Introduction
Lung cancer is the most common malignant tumor in
the world. With the improvement in diagnostic technology, more and
more stage I lung cancer is identified. Video-assisted
thoracoscopic surgery (VATS) was first reported by Levi et
al in 1990 (1). The inital
clinical reports on video-assisted thoracoscopic surgery lobe
ctomy, published from 1993 onward, were either preliminary or
involved a small number of patients, which indicated the
feasibility and safety of VATS lobectomy (2–6).
Since then, VATS has become an attractive surgical procedure for
benign diseases, due to its low invasiveness (7,8). As
the experience with VATS for benign diseases increased, VATS was
gradually adopted for the treatment of lung cancer. Thereafter,
VATS lobectomy for elderly patients and series involving a larger
number of patients were reported (9–14).
VATS lobectomy is now accepted, if not universally, at least as an
alternative surgical option for early-stage lung cancer.
The advantages of VATS lobectomy, which include less
pain and better pulmonary function in the early postoperative
period, have been previously reported. However, there are various
controversies regarding the prognosis of VATS vs. open lobectomy
for stage I lung cancer. Immune system function in the early
postoperative period was found to be better preserved in patients
who underwent VATS lobectomy. Curability after VATS lobectomy was
also reported to be similar to the generally accepted curability
rate after open lobectomy. Several investigators reported that the
survival rate after VATS lobectomy was similar to that after open
lobectomy. Sawada et al (15), Sugiura et al (16), Tashima et al (17) and Flores et al (18) reported that there was no
statistically significant difference in survival between the VATS
and open lobectomy in early-stage lung cancer. However, this
meta-analysis showed that VATS had significantly better survival
rates in terms of overall survival at 5 years.
Materials and methods
Study selection
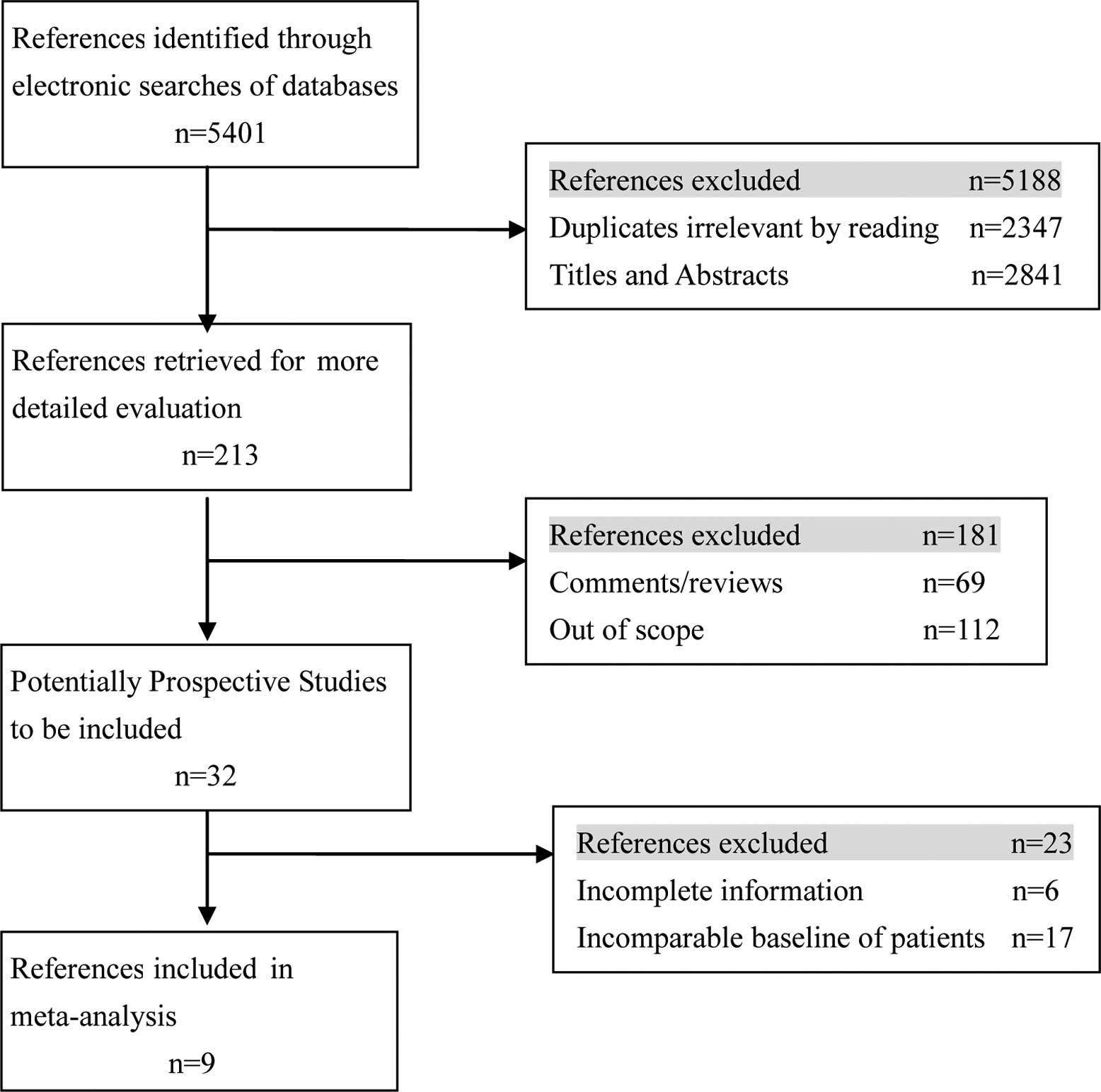
Electronic searches were performed of the MEDLINE,
Cochrane Controlled Trial Register (CENTRAL) and EMBASE databases
until July 2011. The following Mesh search headings were used:
(VATS, video-assisted thoracoscopic or video-assisted thoracoscopic
surgery), (open or conventional or standard lobectomy), (lung
cancer or lung carcinoma), (randomized controlled trials) and
(comparative study) in English (Fig.
1).
Data extraction and quality
assessment
Data were extracted by three independent observers
using standardized forms. The recorded data included the number of
patients, overall survival and local recurrence rates, systemic
recurrence and complications. The quality of all selected articles
was ranked in accordance with the score of the non-randomized
controlled clinical trial quality evaluation standard.
Study selection criteria
Inclusion criteria for this study were as follows:
i) no extrapulmonary metastasis; ii) no previous treatment of lung
cancer; iii) patients with clinical stage or pathological stage I;
iv) good lung function and all patients tolerated open lobectomy;
v) no previous or simultaneous malignancies; vi) patients were
suitable for treatment with either VATS or open lobectomy, and vii)
the baseline characteristics of patients were similar.
Criteria for exclusion
Abstracts, letters, editorials and expert opinions,
reviews without original data, case reports and studies lacking
control groups were excluded. The following studies were also
excluded: i) those dealing with unresectable lung cancer or
recurrence after lobectomy, and ii) those with no clearly reported
outcomes of interest.
Surgical technique
Open lobectomy. One-lung ventilation was used
and the patient was placed in a lateral decubitus position. A
postero-lateral or lateral incision was performed in the 4th or 5th
intercostal spaces. No or one rib adjacent to the thoracotomy was
resected. A rib retractor was used to open the wound and spread the
intercostal spaces. For right lung resection, mediastinal
lymphadenectomy was performed completely; for left lung resection,
the botallo ligament and hillal and carinal lymph nodes were
dissected.
VATS lobectomy. In VATS lobectomy, two or
three trocars were placed for a thoracoscope or instruments. Then,
4- to 8-cm access thoracotomy was placed along the anterior
axillary line in the 4th intercostal space or posterior axillary
line in the 5th intercostal space, and one or two or more access
ports were added. Dissection of the pulmonary vessels and bronchi
are performed in the same manner as in open lobectomy, and moderate
adhesions or absent fissures are not contraindications to VATS
resection. Mediastinal lymph node dissection is also performed in
patients with non-small lung cancer, similar to open lobectomy
techniques (19,20). A rib spreader was not used. The
resected lobe was placed in a plastic bag within the chest and then
extracted intact via the anterior utility port.
Statistical analysis The meta-analysis was
performed using the software package RevMan 5.1.0. Odds ratio (OR)
or mean difference with 95% confidence intervals (95% CI) were
calculated for dichotomous outcomes and continuous outcomes,
respectively. A random-effects model and a fixed-effect model were
used using ‘intention-to-treat’ analysis. If results were not
different between the two models, the random-effects model was
reported, as this model was used for the indirect comparisons. If
results differed between the two models, both results were
reported. Heterogeneity was explored by χ2 test and
I2. I2<25% and I2>50%
reflect a small and large inconsistency, respectively. P<0.05
was considered to indicate a statistically significant
difference.
Publication bias A funnel plot was used to
explore bias. Asymmetry in the funnel plot of the trial size
against treatment effect was used to assess the risk of bias.
Results
Description of studies
Of 1,362 patients in 9 studies, 668 were allocated
to the VATS group, whereas 694 were allocated to the open lobectomy
group to evaluate their therapeutic effects on stage I lung cancer.
Patient characteristics and evaluation index are shown in Table I. Three clinical stage I studies
(23–25) post operatively reported that
pathological N1 and N2 diseases were found in 14 and 17 patients,
respectively, from the open lobectomy group, and in 13 and 14
patients, respectively, from the VATS group. However, no
significant difference was found between the groups (P=0.799).
Patients reported from other studies were pathological stage I.
 | Table I.Characteristics of the included
trials. |
Table I.
Characteristics of the included
trials.
| Author/(Ref.) | Design | Country | Treatment | No. of
patients | Gender (M/F) | Mean age
(years) | Stage | Mean follow-up
(months) |
|---|
| Shiraishi et
al (23) | NRCT | Japan | VATS | 81 | 48/33 | 63.3±10.3 | cIA |
1,309±824b |
| | | OPEN | 79 | 52/27 | 65.9±8.9 | |
1,442±970b |
| Sakuraba et
al (24) | NRCT | Japan | VATS | 84 | 51/33 | 66a | cIA |
24.9a |
| | | OPEN | 56 | 33/23 | 63a | |
35.6a |
| Sawada et al
(15) | NRCT | Japan | VATS | 165 | 76/89 | 65.1±9.4 | pIA+IB |
57.0a |
| | | OPEN | 123 | 78/45 | 65.0±9.7 | |
57.0a |
| Sugi et al
(25) | RCT | Japan | VATS | 48 | 28/20 | 64.9±1.4 | cIA |
1,797b |
| | | OPEN | 52 | 29/23 | 65.9±1.4 | |
1,797b |
| Koizumi et
al (29) | NRCT | Japan | VATS | 39 | - | 74a | pIA+IB |
40.0a |
| | | OPEN | 22 | - | 71a | |
40.0a |
| Shigemura et
al (32) | NRCT | Japan | VATS | 31 | 14/17 | 64±11 | pIA |
38.8a |
| | | OPEN | 55 | 29/26 | 62±9 | |
38.8a |
| Yang et al
(21) | NRCT | China | VATS | 43 | - | 54a | pIA+IB |
68.1a |
| | | OPEN | 98 | - | 54a | |
68.1a |
| Tatsumi and Ueda
(30) | NRCT | Japan | VATS | 118 | 67/51 | 66.2±0.99 | pIA+IB | 31.8±1.42 |
| | | OPEN | 121 | 76/45 | 67.7±0.79 | | 36.9±2.03 |
| Whitson et
al (22) | NRCT | USA | VATS | 59 | 30/29 |
67.1a | cIA+IB |
4.6a |
| | | OPEN | 88 | 45/43 |
64.9a | |
7.9a |
Overall survival rates
One-year survival rates. The meta-analysis
(two trials reported these data) showed that there was no
statistically significant difference in 1-year overall survival
between the VATS and open lobectomy groups (OR=3.21, 95% CI
0.77–13.40; P=0.11), with no evidence of significant heterogeneity
(Table II).
 | Table II.Summary of the results between VATS
and OPEN in the management of stage I lung cancer. |
Table II.
Summary of the results between VATS
and OPEN in the management of stage I lung cancer.
| Variables | No. of studies
furnishing data (Refs.) | Results
| OR (95% CI) | P-value | I2
(%) |
|---|
| VATS (%) | OPEN (%) |
|---|
| Overall
survival | | | | | | |
| 1-year | 2 (20,29) | 97.90 | 91.8 | 3.21
(0.77–13.4) | 0.1100 | 0 |
| 3-year | 2 (22,25,29) | 83.50 | 83.3 | 0.91
(0.49–1.70) | 0.7700 | 0 |
| 5-year | 8 (15,21,23–25,29,30,32) | 87.80 | 80.2 | 2.01
(1.44–2.78) | <0.0001 | 17 |
| Local
recurrence | 6 (23–25,29,30,32) | 4.98 | 8.3 | 0.58
(0.33–1.03) | 0.0600 | 34 |
| Systemic
recurrence | 5 (24,25,29,30,32) | 6.70 | 11.4 | 0.52
(0.29–0.90) | 0.0200 | 0 |
| Complications | 3 (21,22,29,32) | 29.60 | 46.4 | 0.36
(0.23–0.57) | <0.0001 | 59 |
Three-year survival rates. The meta-analysis
(two trials reported these data) showed that there was no
statistically significant difference in 3-year overall survival
between the VATS and open lobectomy groups (OR=0.91, 95% CI
0.49–1.70; P=0.77), with no evidence of significant
heterogeneity.
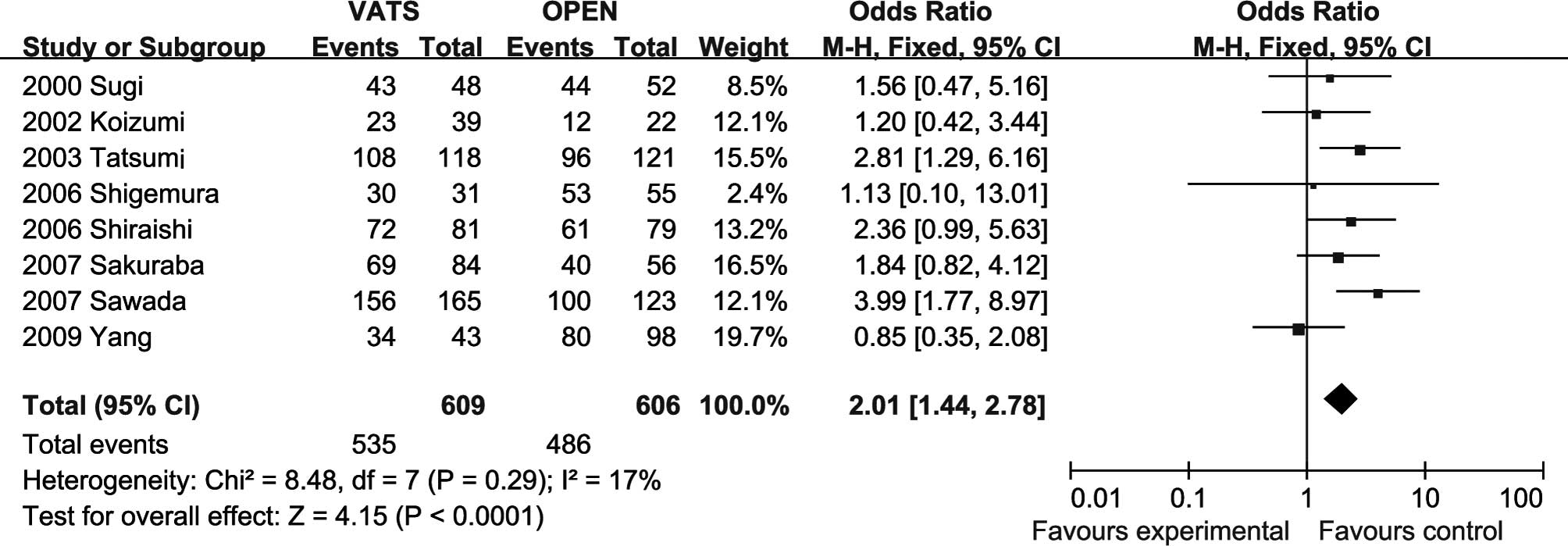
Five-year survival rates. The meta-analysis
(all trials reported these data) showed that the 5-year survival
rate in the open lobectomy group was significantly lower than that
in the VATS group (OR=2.01, 95% CI 1.44–2.78; P<0.0001), with
certain heterogeneity (Fig.
2).
Locoregional recurrence rates
The meta-analysis (six trials reported these data)
showed that there was no significantly statistical difference in
the recurrence rate (OR=0.58, 95% CI 0.33–1.03; P=0.07). However,
in the VATS group (4.98%) the incidence of complications was
significantly less compared to the incidence in the open lobectomy
group (8.31%).
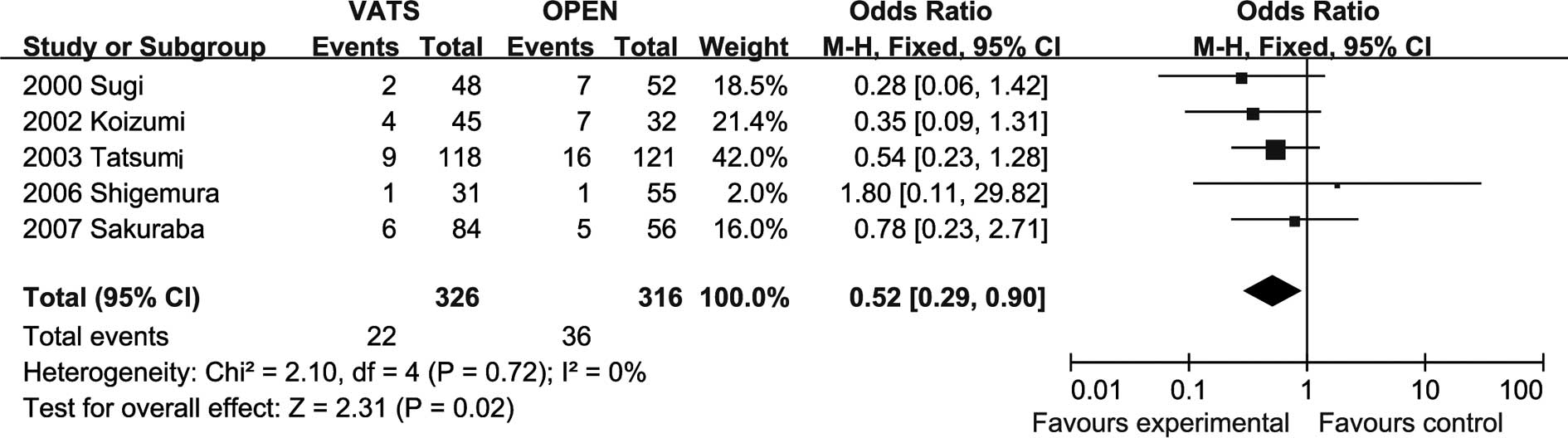
Systemic recurrence rates
The meta-analysis (four trials reported these data)
showed that the recurrence rate in the lobectomy group was
significantly higher than that in the VATS group (OR=0.52, 95% CI
0.23–0.82; P=0.01), with no evidence of significant heterogeneity
(Fig. 3).
Complications
The meta-analysis (three trials reported these data)
showed that there was significant difference in the incidence of
complications favorable to the VATS group (OR=0.36, 95% CI
0.23–0.57; P<0.0001), with certain heterogeneity.
Sensitivity analysis and publication
bias
Publication bias may exist when no significant
findings remain unpublished, thus artificially inflating the
apparent magnitude of an effect.
Complication, survival and recurrence rates
following VATS or open lobectomy for the treatment of stage I lung
cancer were calculated by the fixed-effects model and
random-effects model, respectively. The results were similar and
the combined results were highly reliable.
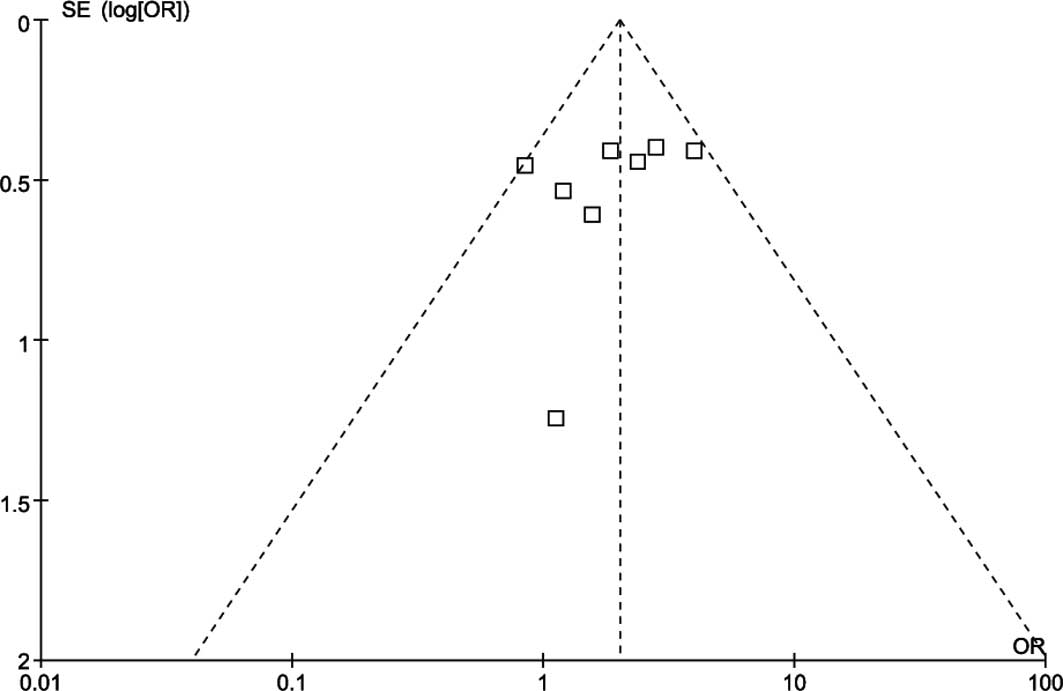
Funnel plots of the study results are shown in
Fig. 4. The funnel plots
concerning the 5-year overall survival rates following VATS or open
lobectomy for the treatment of stage I lung cancer showed
asymmetry, which suggested that there was some publication
bias.
Discussion
Meta-analysis, a quantitative technique for
therapeutic evaluation, may be used when controversy persists after
several trials. We acknowledge that a very limited number of
quality randomized controlled trials (RCTs) were available and
included in this meta-analysis. The main reason for this is that it
remains a challenge to conduct clinical research trials with
randomization support and double blinding, both of which are
effective means of preventing bias and improving the objectivity of
clinical evidence for both the efficacy and the safety of any
approved medical product or procedure or device. Although
meta-analysis has traditionally been applied and is best confined
to RCT, meta-analytical techniques using non-RCT may be a valid
method in certain clinical settings in which either the number or
the sample size of RCT is insufficient (26).
This meta-analysis suggested that the incidence of
complications after VATS for the treatment of stage I lung cancer
were less than that in the open lobectomy group, which obviously
embodied the microinvasive characterization of VATS. VATS is a
minimally invasive technique that has been used in the clinic in
the 1990s. Advocates of VATS lobectomy emphasize that this
minimally invasive procedure is associated with less tissue trauma,
reduced postoperative pain and a shorter hospital stay. McKenna
et al (27) reported the
largest single-institutional series on VATS lobectomy to date. In
their study of 1,100 patients, the mortality rate was 0.8% and the
morbidity rate was 15.3%. Several studies reported that VATS
resulted in superior rates of postoperative morbidity when compared
to thoracotomy. VATS reduced intraoperative blood loss (16,17,28–33),
chest drain duration and length of hospital stay (28,30,32,34,35).
Also, this meta-analysis suggested that the incidence of
complications after VATS was less than that in the open lobectomy
group, which obviously embodied the micro-invasive characterization
of VATS. These facts suggest that, in the hands of experienced
surgeons, VATS lobectomy is a safe procedure. The advantages of
VATS lobectomy have been previously reported, and the survival
rates after VATS lobectomy are at the very least identical to those
after open lobectomy.
Rare reports can be found on the long-term efficacy
for VATS lobectomy, most of which are based on follow-up periods of
2 or 3 years and provide survival rates or predicted 5-year
survival rates calculated by the Kaplan-Meier method. This
meta-analysis showed that VATS had significantly better survival
rates in terms of overall survival at 5 years, but there was no
statistically significant difference in 1.3-year overall survival
between the VATS and open lobectomy groups. This could be partly
explained by the reduced invasiveness and improvements in surgical
techniques. The minimal invasiveness of VATS lobectomy may reduce
the immunosuppressive cytokine effect (36). Craig et al (37) demonstrated that VATS pulmonary
lobectomy is associated with reduced peri-operative changes in
acute phase responses. This finding may have implications for
peri-operative tumor immuno-surveillance in lung cancer patients.
Observations indicate that VATS is associated with better preserved
cellular immunity and less immunosuppression when compared to open
lobectomy during the immediate postoperative period, and less
disturbance of inflammatory and immunomodulatory mediators
following VATS may have additional impact upon tumor biological
behavior (38). The short-term
immunological advantages may improve long-term survival and reduce
systemic recurrence. Additionally, a longer operative duration may
be another factor affecting long-term clinical outcome.
The high rate of recurrence after surgery is the
main cause of late death of patients with lung cancer. The risk
factors include tumor size, insufficient safety margin and tumor
location. This meta-analysis suggests that there is no
statistically significant difference between the two groups. It
proved again that VATS is a safe and acceptable surgical procedure
when compared to open surgery. Also, VATS lobectomy with lymph node
dissection was similar to that carried out in open surgery
(25). Three clinical studies
(23–25) postoperatively reported that
pathological N1 was found in 14 patients from the open group, and
in 13 patients from the VATS group. These results were consistent
with various previous reports in which 10–25% of the T1 patients
were found to have lymph node metastasis (39,40).
Lymph node metastasis is a significant prognostic factor, and the
frequency of metastasis is high for clinical stage I. Therefore, we
do not omit mediastinal lymph node dissection. However, this
meta-analysis showed that systemic recurrence rate in the group
undergoing open lobectomy was significantly higher than that in the
VATS group. This also was partly explained by the reduced
invasiveness and preserved cellular immunity and less
immunosuppression. The higher 5-year overall survival rates and
similar 3-year overall survival rates in the VATS lobectomy group
compared to the open lobectomy group may be explained by the lower
systemic recurrence rates.
A growing body of evidence has shown that patients
with a tumor of 2 cm or less in diameter have a better survival
than those with a tumor of 2.1–3 cm associated with improved
curability of stage IA lung cancers, which we speculate is one of
the reasons for the better survival found in this meta-analysis
(41–43). In addition, the extended survival
may be further exaggerated by the limited number of patients
available (44,45). We considered that patient bias may
be a critical bias, and it should have influence on survival.
However, no studies have mentioned this issue. The pathological
type is also associated with prognosis. Sawada et al
(15) showed that BAC has been
detected more and more frequently, and is generally considered to
be associated with better prognosis than other types of
adenocarcinoma. BAC should be distinct from other adenocarcinomas.
The prognosis of BAC and BAC + papillary adenocarcinoma in VATS was
greater compared to the open lobectomy group. The BAC group showed
a 100% 5-year survival.
Limitations of the study
The conclusions of this meta-analysis are limited by
various factors. First, the number of studies that are included is
small. Only one randomized study (23) met our inclusion criteria. This may
have led to false positive or false negative conclusions (risk of
random errors). Second, the majority of data in the present study
comes from non-RCTs; therefore the overall level of clinical
evidence is low. Even the randomization procedure was unclear or
inadequate in the trials (46).
There is a concern for publication bias in the
studies included. Those surgeons with less than optimal experiences
or outcomes inferior to a thoracotomy would likely be less than
enthusiastic about publishing their data, if they were accepted for
publication at all. However, a firm conclusion about bias is
difficult to reach as the asymmetry of the funnel plot is minimal.
In addition, funnel plots show asymmetry for reasons other than
publication bias. Therefore, our pooled OR may be an overestimate
of the true effect. Due to data constraints, this meta-analysis
could not analyze the quality of life score and was unable to carry
out stratified analyses of other possible confounding factors. If
the method is to be more effective, then larger samples and
randomized controlled studies with longer follow-up are
required.
Furthermore, stage I includes IA and IB. Non-small
lung cancer can be classified into adenocarcinoma, squamous cell
carcinoma, large-cell carcinoma and adenosquamous carcinoma.
However, we failed to separate the analysis because of the limited
data of these trials.
In conclusion, VATS was superior to open lobectomy
in the 5-year overall survival rates of patients with stage I lung
cancer eligible for surgical treatments. VATS lobectomy is a
beneficial alternative to open lobectomy for selected cases of
pulmonary lesions and should be vigorously promoted, although the
procedure is more complex than conventional open lobectomy. The
successful completion of VATS lobectomy depends on adequate patient
selection, instrumentation and experience of the surgeon. However,
the findings have to be carefully interpreted due to the lower
levels of evidence (systematic error and random error). Further
RCTs are warranted to clarify the exact value of VATS and open
lobectomy for stage I lung cancer.
Acknowledgements
The work was supported by the National
Natural Science Foundation of China (Grant No. 30700821) and Liao
Ning BaiQianWan Talents Program (Grant No.2011921038).
References
|
1.
|
Levi JF, Kleinmann P, Riquet M and Debesse
B: Percutaneous parietal pleurectomy for recurrent spontaneous
pneumothorax. Lancet. 336:1577–1578. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kirby TJ, Mack MJ, Landreneau RJ and Rice
TW: Initial experience with video-assisted thoracoscopic lobectomy.
Ann Thorac Surg. 56:1248–1253. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
De Letter J and Proot L: Thoracoscopic
assisted lobectomy. Surg Laparosc Endosc. 5:12–16. 1995.
|
|
4.
|
Walker WS, Carnochan FM and Tin M:
Thoracoscopy assisted pulmonary lobectomy. Thorax. 48:921–924.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Roviaro G, Varoli F, Rebuffat C, Vergani
C, D'Hoore A, Scalambra SM, Maciocco M and Grignani F: Major
pulmonary resections: pneumonectomies and lobectomies. Ann Thorac
Surg. 56:779–783. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kirby TJ and Rice TW: Thoracoscopic
lobectomy. Ann Thorac Surg. 56:784–786. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cole FH Jr, Cole FH, Khandekar A, Maxwell
JM, Pate JW and Walker WA: Video-assisted thoracic surgery: primary
therapy for spontaneous pneumothorax? Ann Thorac Surg. 60:931–935.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sawada S, Watanabe Y and Moriyama S:
Video-assisted thoracoscopic surgery for primary spontaneous
pneumothorax: evaluation of indications and long-term outcome
compared with conservative treatment and open thoracotomy. Chest.
127:2226–2230. 2005. View Article : Google Scholar
|
|
9.
|
Giudicelli R, Thomas P, Lonjon T, Ragni J,
Bulgare JC, Ottomani R and Fuentes P: Major pulmonary resection by
video-assisted mini-thoracotomy. Initial experience in 35 patients.
Eur J Cardiothorac Surg. 8:254–258. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Solaini L, Bagioni P and Grandi U: Role of
video endoscopy in pulmonary surgery: present experience. Eur J
Cardiothorac Surg. 9:65–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
McKenna RJ Jr: Thoracoscopic lobectomy
with mediastinal sampling in 80-year-old patients. Chest.
106:1902–1904. 1994.PubMed/NCBI
|
|
12.
|
Roviaro G, Varoli F, Rebuffat C, Vergani
C, Maciocco M, Scalambra SM, Sonnino D and Gozi G: Video
thoracoscopic staging and treatment of lung cancer. Ann Thorac
Surg. 59:971–974. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kirby TJ, Mack MJ, Landreneau RJ and Rice
TW: Lobectomy – video-assisted thoracic surgery versus
muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc
Surg. 109:997–1002. 1995.
|
|
14.
|
Asamura H, Nakayama H, Kondo H, Tsuchiya R
and Naruke T: Video-assisted lobectomy in the elderly. Chest.
111:1101–1105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sawada S, Komori E, Yamashita M, et al:
Comparison in prognosis after VATS lobectomy and open lobectomy for
stage I lung cancer: retrospective analysis focused on a
histological subgroup. Surg Endosc. 21:1607–1611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sugiura H, Morikawa T, Kaji M, Sasamura Y,
Kondo S and Katoh H: Long-term benefits for the quality of life
after video-assisted thoracoscopic lobectomy in patients with lung
cancer. Surg Laparosc Endosc Percutan Tech. 9:403–408. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tashima T, Yamashita J, Nakano S, et al:
Comparison of video-assisted minithoracotomy and standard open
thoracotomy for the treatment of non-small-cell lung cancer. Minim
Invasive Ther Allied Technol. 14:203–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Flores RM, Park BJ, Dycoco J, et al:
Lobectomy by video-assisted thoracic (VATS) versus thoracotomy for
lung cancer. J Thorac Cardiovasc Surg. 138:11–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
McKenna RJ Jr and Houck WV: New approaches
to the minimally invasive treatment of lung cancer. Curr Opin Pulm
Med. 11:282–286. 2005. View Article : Google Scholar
|
|
20.
|
Shiraishi T, Shirakusa T, Miyoshi T, et
al: A completely thoracoscopic lobectomy/segmentectomy for primary
lung cancer – technique, feasibility, and advantages. Thorac
Cardiovasc Surg. 54:202–207. 2006.PubMed/NCBI
|
|
21.
|
Yang X, Wang S and Qu J: Video-assisted
thoracic surgery (VATS) compares favorably with thoracotomy for the
treatment of lung cancer: a five-year outcome comparison. World J
Surg. 33:1857–1861. 2009.PubMed/NCBI
|
|
22.
|
Whitson BA, Andrade RS, Boettcher A,
Bardales R, Kratzke RA, Dahlberg PS and Maddaus MA: Video-assisted
thoracoscopic surgery is more favorable than thoracotomy for
resection of clinical stage I non-small cell lung cancer. Ann
Thorac Surg. 83:1965–1970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shiraishi T, Shirakusa T, Hiratsuka M,
Yamamoto S and Iwasaki A: Video-assisted thoracoscopic surgery
lobectomy for c-T1N0M0 primary lung cancer: its impact on
locoregional control. Ann Thorac Surg. 82:1021–1026. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sakuraba M, Miyamoto H, Oh S, et al:
Video-assisted thoracoscopic lobectomy vs. conventional lobectomy
via open thoracotomy in patients with clinical stage IA
non-small-cell lung carcinoma. Interact Cardiovasc Thorac Surg.
6:614–617. 2007. View Article : Google Scholar
|
|
25.
|
Sugi K, Kaneda Y and Esato K:
Video-assisted thoracoscopic lobectomy achieves a satisfactory
long-term prognosis in patients with clinical stage IA lung cancer.
World J Surg. 24:27–31. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mathurin P, Raynard B, Dharancy S, et al:
Meta-analysis: evaluation of adjuvant therapy after curative liver
resection for hepatocellular carcinoma. Aliment Pharmacol Ther.
17:1247–1261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
McKenna RJ Jr, Houck W and Fuller CB:
Video-assisted thoracic surgery lobectomy: experience with 1,100
cases. Ann Thorac Surg. 81:421–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ohbuchi T, Morikawa T, Takeuchi E and Kato
H: Lobectomy: video-assisted thoracic surgery versus posterolateral
thoracotomy. Jpn J Thorac Cardiovasc Surg. 46:519–522. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Koizumi K, Haraguchi S, Hirata T, et al:
Video-assisted lobectomy in elderly lung cancer patients. Jpn J
Thorac Cardiovasc Surg. 50:15–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Tatsumi A and Ueda Y: Video-assisted
thoracic surgery for lung cancer: is it a feasible operation for
stage I lung cancer? Jpn J Thorac Cardiovasc Surg. 51:646–650.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Muraoka M, Oka T, Akamine S, et al:
Video-assisted thoracic surgery lobectomy reduces the morbidity
after surgery for stage I non-small-cell lung cancer. Jpn J Thorac
Cardiovasc Surg. 54:49–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Shigemura N, Akashi A, Funaki S, et al:
Long-term outcomes after a variety of video-assisted thoracoscopic
lobectomy approaches for clinical stage IA lung cancer: a
multi-institutional study. J Thorac Cardiovasc Surg. 132:507–512.
2006. View Article : Google Scholar
|
|
33.
|
Tajiri M, Maehara T, Nakayama H and
Sakamoto K: Decreased invasiveness via two methods of thoracoscopic
lobectomy for lung cancer compared with open thoracotomy.
Respirology. 12:207–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Petersen RP, Pham D, Burfeind WR, et al:
Thoracoscopic lobectomy facilitates the delivery of chemotherapy
after resection for lung cancer. Ann Thorac Surg. 83:1245–1250.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Park BJ, Zhang H, Rusch VW and Amar D:
Video-assisted thoracic surgery does not reduce the incidence of
postoperative atrial fibrillation after pulmonary lobectomy. J
Thorac Cardiovasc Surg. 133:775–779. 2007. View Article : Google Scholar
|
|
36.
|
Yim AP, Wan S, Lee TW and Arifi AA: VATS
lobectomy reduces cytokine responses compared with conventional
surgery. Ann Thorac Surg. 70:243–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Craig SR, Leaver HA, Yap PL, Pugh GC and
Walker WS: Acute phasere sponses following minimal access and
conventional thoracic surgery. Eur J Cardiothorac Surg. 20:455–463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ng CS, Wan S, Hui CW, Lee TW, Underwood MJ
and Yim AP: Video-assisted thoracic surgery for early stage lung
cancer – can short-term immunological advantages improve long-term
survival? Ann Thorac Cardiovasc Surg. 12:308–312. 2006.
|
|
39.
|
Naruke T, Goya T, Tsuchiya R and Suematsu
K: The importance of surgery to non-small cell carcinoma of lung
with mediastinal lymph node metastasis. Ann Thorac Surg.
46:603–610. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Hata E, Hayakawa K, Miyamoto H and
Hayashida R: Rationale for extended lymphadenectomy for lung
cancer. Theor Surg. 5:19–25. 1990.
|
|
41.
|
Wisnivesky JP, Yankelevitz D and Henschke
CI: The effect of tumor size on curability of stage I non-small
cell lung cancers. Chest. 126:761–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Sakao Y, Nakazono T, Sakuragi T, Natsuaki
M and Itoh T: Predictive factors for survival in surgically
resected clinical IA peripheral adenocarcinoma of the lung. Ann
Thorac Surg. 77:1157–1162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Okada M, Nishio W, Sakamoto T, Uchino K,
Yuki T, Nakagawa A and Tsubota N: Effect of tumor size on prognosis
in patients with non-small cell lung cancer: the role of
segmentectomy as a type of lesser resection. J Thorac Cardiovasc
Surg. 129:87–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Roviaro G, Varoli F, Vergani C, Nucca O,
Maciocco M and Grignani F: Long-term survival after video
thoracoscopic lobectomy for stage I lung cancer. Chest.
126:725–732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Walker WS, Codispoti M, Soon SY,
Stamenkovic S, Carnochan F and Pugh G: Long-term outcomes following
VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J
Cardiothorac Surg. 23:397–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Wood L, Egger M, Gluud LL, et al:
Empirical evidence of bias in treatment effect estimates in
controlled trials with different interventions and outcomes:
meta-epidemiological study. BMJ. 336:601–605. 2008. View Article : Google Scholar : PubMed/NCBI
|