Introduction
Infertility has been considered to be a worldwide
problem that affects 9–15% of the childbearing population and 55%
of those affected will seek medical advice in the hope of achieving
parenthood (1). The fertilization
process of oocytes appears to be simple. However, multiple
signaling pathways are involved in this process, and numerous
factors can regulate these pathways, including various minerals,
specific proteins and a multitude of nutrients. Unfortunately,
little is known concerning the fertilization process of oocytes.
Many relevant questions must be addressed. For example, many
embryos develop well but cannot achieve implantation, and many
oocytes appear healthy but are unable to be fertilized. We know
that embryos from fertilization to future development require
sufficient nutrition, but the specific nutrients which play an
important role are yet unknown. Although numerous substances have
been demonstrated to exist in oocytes (2), the roles of these substances and the
relevant molecular regulatory mechanisms remain to be clarified.
Additionally, with the increasing popularity of in vitro
fertilization (IVF) as a method of human reproduction in recent
years, a deeper understanding of the molecular composition of
oocytes and their roles may help increase the IVF pregnancy
rate.
During mammalian oogenesis, the oocyte undergoes two
cell cycle arrests at the dictyate or germinal vesicle (GV) stage
and the metaphase II (MII) stage. The primary cellular functions of
ovulated MII-arrested mammalian oocytes are to bind and fuse with a
sperm, successfully reprogram and combine the two haploid genomes,
and facilitate early mitotic divisions until the embryo can
initiate and carry out its own molecular programs. MII oocytes have
been widely used to investigate the effect of osmotic stress on the
developmental competence and to perform various studies, including
routine oocyte cryo-preservation and the reprogramming of somatic
cell nuclei (3). In contrast,
oocytes at GV stage show no reprogramming activity, and zygotes
exhibit low or no reprogramming activity. A comparison of the
proteomes of oocytes at different developmental stages may help
identify the factors responsible for fertilization and
implantation.
Reproductive risk related to certain proteins and
nutrients between the oocyte and zygote is a critical step in
determining fertilization. Some proteins and nutrients are
associated with reproductive risks, which are critical in
determining fertilization. To date, most functional analyses of
oocyte proteins have been limited to proteins that are primarily
expressed in the oocyte. However, functional analysis of other
abundant non-oocyte-restricted proteins in a developmental context
would also be highly productive. In the present study, proteomes of
mouse oocytes at different developmental stages were analyzed using
an LTQ Orbitrap mass spectrometer. Numerous proteins identified in
this study have not been previously reported, and some proteins
were abundantly expressed in the oocytes at different developmental
stages. These proteins may be vital nutritional elements for
developing oocytes.
Materials and methods
The materials and methods for this study are the
same as described previously (4).
The SPF (specific pathogen-free) grade hybrid B6D2F1
(C57BL/6xDBA/2) mice were housed at the animal facility of the
National Institute of Biological Sciences. All studies adhered to
procedures consistent with the National Institute of Biological
Sciences Guide for the Care and Use of Laboratory Animals.
Results
Identification of total proteins
expressed in oocytes at different developmental stages
Over 7,000 oocytes were collected at each
developmental stage from the mouse strain most commonly used as a
recipient for proteome. From these oocytes, we successfully
identified 3,892 peptides in the GV stage, 185,643 peptides in the
MII oocyte and 85,369 peptides in the zygote using an LTQ Orbitrap
mass spectrometer. According to this criterion, the total protein
numbers identified in GV, MII and zygotes were 2,354, 2,973 and
2,082, respectively. Furthermore, various specific proteins,
substances related to metabolism and signaling pathways were
analyzed.
Ybx2
Ybx2, a member of the multifunctional Y-box protein
family, is implicated to play a key role in repressing the
translation of paternal mRNAs. The expression of Ybx2 in mouse
oocytes has been demonstrated previously (5). Here, the expression of Ybx2 was
detected in mouse oocytes and zygotes. The results showed that Ybx2
was a very abundant protein in GV oocytes, and its level gradually
decreased with the development of oocytes. The expression of Ybx2
in zygotes was very low. The decreased expression of Ybx2 protein
is essential for oocyte maturation and fertilization.
Heat shock-related genes
The expression of heat shock proteins (HSPs)
increases when cells are exposed to elevated temperatures or other
stress (6), and HSPs function as
molecular chaperones, which play a critical role in protein folding
and unfolding, intracellular trafficking of proteins and coping
with proteins denatured by heat or other stresses (7). Sixteen HSPs (Hspa8, Hsp90aa1, Hspa2,
Hspa4, Hspa1b, Hspa9, Trap1, Hsbp1, Hspe1, Hspa4l Isoform 1, Hspa4l
Isoform 2, Hspa14 Isoform 1, Hspa14 Isoform 2, Hspd1, Ahsa1 and
Hsph1) were found to be expressed in the different developmental
stages of oocytes; most exhibited high expression in the MII
oocytes, but low expression in the zygotes. Hspa8, Hsp90aa1 and
Hspd1 were highly expressed in all three stages and their
expression levels significantly decreased in the zygotes. Hspa9 was
identified only in the GV stage. When oocytes develop into zygotes,
oocytes consume a plentitude of nutrition previously stored in the
GV and MII stage. The expression of certain HSPs had a downward
trend, confirming the fact that oocytes consume HSPs in order to
adapt to adverse environments.
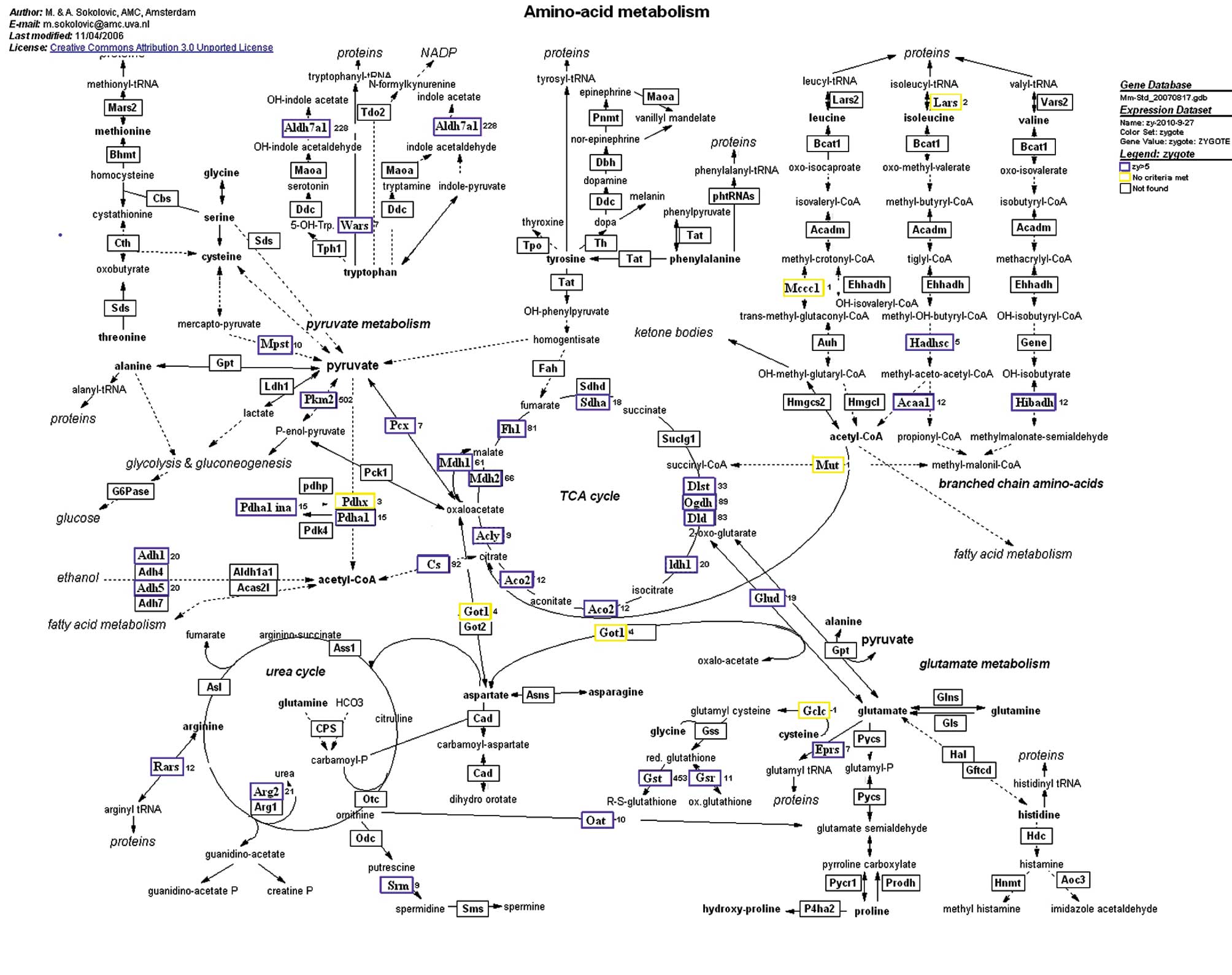
Amino acid metabolism
Amino acids are vital nutritional elements and this
dependency increases throughout the pre-implantation period
(8). The presence of amino acids
in maturation media increases the success rate of IVF cycles and
the number of embryos that reach the blastocyst stage (9). We found that proteins involved in
amino acid metabolism were highly enriched in the zygotes (Fig. 1), where they may play essential
roles in the development of mammalian pre-implantation embryos.
Forty proteins were identified in zygotes and 33 exhibited high
expression. In amino acid metabolism, zygotes express many key
factors involved in energy, including Aldh7a1 and Pkm2. Aldh7a1 was
detected in mouse embryos and extraembryonic cells; it regulates
energy production through altering aldehyde dehydrogenase (NAD)
activity (10). Amino acids
provide energy for fertilization and the developmental process of
zygotes. Therefore, amino acid metabolism is critical for
pregnancy.
Pyruvate metabolism
Much attention has been paid to pyruvate metabolism,
since it is an important energy source for the development of
oocytes. PKM2 exhibited high expression in MII oocytes. PKM2,
belonging to pyruvate kinase, is considered to be a protein
associated with energy metabolism (11). The fertilization of oocytes
requires the involvement of energy metabolism in which PKM2 is
consumed. Apart from PKM2, numerous proteins associated with
pyruvate metabolism were detected in our study. Thus, pyruvate
metabolism not only provides energy, but also plays an important
role in the development of oocytes. However, detailed effects of
pyruvate remain to be clarified.
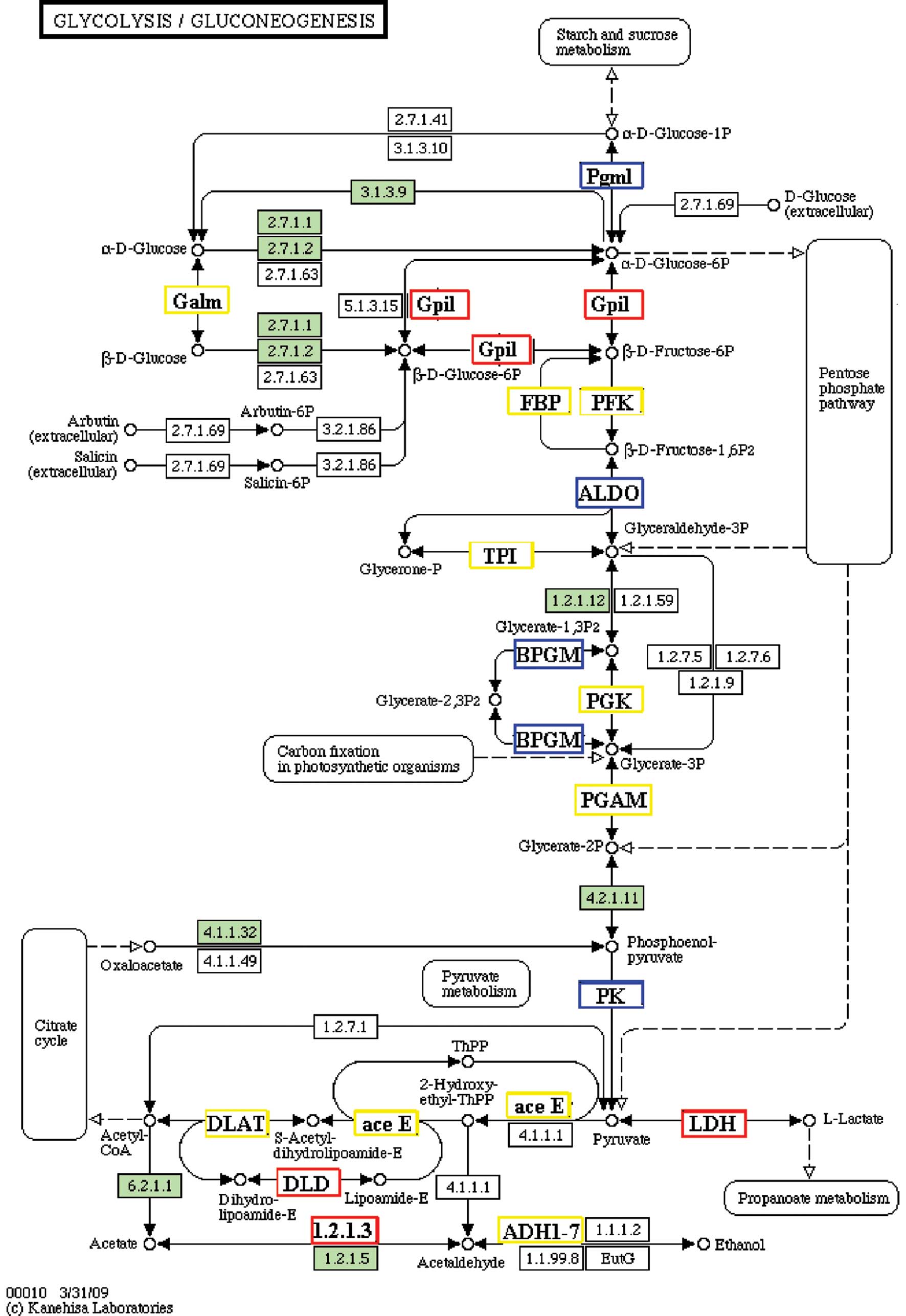
Glycolysis or gluconeogenesis
Glycolysis is a decomposition process to provide
energy; gluconeogenesis is a synthesis process to store energy.
Since oocytes themselves are unable to take up L-alanine and poorly
metabolize glucose for energy production, they obtain these amino
acids and products of glycolysis, which are essential for their
development and function (12). In
our study, many proteins were detected in both pathways at all
developmental stages of oocytes (Fig.
2). There are always different proteins exhibiting high
expression at every stage of oocyte development. It appears that
glycolysis and gluconeogenesis are both important metabolic
processes in the development of oocytes.
Discussion
During oogenesis in mammals, the oocyte grows by
more than two orders of magnitude, produces large quantities of a
myriad of macromolecules and undergoes a complex series of
morphologic and developmental changes (13). A tremendous energy toll is required
for the developmental process of oocytes. Oocytes must meet their
energy requirements during development by modulating a number of
metabolic pathways that generate ATP. Additionally, nutrition is
essential for sustaining life, and lack of various nutritional
components may induce fertilization failure or embryonic dysplasia.
Therefore, the relationship between nutrients and embryonic
development was addressed in the present study. Compared with the
methods previously used, in the present study, more accurate
semi-quantitative MS proteomic analysis was employed to detect the
composition of the nutrients found in oocytes and zygotes (14). The results obtained from our recent
proteomic studies have provided a significant molecular insight
into the processes of oocyte maturation, fertilization and
pre-implantation development.
Based on the evidence obtained from previous
metabolic labeling experiments and recent proteomic analysis, it
appears that synthesis of these proteins begins during oocyte
growth, and these factors are then stored in the egg for future
utilization following fertilization (15). We found that a number of proteins
were highly expressed in MII oocytes or in GV stage oocytes, but
exhibited low expression in zygotes, further supporting this
hypothesis. In the present study, we identified Astl that has never
been associated with embryonic development. It should be noted that
the oocytes stored many nutrients for further development, and the
expression of Astl was increased 100-fold in the MII oocytes when
compared with that in the zygotes, indicating that Astl is a
crucial factor in early embryo development.
Twelve selenium (Se)-binding proteins were detected
in this study, and they were expressed in different stages. Se has
been demonstrated to be an essential element for normal testicular
development, spermatogenesis and spermatozoa motility, indicating
its essential effect for fertility (16). It has been reported that Se
deficiency lowers the reproduction rates in humans as well as in
animals (17). Previous studies
have shown that mouse pre-implantation embryos exposed to oxidative
stress normally implant when cultured with insulin-like growth
factor I and II, epidermal growth factor, insulin, transferrin and
selenium (18). Therefore, 12
Se-binding proteins may be critical for the developing of oocytes.
Human reproductive cells are also very sensitive to environmental
changes. For example, the expression of HSPs has been reported to
exhibit differential expression levels at different stages of
development during spermatogenesis. Therefore, HSPs were indicated
to play a significant role in the oogenesis and development of
oocytes. However, more detailed studies are needed to clarify this
issue. Numerous proteins involved in energy metabolism were
detected, and some of these proteins were significant
differentially expressed in oocytes or zygotes. These proteins
found to have increased expression only in MII oocytes may be
special factors for storage in the MII oocytes, in order to prepare
for the period of fertilization. The fertilization of oocytes
requires energy; thus energy metabolism is extremely important.
Proteins such as, Aldh7a1, Pkm2 and Ckb that are highly expressed
in zygotes may be associated with energy provisions for the
development of oocytes.
In recent years, IVF has become a major treatment
method for infertility when other methods of assisted reproductive
technologies fail. Despite recent advances in IVF technology, many
important issues associated with IVF need to be resolved. For
example, low implantation rates and high multiple pregnancy rates
may lead to our inability to accurately assess the reproductive
potential of individual embryos. Development of non-invasive
predictors is crucial to overcome the limitations of morphologic
observation as assessment of the reproductive potential of an
embryo. Our recent results provide the possibility of selection of
the most viable embryos using amino acid and carbohydrate
metabolism as predictors. In summary, our recent study provides a
valuable basis and novel method for the increased understanding of
the molecular mechanisms of early embryonic development and
non-invasive quality assessment of embryos.
Acknowledgements
This project is supported by the China
Postdoctoral Science Foundation.
References
|
1.
|
Gurunath S, Pandian Z, Anderson RA and
Bhattacharya S: Defining infertility - a systematic review of
prevalence studies. Hum Reprod Update. 17:575–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yurttas P, Morency E and Coonrod SA: Use
of proteomics to identify highly abundant maternal factors that
drive the egg-to-embryo transition. Reproduction. 139:809–823.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Boiso I, Marti M, Santalo J, Ponsa M,
Barri PN and Veiga A: A confocal microscopy analysis of the spindle
and chromosome configurations of human oocytes cryopreserved at the
germinal vesicle and metaphase II stage. Hum Reprod. 17:1885–1891.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wang S, Ou D, Yin J, Wu G and Wang J:
Proteome of mouse oocytes at different developmental stages. Proc
Natl Acad Sci USA. 107:17639–17644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yu J, Hecht NB and Schultz RM: Expression
of MSY2 in mouse oocytes and preimplantation embryos. Biol Reprod.
65:1260–1270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
De Maio A: Heat shock proteins: facts,
thoughts, and dreams. Shock. 11:1–12. 1999.
|
|
7.
|
Akerfelt M, Vihervaara A, Laiho A, Conter
A, Christians ES, Sistonen L and Henriksson E: Heat shock
transcription factor 1 localizes to sex chromatin during meiotic
repression. J Biol Chem. 285:34469–34476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Leese HJ, Sturmey RG, Baumann CG and
McEvoy TG: Embryo viability and metabolism: obeying the quiet
rules. Hum Reprod. 22:3047–3050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gardner DK, Lane M, Spitzer A and Batt PA:
Enhanced rates of cleavage and development for sheep zygotes
cultured to the blastocyst stage in vitro in the absence of serum
and somatic cells: amino acids, vitamins, and culturing embryos in
groups stimulate development. Biol Reprod. 50:390–400. 1994.
View Article : Google Scholar
|
|
10.
|
Sorolla MA, Rodriguez-Colman MJ, Tamarit
J, Ortega Z, Lucas JJ, Ferrer I, Ros J and Cabiscol E: Protein
oxidation in Huntington disease affects energy production and
vitamin B6 metabolism. Free Radic Biol Med. 49:612–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Macintyre AN and Rathmell JC: PKM2 and the
tricky balance of growth and energy in cancer. Mol Cell.
42:713–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Eppig J: Mouse oocytes control metabolic
co-operativity between oocytes and cumulus cells. Reprod Fertil
Dev. 17:1–2. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Borrell C, Rué M, I Pasarin M, Benach J
and E Kunst A: The measurement of inequalities in health. Gac
Sanit. 14:20–33. 2000.
|
|
14.
|
Malmstrom J, Beck M, Schmidt A, Lange V,
Deutsch EW and Aebersold R: Proteome-wide cellular protein
concentrations of the human pathogen Leptospira interrogans.
Nature. 460:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tsujii H, Taniguchi N and Hamano K:
Development of Mongolian gerbil embryos in chemically defined
media: effects of osmolarity, glucose and phosphate. J Reprod Dev.
50:653–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hawkes WC and Turek PJ: Effects of dietary
selenium on sperm motility in healthy men. J Androl. 22:764–772.
2001.PubMed/NCBI
|
|
17.
|
Tang CC, Chen HN and Rui HF: The effects
of selenium on gestation, fertility, and offspring in mice. Biol
Trace Elem Res. 30:227–231. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Meldrum DR: Factors affecting embryo
implantation after human in vitro fertilization. Am J Obstet
Gynecol. 165:1896–1897. 1991. View Article : Google Scholar : PubMed/NCBI
|