Introduction
Glutamate, a normal constituent of retina, is the
primary chemical signal used by ganglion cells, photoreceptors and
bipolar cells. However, excessive stimulation by glutamate results
in neuronal injury and degeneration (1,2).
Müller cells, the major glial cells of the retina,
provide functional and structural support to the retinal neurons
and constitute a functional link between neurons and vessels. Among
all the roles, Müller cells play an important role in keeping the
extracellular levels of neurotransmitters low and regulating
synaptic transmission, such as glutamate (3–6).
Müller cells participate in glutamate metabolism by glutamate
aspartate transporter (GLAST) and glutamine synthetase (GS). GLAST
transports glutamate into Müller cells, and GS is the enzyme which
converts glutamate to glutamine inside Müller cells (7–9). In
several pathological states, such as hypoxia, edema and injury,
Müller cells can be rapidly activated, remove metabolic waste and
maintain the balance of the retinal extra-cellular environment to
protect retinal ganglion cells (RGCs).
Adenosine is considered a reactive metabolite
involved in cellular communication during periods of certain
pathological states. In the eye, adenosine levels have been shown
to increase during periods of retinal ischemia and hypoxia
(10,11). Four adenosine receptor subtypes,
A1, A2A, A2B and A3,
have been identified on the basis of their molecular and
pharmacological characteristics (12,13).
It is well known that A2A receptors are promoters of
excitotoxicity by directly stimulating glutamate outflow,
inhibiting glutamate uptake from neurons and glial cells in the
central nervous system (14,15).
In recent years, the neuroprotection afforded by A2A
blockade has been observed in animal models of several
neurodegenerative disorders, such as Huntington’s disease,
Alzheimer’s disease, epilepsy and excitotoxic conditions, including
ischemia and trauma (15–17).
The aim of this study was to investigate whether
A2A receptor (A2AR) antagonist (SCH 442416)
modulates the expression of GS and GLAST in retinal Müller cells
under hypoxic conditions in vitro.
Materials and methods
Drugs
The A2AR (SCH 442416),
2-(2-furanyl)-7-[3-(4-methoxyphernyl)
propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo
[1,5-c]pyrimidin-5-amine was purchased from Tocris Bioscience.
Cell separation and culture
Eye balls from post-natal day 0–3 Sprague-Dawley
rats (Slaccas Laboratory Animal Co., Ltd.) were enucleated, and the
retina of each was dissected free and stored on ice in D-Hank’s
solution (Anresco). Tissue was dissociated by centrifugation and
incubated for 15 min at 37°C in phosphate-buffered saline (PBS)
containing 0.125% trypsin (Anresco).
Finally, the cell suspension was cultured in T75
culture flasks at 37°C in humidified air containing 5%
CO2. After initial primary outgrowth, the cell culture
medium was replaced every 48 h, and maintained in DMEM/F12 medium
(Gibco) supplemented with 2 mM glutamine, 100 U/ml penicillin, 100
μg/ml streptomycin and 10% fetal bovine serum (FBS;
Sijiqing).
After 5–8 days, all the flasks were shaken at 37°C,
at 100 rpm for 1 h, and the cell culture medium was refreshed. By
shaking, other types of cells (microglial cells, RGCs), which
initially adhered to the surface of the Müller cells, were rinsed
off, and a purified flat cell population was obtained. For
passaging, cell cultures were incubated at 37°C with PBS containing
0.125% trypsin. Experiments were performed after second passage
when the cell confluence was 80–90%.
Cell proliferation in normoxia vs.
hypoxia
SCH 442416 was added to the cell culture medium at
the final concentrations of 0.1, 1 and 10 μM.
Müller cells were spread out in 6-well plates at
5x105/ml 24 h prior to the induction of hypoxia. For
hypoxia, the medium was changed to serum- and SCH 442416-free DMEM,
and the cultures were transferred to a humidified hypoxia chamber
(37°C, 94% nitrogen, 1% O2, 5% CO2) for 24 h.
For normoxia, the medium was changed to serum-free DMEM and the
cultures were placed in a humidified hypoxia chamber (37°C, 20%
O2, 5% CO2) for 24 h. After 24 h of
incubation, the cells were analyzed.
Immunocytochemistry
Müller cells, which were cultured under hypoxic
conditions for 24 h, were fixed in 4% paraformaldehyde for 10 min.
The coverslips were incubated overnight in the primary antibodies
anti-GFAP (1:200, polyclonal mouse anti-GFAP antibody; Abcam),
anti-GS (1:5,000, polyclonal rabbit anti-GS antibody; Abcam) at
4°C. Then, the coverslips were immunolabeled with fluoroscein
isothiocyanate (FITC; 1:200; Invitrogen) or Cy3 (1:200;
Biolegend)-linked anti-mouse or anti-rabbit IgG. The labeled cells
were visualized and processed by laser confocal microscopy
(Leica).
Gene expression analysis by quantitative
real-time PCR
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen). Each RNA sample was quality-controlled for
DNA and protein contamination. The cDNAs were reverse-transcribed
according to the manufacturer’s instructions. To analyze GS and
GLAST mRNA expression, the quantitative real-time PCR method was
used. The primer sequences were as follows: GS, sense
5′-ccgctcttcgtctcgttc-3′, antisense 5′-ctgcttgatgcctttgtt-3′;
GLAST, sense 5′-cctatgtggcagtcgttt-3′, antisense
5′-ctgtgatgggctggctaa-3′; β-actin, sense 5′-cccatctatgagggt
tacgc-3′, antisense 5′-tttaatgtcacgcacgatttc-3′. Different mRNA
levels were subsequently normalized to β-actin mRNA levels.
Statistical analysis
Data are reported as the means ± SEM, and were
analyzed by one-way ANOVA followed by LSD test for multiple
comparison. Differences were considered statistically significant
at P<0.05.
Results
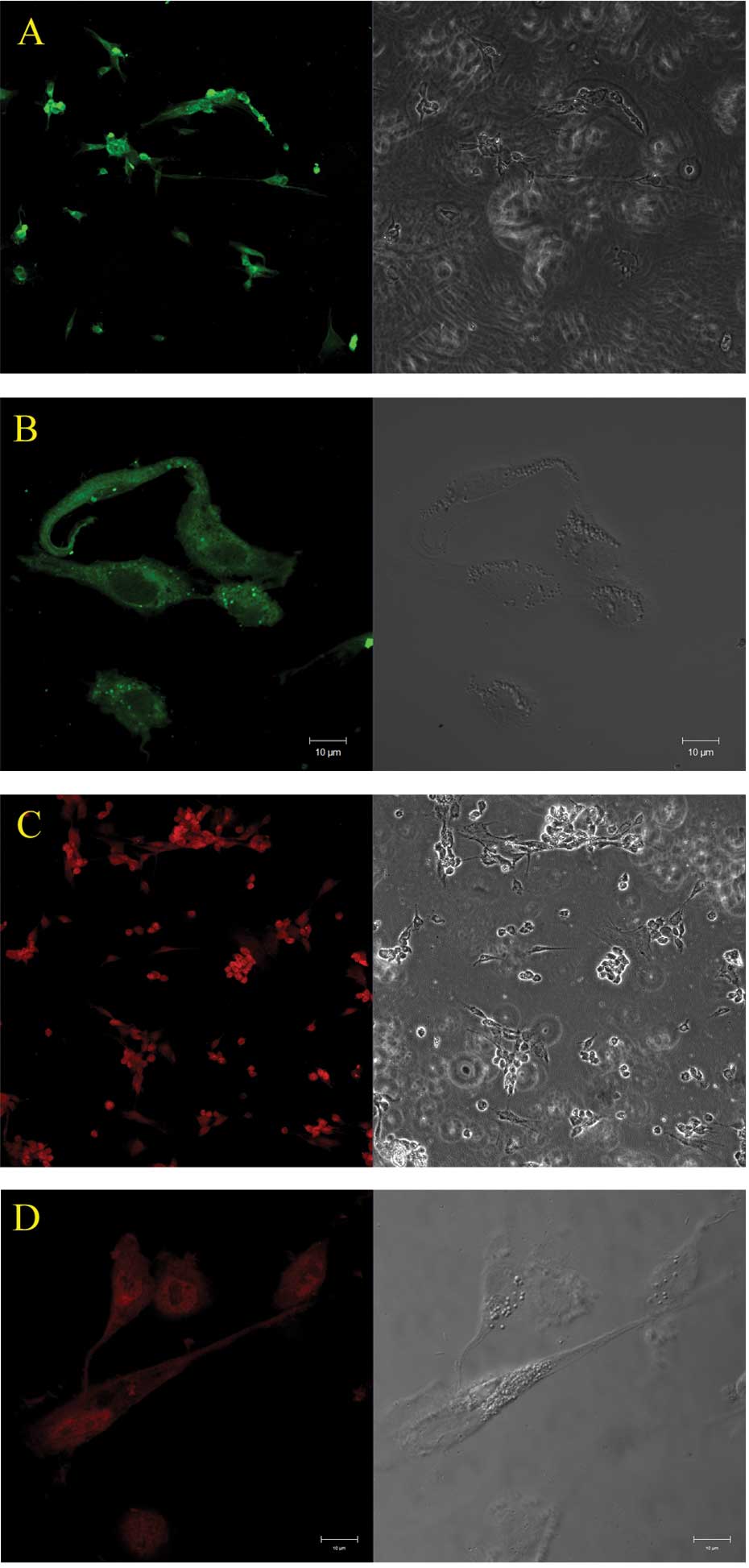
Expression of cytoskeletal proteins in
the cultured Müller cells
The cultured Müller cells under hypoxic conditions
showed positive labeling for GS and GFAP, the molecular markers for
Müller cells in the retina (Fig.
1).
GS and GFAP are two important cytoskeletal proteins
in retinal Müller cells. Fig. 1
shows the expression of these proteins by immunocytochemical
staining. In normal retina, Müller cells express little or no GFAP,
but become strong when the retina is damaged. GS is predominantly
expressed in the retina and has been used as a specific marker for
Müller cells (18–20). In our study, >90% of cells in
the culture system showed positive markers for GS and GFAP,
therefore these cells were identified as Müller cells.
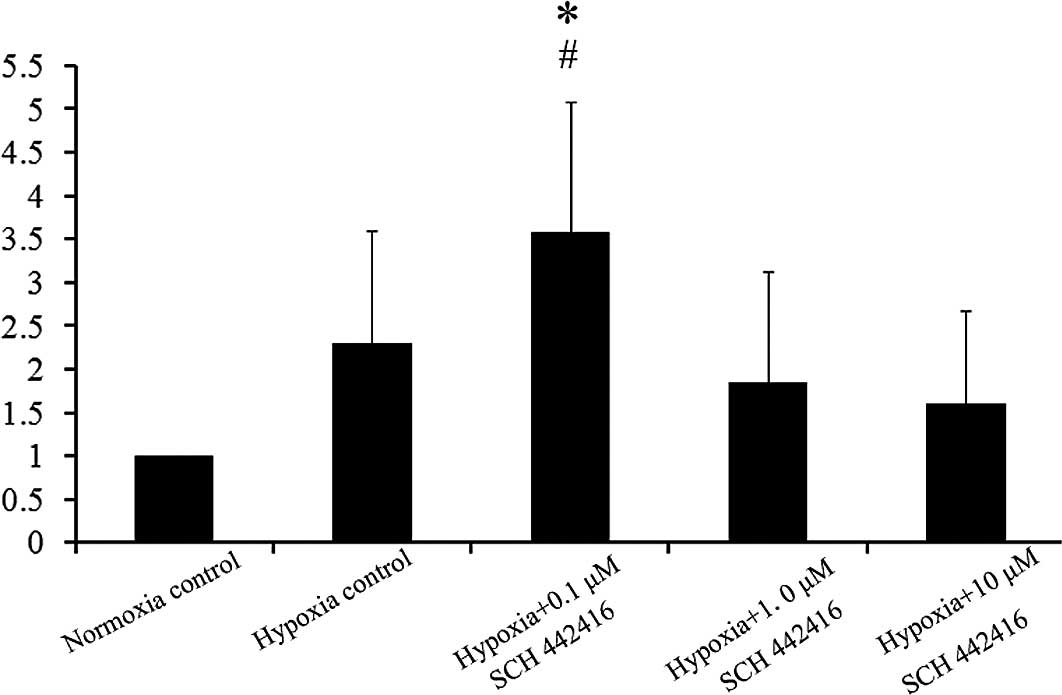
Effect of SCH 442416 on the mRNA
expression of GLAST in the cultured Müller cells
In the present study, we chose different
concentrations of SCH 442416 to carry out the experiment (0.1, 1
and 10 μM). The mRNA expression of GS was compared among
Müller cells incubated with the different concentrations of SCH
442416 cultured under hypoxic conditions. Real-time PCR showed that
the mRNA expression of GLAST was increased significantly when
Müller cells were cultured with 0.1 μM SCH 442416 under
hypoxic conditions, compared to the normoxia control and hypoxia
control (Fig. 2).
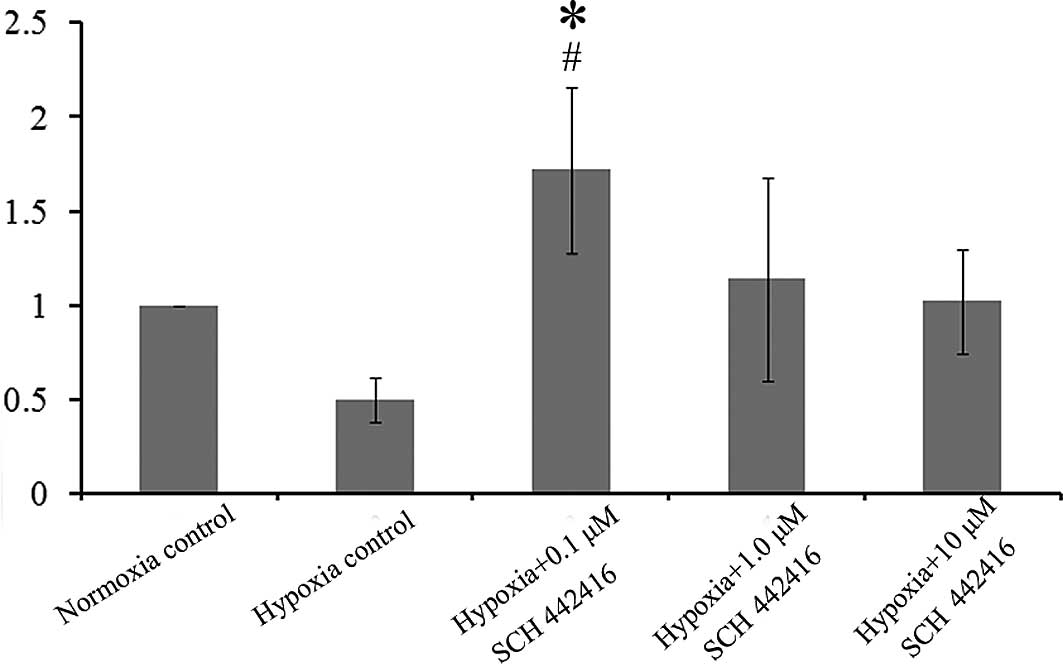
Effect of SCH 442416 on the mRNA
expression of GS in the cultured Müller cells
Real-time PCR showed that the mRNA levels of GS were
decreased in the hypoxia control compared to the normoxia control.
However, the mRNA expression of GS was increased significantly when
Müller cells were cultured with 0.1 μM SCH 442416 under
hypoxic conditions, compared to the normoxia control and hypoxia
control (Fig. 3).
Discussion
The results of the present study showed that Müller
cells increased the mRNA expression of GLAST under hypoxic
conditions. Cells treated with 0.1 μM SCH 442416 showed a
further significant increase in the mRNA expression of GLAST in
vitro (2). Although the mRNA
expression of GS was decreased under hypoxic conditions, the mRNA
expression was increased when Müller cells were treated with 0.1
μM SCH 442416.
Hypoxia certainly plays a central role in retinal
diseases, such as diabetes, retinal vascular occlusion and
glaucoma. It is an important cause of central neuronal damage
(21,22). In normal retina, Müller cells
express little or no GFAP, but the expression becomes strong when
the retina was damaged. Our study also confirmed that hypoxia
results in the increased expression of GFAP.
GS is a major enzyme involved in the metabolism of
glutamate in glial cells. GS catalyzes the amidation of glutamate
to glutamine, which is an essential part of the cycling of the
transmitter pool of glutamate between neurons and glia. Decreased
GS activity leads to neuronal damage by allowing extracellular
glutamate to accumulate. Decreased GS activity has also been
reported after hypoxia or ischemia in the brain (24,25).
However, research has revealed that there is a slight change in the
expression of GS and even increased GS activity in some retinal
damage (26–28). In our study, we found that the mRNA
expression of GS was decreased in hypoxia, nevertheless, there was
no statistical significance. A low concentration of A2AR
antagonist reversed these changes (Fig. 3).
GLAST is the predominant glutamate transporter in
Müller cells. Several studies have indicated that GLAST is
upregulated by hypoxia (29,30),
while others observed the opposite phenomenon (31,32).
In our experiment, we detected GLAST mRNA upregulation in hypoxia,
and a low concentration of the A2AR antagonist caused a
further increase.
According to the glutamate cycle, we presume that
the increase in GS and GLAST accelerates the transport and
clearance of glutamate in the retina to protect the neurons. Our
results suggest that the A2AR antagonist was capable of
significantly upregulating GS and GLAST, and maintained glutamate
homeostasis by regulating the glutamate uptake and metabolism of
Müller cells under hypoxic conditions.
In recent years, the A2AR antagonist has
been viewed as an attractive option to improve the treatment of
neurological disorders (33,34).
Based on the results described here, we regard the A2AR
antagonist as a new option for the neuroprotection of retinal
Müller cells under hypoxic conditions. These data provide
additional evidence for constructing a model of protection against
hypoxia in the retina, which invites future studies to explore the
function of the A2AR antagonist in ophthalmology.
Acknowledgements
This study was funded by Shanghai
Leading Academic Discipline Project (S30205), Joint research
Project of Shanghai Municipal Level for Emerging Cutting-edge
Technology (SHDC12010107), Projects of Shanghai Muncipal Health
Bureau (2008159) and National Natural Science Foundation of China
(81070760).
References
|
1.
|
Kalloniatis M and Napper G: Retinal
neurochemical changes following application of glutamate as a
metabolic substrate. Clin Exp Optom. 85:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Barabas P, Kovacs I, Kardos J, et al:
Exogenous glutamate and taurine exert differential actions on
light-induced release of two endogenous amino acids in isolated rat
retina. J Neurosci Res. 73:731–736. 2003. View Article : Google Scholar
|
|
3.
|
Walsh N, Valter K and Stone J: Cellular
and subcellular patterns of expression of bFGF and CNTF in the
normal and light stressed adult rat retina. Exp Eye Res.
72:495–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Harada T, Harada C and Kohsaka S:
Microglia-Müller glia cell interactions control neurotrophic factor
production during light-induced retinal degeneration. J Neurosci.
22:9228–9236. 2002.
|
|
5.
|
Pow D and Crook D: Direct
immunocytochemical evidence for the transfer of glutamine from
glial cells to neurons: use of specific antibodies directed against
the D-steroisomers of glutamate and glutamine. Neuroscience.
70:295–302. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Newman E and Reichenbach A: The Müller
cell: a functional element of the retina. Trends Neurosci.
19:307–312. 1996.
|
|
7.
|
Otori Y, Shimada S, Tanaka K, Ishimoto I,
Tano Y and Tohyama M: Marked increase in glutamate-aspartate
transporter (GLAST/GluT-1) mRNA following transient retinal
ischemia. Brain Res Mol Brain Res. 27:310–314. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Iandiev I, Wurm A, Hollborn M, et al:
Müller cell response to blue light injury of the rat retina. Invest
Ophthalmol Vis Sci. 49:3559–3567. 2008.
|
|
9.
|
Woldemussie E, Wijono M and Ruiz G: Müller
cell response to laser induced increase inintraocular pressure in
rats. Glia. 47:109–119. 2004.
|
|
10.
|
Roth S, Rosenbaum P, Osinski J, et al:
Ischemia induces significant changes in purine nucleoside
concentration in the retina-choroid in rats. Exp Eye Res.
65:771–779. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Taomoto M, McLeod D, Merges C, et al:
Localization of adenosine A2a receptor in retinal development and
oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 41:230–242.
2000.PubMed/NCBI
|
|
12.
|
Daines B, Kent A, McAleer M, et al:
Intraocular adenosine levels in normal and ocular-hypertensive
patients. J Ocul Pharmacol. 19:113–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ralevia V and Burnstock G: Receptors fro
purines and pyrimidines. Pharmacol Rev. 50:413–492. 1998.
|
|
14.
|
Patemiti I, Melani A, Cipriani S, et al:
Selective adenosine A2A receptor agonists and antagonists protect
against spinal cord injury through peripheral and central effects.
J Neuroinflammation. 8:312011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sebastião A and Ribeiro J: Triggering
neurotrophic factor actions through adenosine A2A receptor
activation: implications for neuroprotection. Br J Pharmacol.
158:15–22. 2009.PubMed/NCBI
|
|
16.
|
Leite M, Wilhelm E, Jesse C, et al:
Protective effect of caffeine and a selective A2A receptor
antagonist on impairment of memory and oxidative stress of aged
rats. Exp Gerontol. 46:309–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Marcellino D, Lindqvist E, Schneider M, et
al: Chronic A2A antagonist treatment alleviates Parkinsonian
locomotor deficiency in MitoPark mice. Neurobiol Dis. 40:460–466.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rauen T and Wiessner M: Fine tuning of
glutamate uptake and degradation in glial cells: common
transcriptional regulation of GLAST1 and GS. Neurochem Int.
37:179–189. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shen X, Zhong Y, Xie B, et al: Pigment
epithelium derived factor as an anti-inflammatory factor against
decrease of glutamine synthetase expression in retinal Müller cells
under high glucose conditions. Graefes Arch Clin Exp Ophthalmol.
248:1127–1136. 2010.
|
|
20.
|
Shen F, Chen B, Danias J, et al:
Glutamate-induced glutamine synthetase expression in retinal Müller
cells after short-term ocular hypertension in the rat. Invest
Ophthalmol Vis Sci. 45:3107–3112. 2004.
|
|
21.
|
Kitano S, Morgan J and Caprioli J: Hypoxic
and excitotoxic damage to cultured rat retinal ganglion cells. Exp
Eye Res. 63:105–112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Choi D: Glutamate neurotoxicity and
diseases of the nervous system. Neuron. 1:623–634. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Grosche J, Härtig W and Reichenbach A:
Expression of glial fibrillary acidic protein (GFAP), glutamine
synthetase (GS), and Bcl-2 protooncogene protein by Müller (glial)
cells in retinal light damage of rats. Neurosci Lett. 185:119–122.
1995.PubMed/NCBI
|
|
24.
|
Chen C, Alyahya K, Gionfriddo J, et al:
Loss of glutamine synthetase immunoreactivity from the retina in
canine primary glaucoma. Vet Ophthalmol. 11:150–157. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Krajnc D, Neff N and Hadjiconstantinou M:
Glutamate, glutamine and glutamine synthetase in the neonatal rat
brain following hypoxia. Brain Res. 707:134–137. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Woldemussie E, Wijono M and Ruiz G: Müller
cell response to laser-induced increase in intraocular pressure in
rats. Glia. 47:109–119. 2004.
|
|
27.
|
Zhang S, Wang H, Lu Q, et al: Detection of
early neuron degeneration and accompanying glial responses in the
visual pathway in a rat model of acute intraocular hypertension.
Brain Res. 1303:131–143. 2009. View Article : Google Scholar
|
|
28.
|
Ishikawa M, Yoshitomi T, Zorumski C and
Izumi Y: Effects of acutely elevated hydrostatic pressure in a rat
ex vivo retinal preparation. Invest Ophthalmol Vis Sci.
51:6414–6423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Imasawa M, Kashiwagi K, Iizuka Y, et al:
Different expression role among glutamate transporters in rat
retinal glial cells under various culture conditions. Brain Res Mol
Brain Res. 142:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bringmann A, Skatchkov S, Pannicke T, et
al: Müller glial cells in anuran retina. Microsc Res Tech.
50:384–393. 2000.
|
|
31.
|
Pow D, Naidoo T, Lingwood B, et al: Loss
of glial glutamate transporters and induction of neuronal
expression of GLT-1B in the hypoxic neonatal pig brain. Brain Res
Dev Brain Res. 153:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Nickell J, Salvatore M, Pomerleau F, et
al: Reduced plasma membrane surface expression of GLAST mediates
decreased glutamate regulation in the aged striatum. Neurobiol
Aging. 28:1737–1748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pepponi R, Ferrante A, Ferretti R, Martire
A and Popoli P: Region-specific neuroprotective effect of ZM 241385
towards glutamate uptake inhibition in cultured neurons. Eur J
Pharmacol. 617:28–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Morelli M, Carta A and Jenner P: Adenosine
A2A receptors and Parkinson’s disease. Handb Exp Pharmacol.
193:589–615. 2009.
|