Introduction
Constipation is a common digestive disease in
children, which accounts for 3–5% of general pediatric visits and
up to 25% of children with gastroenterological disorders (1). Constipation in children is presented
as a decrease in stool frequency, firm or hard stools, difficult
and painful defecation and voluntary stool retention. Constipation
affects the emotion, appetite and quality of life of afflicted
children. Conventional treatments for constipation include bowel
habit training, intake of fiber-enriched food, increased drinking
of water and physical exercise, and the use of various laxatives
and stool softeners (2).
Polyethylene glycol 4000 (PEG 4000, Forlax) is a non-toxic,
hydrosoluble, high-molecular polymer, which is not absorbed in the
gastrointestinal tract following oral administration. PEG 4000 acts
as an osmotic agent that increases fecal water content. A number of
clinical studies have demonstrated that PEG 4000 is effective in
the treatment of constipation in adults and children (3–7). In
order to investigate the application of PEG 4000 in Chinese
children, we conducted a multicenter study involving 216 children
(8–18 years of age) suffering from constipation from 7 hospitals in
China. This study aimed to investigate the efficacy and safety of
PEG 4000 treatment compared to that of lactulose treatment.
Patients and methods
Patients
Patients were selected from 227 children (8–18 years
of age) who visited pediatric clinics with symptoms of constipation
at the 7 participating hospitals in China from July 2004 to March
2005. Subjects were selected based on: i) symptoms which consisted
of weekly stool frequency of 2 or less and stool consistency type
1–3 (Bristol Stool Scale) for at least 2 weeks; ii) no presence of
organic or systemic disease; iii) no initiation of any treatment
for constipation or administration of drugs affecting
gastrointestinal movements; iv) obtainment of informed consent by
patients and parents or guardians. The procedures were approved by
the Ethics Committee for Research and Education of the Fourth
Military Medical University, Xi’an, China. Among the 227 patients,
11 cases were excluded due to lack of medical records. A total of
216 subjects of either gender were randomly assigned into two
groups. The PEG 4000 treatment group consisted of 105 cases and the
control lactulose treatment group consisted of 111 cases.
Methods
Treatments
Patients in the PEG 4000 treatment group received 20
g of PEG 4000 dissolved in a glass of water or drink each morning
before breakfast for two weeks. Patients in the lactulose treatment
group received 15 ml of lactulose (10 g) oral solution (Duphlac)
per day after breakfast for the first 3 days, and then 10 ml (6.7
g) per day for the following 11 days.
Study design
A blind randomized study design was adopted to
reduce possible deviation in the statistical testing. First, a
biostatistician constructed random digit tables using statistical
software SAS v8.2. Then PEG 4000 and lactulose were digitally
labeled. The researchers in each study center received
corresponding drugs from drug administrators according to the
patient sequence.
Assessment of the treatments
The efficacy and safety of the PEG 4000 treatment
were compared with the lactulose treatment according to the
following primary and secondary parameters.
The examined primary efficacy parameters included
weekly stool frequency and stool consistency. The stool consistency
was classified into types according to the Bristol Stool Scale: 1,
separate hard lumps; 2, sausage-shaped, but lumpy; 3, like a
sausage but with cracks; 4, like sausage or snake, smooth and soft;
5, soft blobs with clear-cut edges; 6, fluffy pieces with ragged
edges, a mushy stool; 7, watery without solid pieces.
The examined secondary efficacy parameters included
the remission rate of abdominal pain and the rate of clinical
remission. Clinical remission was achieved when the weekly stool
frequency became >3 and stool consistency was normalized
(Bristol type 4–6).
Safety of the treatments was evaluated by clinical
observation and laboratory tests. Patients were monitored for
adverse symptoms, including abdominal pain, diarrhea and blood in
the stools. Laboratory tests, including full blood counts, urine
tests, liver function tests (transaminases eg., AST and alkaline
phosphatase), renal function (creatinine and blood urea nitrogen),
blood glucose, and serum concentrations of sodium, potassium,
calcium and phosphorus, were conducted. Blood pressure, pulse,
height and weight were also monitored by physical examinations.
Statistical analysis
Data were analyzed by statistical software SAS v8.2.
Differences in the stool frequencies between the two groups were
examined by the Wilcoxon rank-sum test. The Cochran Mantel Haenszel
χ2 test was used to compare the stool consistencies, the
normal rate of stool frequency, and the normal rate of stool
consistency. One-way ANOVA tests were used to compare the age,
weight and height between the two groups, while the χ2
test was used to compare the gender proportion between the two
groups.
The clinical remission rates and remission rates of
abdominal pain were compared between the two group by using
χ2 tests. Using descriptive statistics, a safety
evaluation was made according to results from laboratory tests,
physical examinations, vital signs and adverse events.
Results
Study population
As shown in Table
I, there were no significant differences in the gender, age,
weight and height of the patients between the PEG 4000 and the
lactulose group.
 | Table I.Comparison of the study
population. |
Table I.
Comparison of the study
population.
| Characteristics | PEG group
(n=105) | Lactulose group
(n=111) | Statistics | P-value |
|---|
| Gender | | | | |
| Male | 43 (40.95%) | 47 (42.34%) |
χ2=0.043 | 0.8359 |
| Female | 62 (59.05%) | 64 (57.66%) | | |
| Age (years) | 11.29±2.80 | 11.20±2.75 | F=0.055 | 0.8149 |
| Weight (kg) | 36.23±11.65 | 35.74±10.99 | F=0.104 | 0.7477 |
| Height (cm) | 143.57±14.21 | 144.22±14.12 | F=0.115 | 0.7348 |
Effect of PEG 4000 and lactulose on stool
frequency and stool consistency
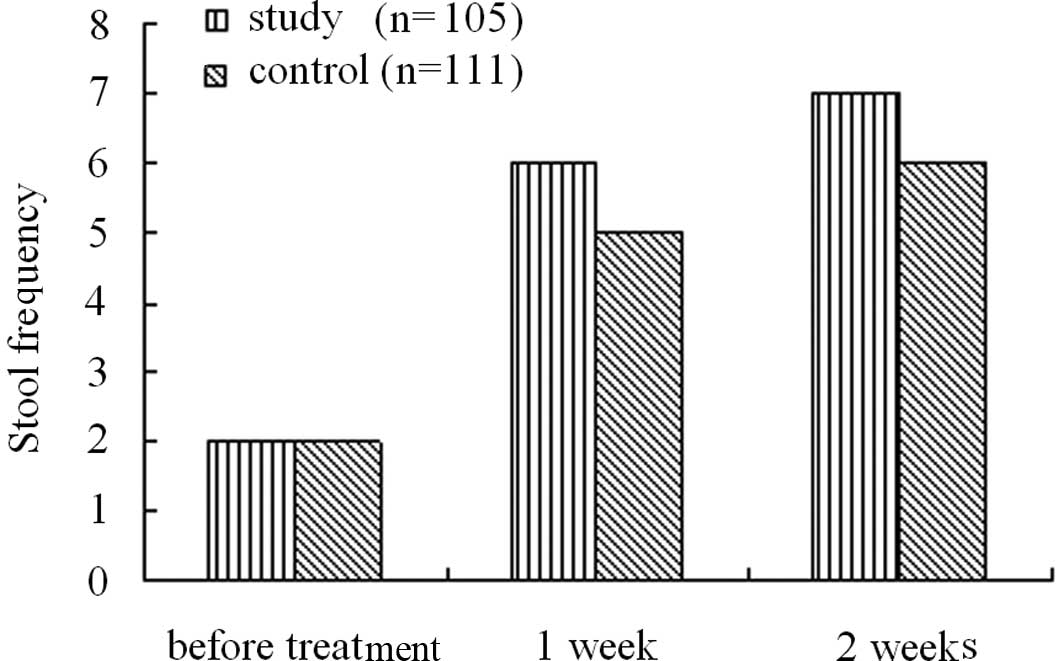
Among the patients, the course of disease ranged
from 2 to 887 weeks with a median of 104.36 weeks. Prior to
treatment, the patients in the two groups had a median stool
frequency of 2 per week. Upon PEG 4000 treatment, the median stool
frequency significantly increased to 6 times per week following one
week of treatment, and 7 times per week following two weeks of
treatment. In the lactulose group, the median stool frequency
following one week of treatment increased to 5 times per week, and
following two weeks of treatment increased to 6 times per week.
Statistical analyses demonstrated that the median stool frequency
both one and two weeks following treatment in the PEG group was
significantly higher than that in the lactulose group (Fig. 1).
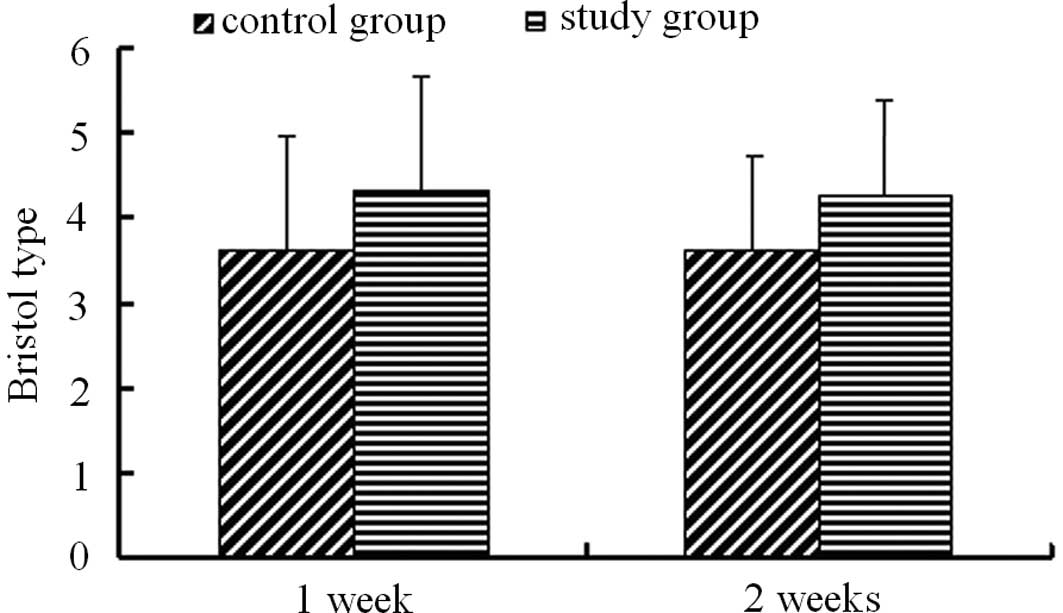
Prior to treatment, the stool consistencies of all
enrolled patients ranged from type 1 to 3 on the Bristol Stool
Scale. A total of 49.52% patients in the PEG group and 40.54%
patients in the lactulose group were classified as Bristol type 2.
There was no significant difference in the stool frequencies
between the two groups prior to treatment. Treatment of PEG 4000
markedly increased the stool consistency to Bristol type 4.34±1.11
following one week of treatment and 4.26±0.89 following two weeks
of treatment. Lactulose treatment also improved the stool
consistency to 3.64±1.33 following one week of treatment and
3.63±1.33 following two weeks. However, the increase in stool
consistency by lactulose was significantly less than that by the
PEG 4000 treatment (Fig. 2).
Effect of PEG 4000 and lactulose on
secondary efficacy parameters
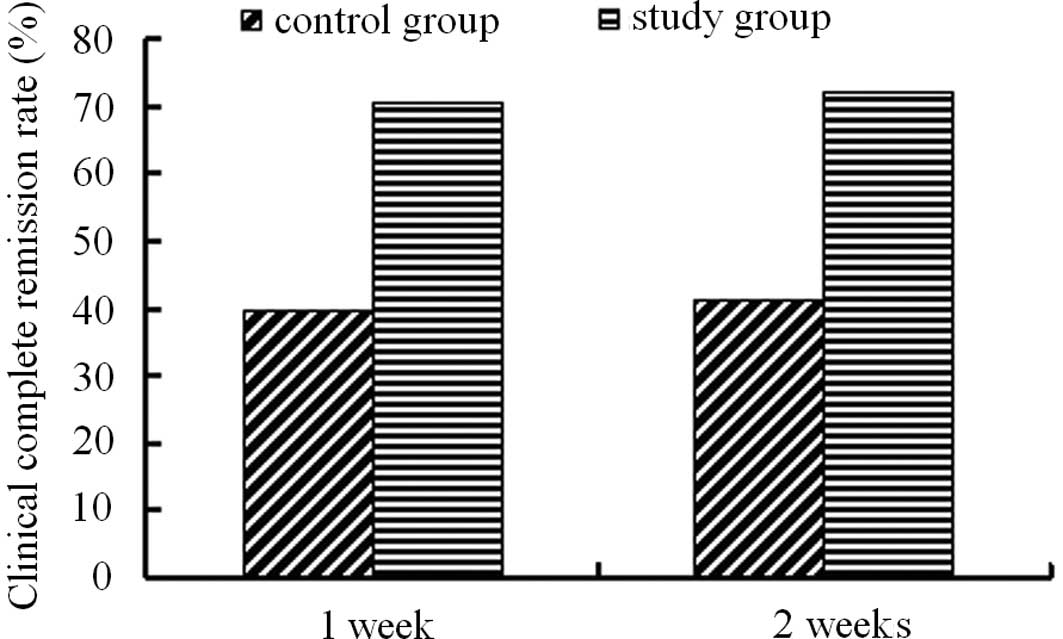
Clinical remission rate
Following one week of treatment, the clinical
remission rate was 70.48% in the PEG group and 39.64% in the
lactulose group. Following two weeks of treatment, the remission
rate was 72.38% in the PEG group and 41.44% in the lactulose group.
PEG 4000 treatment demonstrated a significantly higher remission
rate than that of lactulose treatment (Fig. 3).
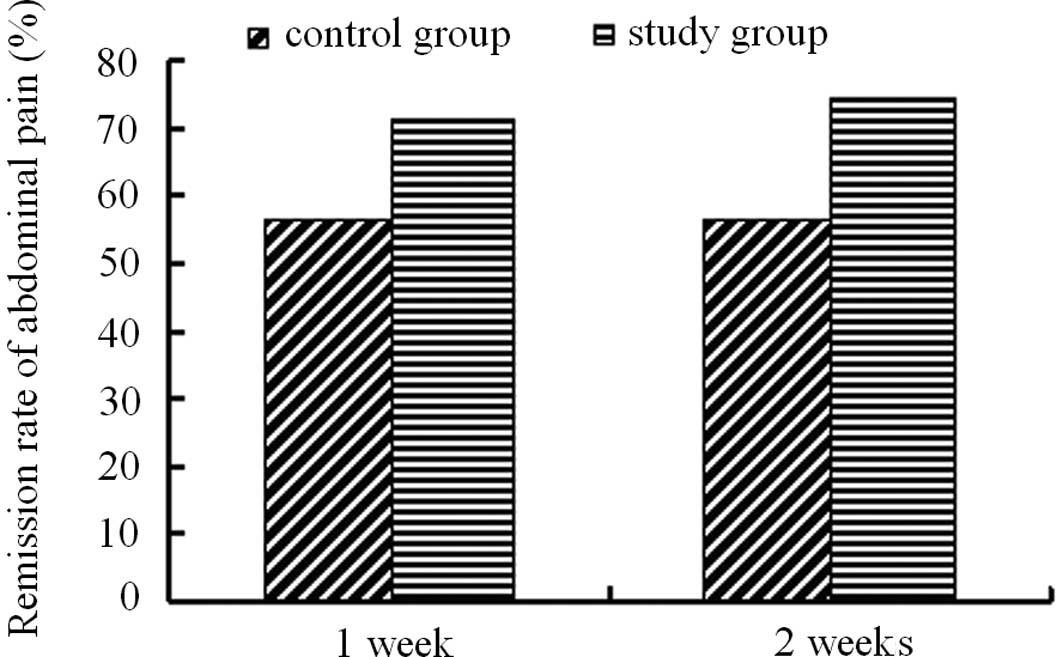
Remission rate of abdominal pain
Prior to treatment, among the 105 cases in the PEG
group (60.0%), there were 63 patients associated with abdominal
pain. While among the 111 patients in the lactulose group, 60
patients were associated with abdominal pain (54.1%). Following one
week of treatment, the abdominal pain in 45 patients of the PEG
group disappeared (71.43%), while 34 patients in the lactulose
group described the disappearance of abdominal pain (56.67%).
Following two weeks of treatment, the disappearance of abdominal
pain was 74.60% (47 patients) in the PEG group and 56.67% (34
patients) in the lactulose group. Treatment with PEG 4000 resulted
in increased abdominal pain remission than that of lactulose
treatment (Fig. 4).
Safety evaluation
Adverse effects
There were no significant adverse effects following
the treatments of PEG 4000 and lactulose. In the PEG group, one
patient developed diarrhea and another developed abdominal pain.
Diarrhea may have been related to PEG 4000 treatment since it
resolved following withdrawal of PEG 4000. There were no adverse
effects in the lactulose group.
Laboratory tests
Serum concentrations of sodium, potassium, calcium
and glucose were measured, and full blood counts, urine tests and
blood tests for renal and liver function were performed before and
after treatment. All patients in the study demonstrated normal
results in the above tests before and after treatment, except one
patient in the lactulose group who had a slightly increased level
of AST (58 units/l). However, due to loss of contact following the
two weeks of treatment, the liver function test on this patient was
not followed up.
Physical examinations
No evident changes in blood pressure, pulse, height
and weight or abnormalities following physical examinations were
observed.
Discussion
Children with chronic constipation often receive
long-term laxative treatment. Thus, a safe, effective and
non-stimulant laxative is preferred. PEG 4000 is tasteless,
non-toxic, hydrosoluble, non-absorbable and is not metabolized by
colonic bacteria. It acts as a pure osmotic agent that adds
moisture to stools to produce ‘hypocatharsis’. With the addition of
favorable flavors, it is palatable and increases children
compliance with the treatment. Lactulose is a synthetic
disaccharide, which is non-absorbable, but is metabolized by
colonic bacteria to produce gas, resulting in abdominal discomfort.
Lactulose is well known as an effective laxative for the treatment
of constipation in adults and children (8). PEG 4000 was first registered for
clinical trials in China in 2003. The present study aimed to
evaluate the safety and efficacy of PEG 4000 treatment compared to
lactulose for the constipation of Chinese children over 8 years of
age.
This randomized, controlled study was conducted in 7
hospitals in China. A total of 216 children with constipation were
investigated, with 105 cases in the PEG group and 111 in the
lactulose group. PEG 4000 and lactulose treatments significantly
increased the weekly stool frequency and stool consistency of the
patients. Following one week of treatment, the weekly stool
frequencies in the PEG 4000 treatment group and lactulose treatment
group achieved 6 and 5 times, respectively. Following two weeks of
treatment, the stool frequencies further increased to 7 and 6
times, respectively. At the end of the treatment, the rate of
normal stool consistency (Bristol type >3) reached 83.70% in the
PEG group and 60.76% in the lactulose group. The clinical remission
rates achieved 72.38% in the PEG group and 41.44% in the lactulose
group. PEG 4000 and lactulose treatment were effective in the
treatment of constipation in children, with significantly improved
results for the PEG 4000 treatment.
PEG 4000 and lactulose treatment markedly reduced
abdominal pain in children with constipation. Among the 216 cases,
there were 123 patients with abdominal pain prior to treatment; 63
cases in the PEG group and 60 in the lactulose group. Following two
weeks of treatment, the remission rates of the abdominal pain
reached 74.60% in the PEG group and 56.67% in the lactulose group.
The resolution of abdominal pain was markedly higher with PEG 4000
treatment than with lactulose treatment.
No significant clinical adverse effects or
abnormalities in the laboratory tests were observed in the two
treatment groups. The main adverse effects of PEG 4000 and
lactulose were diarrhea, abdominal pain and abdominal distention.
As PEG 4000 is not absorbed in the gastrointestinal tract it may
not have systematic toxicity following oral administration. Thus,
PEG 4000 is safe for the treatment of constipation in children
(9,10).
In this study, we observed that each gram of PEG
4000 could retain 2.7 g of water (by means of hydrogen bonding).
This feature of PEG 4000 leads to an increase in fecal water
content, thus it acts as a stool softener facilitating defecation.
Soft feces could be formed 24–48 h following treatment. A large
number of children with constipation returned to normal defecation
following 5 days of treatment. The majority of patients recovered
from constipation following 2 to 3 weeks of therapy.
In conclusion, following two weeks of treatment, PEG
4000 and lactulose were effective and safe for the treatment of
constipation in children. PEG 4000 therapy demonstrated a higher
rate of success compared to lactulose treatment, thus suggesting
that PEG 4000 is a promising new therapy for constipation in
Chinese children.
Acknowledgements
The authors express their gratitude to
Dr Michael Nowicki (University of Mississippi Medical Center, MS,
USA) for his critiques, valuable suggestions and help in preparing
the manuscript. This work was supported by the National Natural
Science Foundation of China (30800417, 81170331).
References
|
1.
|
Rasquin A, Di Lorenzo C, Forbes D, et al:
Childhood functional gastrointestinal disorders: child/adolescent.
Gastroenterology. 130:1527–1537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wang MG and Wang BX: Initial therapy of
constipation in children. J Appl Clin Pediatr. 21:446–448.
2006.
|
|
3.
|
Corazziari E, Badiali D, Habib FI, et al:
Small volume isosmotic polyethylene glycol electrolyte balanced
solution (PMF-100) in treatment of chronic nonorganic constipation.
Dig Dis Sci. 41:1636–1642. 1996.
|
|
4.
|
Attar A, Lémann M, Ferguson A, et al:
Comparison of a low dose polyethylene glycol electrolyte solution
with lactulose for treatment of chronic constipation. Gut.
44:226–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
DiPalma JA, DeRidder PH, Orlando RC, et
al: A randomized, placebo-controlled, multicenter study of the
safety and efficacy of a new polyethylene glycol laxative. Am J
Gastroenterol. 95:446–450. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Loening-Baucke V: Polyethylene glycol
without electrolytes for children with constipation and encopresis.
J Pediatr Gastroenterol Nutr. 34:372–377. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pashankar DS and Bishop WP: Efficacy and
optimal dose of daily polyethylene glycol 3350 for treatment of
constipation and encopresis in children. J Pediatr. 139:428–432.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Freedman MD, Schwartz HJ, Roby R and
Fleisher S: Tolerance and efficacy of polyethylene glycol
3350/electrolyte solution versus lactulose in relieving opiate
induced constipation: a double-blinded placebo-controlled trial. J
Clin Pharmacol. 37:904–907. 1997. View Article : Google Scholar
|
|
9.
|
Kinservik MA and Friedhoff MM: The
efficacy and safety of polyethylene glycol 3350 in the treatment of
constipation in children. Pediatr Nurs. 30:232–237. 2004.PubMed/NCBI
|
|
10.
|
Pashankar DS, Loening-Baucke V and Bishop
WP: Safety of polyethylene glycol 330 for the treatment of chronic
constipation in children. Arch Pediatr Adolesc Med. 155:661–664.
2003. View Article : Google Scholar
|