizumab might be promising
for other fluoropyrimidines that do not contain tegafur.
In conclusion, we have shown that the combination of
S-1 with targeted agents (tyrosine kinase inhibitors or targeted
antibodies) is feasible and exerts a potentiated antitumor effect
as a result of TS inhibition. These preclinical studies suggest the
utility of further clinical trials of S-1-based combination therapy
with targeted agents.
Abbreviations:
|
DIF
|
DPD inhibitory fluoropyrimidine;
|
|
DPD
|
dihydropyrimidine dehydrogenase;
|
|
EGFR
|
epidermal growth factor receptor;
|
|
GDP
|
growth delay period;
|
|
HPMC
|
hydroxypropyl methylcellulose;
|
|
NSCLC
|
non-small cell lung cancer;
|
|
RTV
|
relative tumor volume;
|
|
S-1
|
tegafur-gimeracil-oteracil;
|
|
TGI
|
tumor growth inhibition;
|
|
TS
|
thymidylate synthase;
|
|
UFT
|
uracil and tegafur;
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1.
|
Popov I, Milicević M and Radosević-Jelić
LJ: The addition of bevacizumab to fluoropyrimidine, irinotecan and
oxaliplatin-based therapy improves survival for patients with
metastatic colorectal cancer (CRC): combined analysis of efficacy.
Acta Chir Iugosl. 55:11–16. 2008. View Article : Google Scholar
|
|
2.
|
Meyerhardt JA, Stuart K, Fuchs CS, Zhu AX,
Earle CC, Bhargava P, Blaszkowsky L, Enzinger P, Mayer RJ, Battu S,
et al: Phase II study of FOLFOX, bevacizumab and erlotinib as
first-line therapy for patients with metastatic colorectal cancer.
Ann Oncol. 18:1185–1189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Boccia RV, Cosgriff TM, Headley DL,
Badarinath S and Dakhil SR: A phase II trial of FOLFOX6 and
cetuximab in the first-line treatment of patients with metastatic
colorectal cancer. Clin Colorectal Cancer. 9:102–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Townsley CA, Major P, Siu LL, Dancey J,
Chen E, Pond GR, Nicklee T, Ho J, Hedley D, Tsao M, et al: Phase II
study of erlotinib (OSI-774) in patients with metastatic colorectal
cancer. Br J Cancer. 94:1136–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dal Lago L, D’Hondt V and Awada A:
Selected combination therapy with sorafenib: a review of clinical
data and perspectives in advanced solid tumors. Oncologist.
13:845–858. 2008.PubMed/NCBI
|
|
6.
|
Shirasaka T, Nakano K, Takechi T, Satake
H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.
|
|
7.
|
Fukushima M, Satake H, Uchida J, Shimamoto
Y, Kato T, Takechi T, Okabe H, Fujioka A, Nakano K, Ohshimo H, et
al: Preclinical antitumor efficacy of S-1: A new oral formulation
of 5-fluorouracil on human tumor xenografts. Int J Oncol.
13:693–698. 1998.PubMed/NCBI
|
|
8.
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ohtsu A, Baba H, Sakata Y, Mitachi Y,
Horikoshi N, Sugimachi K and Taguchi T: Phase II study of S-1, a
novel oral fluoropyrimidine derivative, in patients with metastatic
colorectal carcinoma. Br J Cancer. 83:141–145. 2000.PubMed/NCBI
|
|
10.
|
Saeki T, Takashima S, Sano M, Horikoshi N,
Miura S, Shimizu S, Morimoto K, Kimura M, Aoyama H, Ota J, et al: A
phase II study of S-1 in patients with metastatic breast cancer - a
Japanese trial by the S-1 Cooperative Study Group, Breast Cancer
Working Group. Breast Cancer. 11:194–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Muro K, Boku N, Shimada Y, Tsuji A,
Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: a randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nakashima K, Hironaka S, Boku N, Onozawa
Y, Fukutomi A, Yamazaki K, Yasui H, Taku K, Kojima T and Machida N:
Irinotecan plus cisplatin therapy and S-1 plus cisplatin therapy
for advanced or recurrent gastric cancer in a single institution.
Jpn J Clin Oncol. 38:810–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Koizumi W, Tanabe S, Saigenji K, Ohtsu A,
Boku N, Nagashima F, Shirao K, Matsumura Y and Gotoh M: Phase I/II
study of S-1 combined with cisplatin in patients with advanced
gastric cancer. Br J Cancer. 89:2207–2212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Narahara H, Fujitani K, Takiuchi H,
Sugimoto N, Inoue K, Uedo N, Tsukuma H, Tsujinaka T, Furukawa H and
Taguchi T: Phase II study of a combination of S-1 and paclitaxel in
patients with unresectable or metastatic gastric cancer. Oncology.
74:37–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ishigami H, Kitayama J, Kaisaki S,
Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H
and Nagawa H: Phase II study of weekly intravenous and
intraperitoneal paclitaxel combined with S-1 for advanced gastric
cancer with peritoneal metastasis. Ann Oncol. 21:67–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Okabe T, Okamoto I, Tsukioka S, Uchida J,
Hatashita E, Yamada Y, Yoshida T, Nishio K, Fukuoka M, Jänne PA and
Nakagawa K: Addition of S-1 to the epidermal growth factor receptor
inhibitor gefitinib overcomes gefitinib resistance in non-small
cell lung cancer cell lines with MET amplification. Clin Cancer
Res. 15:907–913. 2009. View Article : Google Scholar
|
|
17.
|
Tanizaki J, Okamoto I, Takezawa K,
Tsukioka S, Uchida J, Kiniwa M, Fukuoka M and Nakagawa K:
Synergistic antitumor effect of S-1 and HER2-targeting agents in
gastric cancer with HER2 amplification. Mol Cancer Ther.
9:1198–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fukushima M, Fujioka A, Uchida J, Nakagawa
F and Takechi T: Thymidylate synthase (TS) and ribonucleotide
reductase (RNR) may be involved in acquired resistance to
5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J
Cancer. 37:1681–1687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ikediobi ON, Davies H, Bignell G, Edkins
S, Stevens C, O’Meara S, Santarius T, Avis T, Barthorpe S,
Brackenbury L, et al: Mutation analysis of 24 known cancer genes in
the NCI-60 cell line set. Mol Cancer Ther. 5:2606–2612. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Higgins B, Kolinsky K, Smith M, Beck G,
Rashed M, Adames V, Linn M, Wheeldon E, Gand L, Birnboeck H and
Hoffmann G: Antitumor activity of erlotinib (OSI-774, Tarceva)
alone or in combination in human non-small cell lung cancer tumor
xenograft models. Anticancer Drugs. 15:503–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Arao T, Fukumoto H, Takeda M, Tamura T,
Saijo N and Nishio K: Small in-frame deletion in the epidermal
growth factor receptor as a target for ZD6474. Cancer Res.
64:9101–9104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Prewett MC, Hooper AT, Bassi R, Ellis LM,
Waksal HW and Hicklin DJ: Enhanced antitumor activity of
anti-epidermal growth factor receptor monoclonal antibody IMC-C225
in combination with irinotecan (CPT-11) against human colorectal
tumor xenografts. Clin Cancer Res. 8:994–1003. 2002.
|
|
23.
|
Fox WD, Higgins B, Maiese KM, Drobnjak M,
Cordon-Cardo C, Scher HI and Agus DB: Antibody to vascular
endothelial growth factor slows growth of an androgen-independent
xenograft model of prostate cancer. Clin Cancer Res. 8:3226–3231.
2002.PubMed/NCBI
|
|
24.
|
Balin-Gauthier D, Delord JP, Rochaix P,
Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P and Allal C: In
vivo and in vitro antitumor activity of oxaliplatin in combination
with cetuximab in human colorectal tumor cell lines expressing
different level of EGFR. Cancer Chemother Pharmacol. 7:709–718.
2006. View Article : Google Scholar
|
|
25.
|
Bauer P, Röhmel J, Maurer W and Hothorn L:
Testing strategies in multi-dose experiments including active
control. Stat Med. 17:2133–2146. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Malet-Martino M and Martino R: Clinical
studies of three oral prodrugs of 5-fluorouracil (capecitabine,
UFT, S-1): a review. Oncologist. 288–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Peters GJ, van der Wilt CL, van Triest B,
Codacci-Pisanelli G, Johnston PG, van Groeningen CJ and Pinedo HM:
Thymidylate synthase and drug resistance. Eur J Cancer.
31A:1299–1305. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kadota K, Huang CL, Liu D, Yokomise H,
Haba R and Wada H: Combined therapy with a thymidylate
synthase-inhibiting vector and S-1 has effective antitumor activity
against 5-FU-resistant tumors. Int J Oncol. 38:355–363.
2011.PubMed/NCBI
|
|
29.
|
Skvortsov S, Sarg B, Lindner H, Lukas P,
Hilbe W, Zwierzina H and Skvortsova I: Cetuximab inhibits
thymidylate synthase in colorectal cells expressing epidermal
growth factor receptor. Proteomics Clin Appl. 2:908–914. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Giovannetti E, Lemos C, Tekle C, Smid K,
Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G and
Peters GJ: Molecular mechanisms underlying the synergistic
interaction of erlotinib, an epidermal growth factor receptor
tyrosine kinase inhibitor, with the multitargeted antifolate
pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol.
73:1290–1300. 2008. View Article : Google Scholar
|
|
31.
|
Takeuchi A, Shiota M, Tatsugami K,
Yokomizo A, Eto M, Inokuchi J, Kuroiwa K, Kiyoshima K and Naito S:
Sorafenib augments cytotoxic effect of S-1 in vitro and in vivo
through TS suppression. Cancer Chemother Pharmacol. 68:1557–1564.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Carloni S, Fabbri F, Brigliadori G, Ulivi
P, Silvestrini R, Amadori D and Zoli W: Tyrosine kinase inhibitors
gefitinib, lapatinib and sorafenib induce rapid functional
alterations in breast cancer cells. Curr Cancer Drug Targets.
10:422–431. 2010. View Article : Google Scholar
|
|
33.
|
Kobunai T, Watanabe T and Fukusato T:
Antitumor activity of S-1 in combination with cetuximab on human
gastric cancer cell lines in vivo. Anticancer Res. 31:3691–3696.
2011.PubMed/NCBI
|
|
34.
|
Ooyama A, Takechi T, Toda E, Nagase H,
Okayama Y, Kitazato K, Sugimoto Y, Oka T and Fukushima M: Gene
expression analysis using human cancer xenografts to identify novel
predictive marker genes for the efficacy of 5-fluorouracil-based
drugs. Cancer Sci. 97:510–522. 2006. View Article : Google Scholar
|
|
35.
|
Fukui Y, Oka T, Nagayama S, Danenberg PV,
Danenberg KD and Fukushima M: Thymidylate synthase,
dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase
mRNA and protein expression levels in solid tumors in large scale
population analysis. Int J Mol Med. 22:709–716. 2008.
|
|
36.
|
Tang TC, Man S, Xu P, Francia G, Hashimoto
K, Emmenegger U and Kerbel RS: Development of a resistance-like
phenotype to sorafenib by human hepatocellular carcinoma cells is
reversible and can be delayed by metronomic UFT chemotherapy.
Neoplasia. 12:928–940. 2010.PubMed/NCBI
|
|
37.
|
Basaki Y, Chikahisa L, Aoyagi K, Miyadera
K, Yonekura K, Hashimoto A, Okabe S, Wierzba K and Yamada Y:
γ-hydroxybutyric acid and 5-fluorouracil, metabolites of UFT,
inhibit the angiogenesis induced by vascular endothelial growth
factor. Angiogenesis. 4:163–173. 2001.
|