Introduction
Primary lung cancer is one of the most common types
of cancer worldwide, and non-small cell lung cancers (NSCLCs)
account for approximately 85% of all primary lung cancers. Surgical
resection is the only potentially curative treatment for patients
with early disease. However, the 5-year survival rate after
surgical resection remains unsatisfactory. Improved survival of
patients with NSCLC requires better clinical predictors of outcomes
and of response to specific therapeutic interventions.
Insulin-like growth factor-1 receptor (IGF-1R) is a
trans-membrane heterotetrametric protein encoded by the
IGF-1R gene located on chromosome 15q25-q26. IGF-1R promotes
oncogenic transformation, growth and survival of cancer cells
(1–4). The binding of insulin-like growth
factor (IGF)-1 and IGF-2 to the extracellular subunit domain of
IGF-1R activates the tyrosine kinase activity of IGF-1R and
triggers a cascade of reactions involving signal transduction
pathways, including components such as Ras, Raf, mitogen-activated
protein kinase and phosphoinositol-3-kinase (PI3K)/AKT/BAD
(Bcl-xL/Bcl2-associated death promoter) (5). IGFs are synthesized together with six
molecular species of specific binding proteins [IGF binding protein
(IGFBP)-1 to -6]. IGFBPs modulate IGF-1 and IGF-2 bioavailability
in both circulation and the cellular microenvironment. In several
malignancies, IGF-1R overexpression promotes tumor growth,
progression, invasion and metastasis (6). Increased metastatic activity was
reported in mice after intrasplenic injection of lung cancer cell
lines transfected with IGF-1R (7).
Matrix metalloproteinases (MMPs) are a family of
highly conserved enzymes that are capable of degrading the
extra-cellular matrix (ECM). Over 25 well-characterized members of
this proteinase family have been identified. MMPs play key roles
not only in normal processes, but also in tissue remodeling
associated with inflammatory disease, cancer invasion and
metastasis (8,9). Substantial evidence indicates that
overexpression of MMPs correlates with a more aggressive tumor-cell
phenotype, as well as with poor outcomes in patients with
cancer.
MMP-7 is the smallest (28 kDa) member of the MMP
family. It has broad substrate specificity against ECM components
and is produced by tumor cells. The functions of MMP-7 include
destruction of basement membrane components, which is a crucial
event in tumor cell invasion and metastasis. Increased expression
of MMP-7 in cancer cells is associated with tumor progression and
metastasis in various types of cancer (10,11).
To date, few studies have examined the expression of MMP-7 in lung
cancer (12–14).
Miyamoto et al (15) reported that MMP-7 possesses IGFBP-3
protease activity. MMP-7-induced proteolysis of IGFBP-3 plays a
crucial role in regulating IGF-I bioavailability and promotes cell
survival. These findings suggest that MMP-7 may augment
carcinogenesis and the progression of tumors that express IGF-1R.
Adachi et al (16) found
that IGF/IGF-1R upregulated MMP-7 expression in a gastrointestinal
cancer cell line, suggesting that a positive feedback loop
involving IGF-1R and MMP-7 may have a part in tumor
progression.
The aim of this study was to evaluate the expression
levels of IGF-1R and MMP-7 in resected NSCLC, and to examine the
relations of such levels to clinical characteristics and
survival.
Patients and methods
Patients
This study was performed in 78 consecutive patients
with pathological (p)-stage I to III NSCLC who underwent complete
tumor resection and nodal dissection without any pre-operative
therapy at the Respiratory Center, Yokohama City University Medical
Center, between January 1, 2000 and November 30, 2003.
The subjects were 54 men and 24 women with a mean
age of 64.7 years (range 19–82; median 65) (Table I). The most common histological
type of tumor was adenocarcinoma (57.7%; 45 cases), followed by
squamous cell carcinoma (33.3%; 26 cases), large-cell carcinoma
(6.4%; 5 cases), typical carcinoid (1 case) and pulmonary blastoma
(1 case). The stage of the primary tumor was T1 in 35 patients
(44.9%), T2 in 29 (37.1%), T3 in 9 (11.5%) and T4 in 5 (6.4%).
Thirty-eight (48.7%) patients had no metastasis to regional lymph
nodes (N0), whereas 11 (14.1%) had metastatic involvement of the
hilar lymph nodes (N1), and 29 (37.1%) had metastases to the
mediastinal nodes (N2, N3). Thirty-two tumors (41.0%) were
classified as stage I, 15 (19.2%) were stage II and 31 (39.7%) were
stage III. At the end of follow-up, 36 patients (46.1%) were alive
and 42 (53.8%) had died.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. of patients
(%) |
|---|
| Total | 78 (100) |
| Age (mean ± SD),
years | 64.7±10.8 |
| Gender | |
| Male | 54 (69.2) |
| Female | 24 (30.7) |
| Histological
type | |
| Adenocarcinoma | 45 (57.7) |
| Squamous cell
carcinoma | 26 (33.3) |
| Large-cell
carcinoma | 5 (6.4) |
| Typical
carcinoid | 1 (1.3) |
| Pulmonary
blastoma | 1 (1.3) |
| Pathological
stage | |
| I | 32 (41.0) |
| II | 15 (19.2) |
| III | 31 (39.7) |
| Smoking status | |
| Smoker | 56 (71.8) |
| Non-smoker | 22 (28.2) |
| T-factor | |
| T1 | 35 (44.9) |
| T2 | 29 (37.2) |
| T3 | 9 (11.5) |
| T4 | 5 (6.4) |
| N-factor | |
| N0 | 38 (48.7) |
| N1 | 11 (14.1) |
| N2 | 28 (35.9) |
| N3 | 1 (1.3) |
| Recurrence | |
| (+) | 36 (46.2) |
| (−) | 42 (53.8) |
Histological subgroups were determined according to
the World Health Organization classification. Pathological
tumor-node-metastasis classification and staging were assigned in
accordance with the International Staging System. The mean
follow-up was 1,466 days (range 106–3,328). Informed consent was
obtained from each patient and the Yokohama City Medical Committee
approved this study.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue specimens
were cut into 4-μm thick sections and mounted on slides. The
sections were deparaffinized and rehydrated.
For IGF-1R, the slides were heated in a microwave
for 10 min in a 10-μmol/l citrate buffer solution at pH 6.0
and cooled to room temperature for 20 min. After quenching the
endogenous peroxidase activity with 3% H2O2
for 5 min, the sections were incubated for 60 min at room
temperature with the primary antibody diluted at 1:100 for IGF-1R
(a rabbit polyclonal antibody, clone 1161; Signalway Antibody,
Pearland, TX, USA). Peroxidase-Labeled Polymer EnVision+ kit (Dako,
Glostrup, Denmark) was used for specific staining.
For MMP-7, after the endogenous peroxidase activity
was blocked, the sections were incubated for 90 min at room
temperature with the primary antibody for MMP-7 (a mouse monoclonal
antibody, clone 141-7B2; Daiichi Fine Chemicals, Toyama, Japan),
diluted at 20 μg/ml. Endogenous biotin was blocked by Dako’s
Biotin Blocking system (Dako), according to the manufacturer’s
specifications. After rinsing, specific staining was visualized
with the use of an LSAB+ system-HRP system (Dako).
Color was produced by the application of
3,3’-diaminobenzidine for 10 min. The sections were counterstained
with Meyer’s hematoxylin (Muto Pure Chemicals, Tokyo, Japan).
All sections were scored semi-quantitatively and
qualitatively, without knowledge of the clinical data. Expression
levels were measured by immunohistochemical analysis on the basis
of staining intensity, scored from 0 to 4 as follows: 0, negative;
1, trace; 2, weak; 3, moderate; and 4, strong. The percentage of
stained cells (0–100%) was multiplied by the staining intensity
(0–4). The final score ranged from 0 to 400. Staining of the sample
was considered high when the score was equal to the median value or
higher, or was otherwise considered as low.
Statistical analysis
Univariate analysis was performed by the
χ2 test and Mann-Whitney U test. Continuous data were
compared using the Student’s t-test. The postoperative survival
rate was analyzed by the Kaplan-Meier method, and differences in
survival rates were assessed with the log-rank test. A Cox
proportional hazard regression model was used for multivariate
analyses. Death from any cause was included in the calculation of
postoperative survival. Differences were considered significant at
P<0.05. All statistical manipulations were performed using the
SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Expression of IGF-1R and MMP-7 according
to immunohistochemical analysis
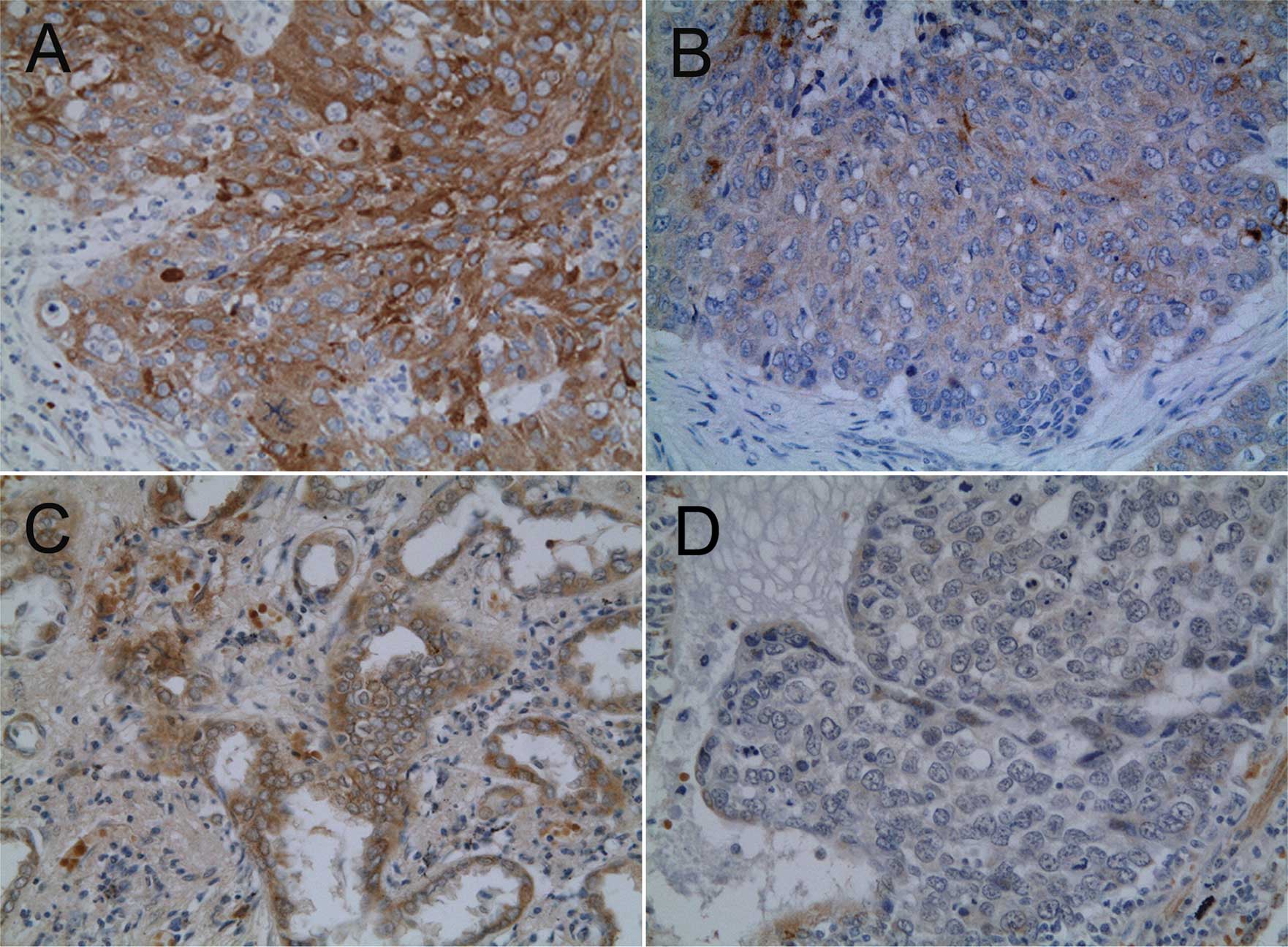
Immunohistochemical expression of IGF-1R was
detected in the tumor cell membrane. Staining for MMP-7 was
characterized by a heterogeneous cytoplasmic pattern (Fig. 1). Among the 78 carcinomas studied,
median expression scores were 210 for IGF-1R and 140 for MMP-7. A
total of 41 (52.6%) carcinomas were classified as IGF-1R-high, and
42 (53.8%) were classified as MMP-7-high.
Relation between expression of IGF-1R and
MMP-7 and clinicopathological factors
High expression of IGF-1R was related to lymph node
metastasis (P=0.034) and recurrence (P=0.006). MMP-7 expression
status did not significantly correlate with any clinicopathological
factor (Table II). There was no
significant correlation between the expression of IGF-1R and that
of MMP-7 (P=0.184).
 | Table II.Characteristics of the NSCLC patients
and IGF-1R and MMP-7 expression. |
Table II.
Characteristics of the NSCLC patients
and IGF-1R and MMP-7 expression.
| IGF-1R expression
| MMP-7 expression
|
|---|
| High | Low | P-value | High | Low | P-value |
|---|
| No. of patients | 41 | 37 | | 42 | 36 | |
| Age (mean ± SD) | 64.8±10.0 | 64.7±11.7 | 0.960 | 65.3±10.0 | 64.3±11.5 | 0.687 |
| Gender | | | | | | |
| Male | 27 | 27 | 0.496 | 27 | 27 | 0.307 |
| Female | 14 | 10 | | 15 | 9 | |
| Histological
type | | | | | | |
| Adenocarcinoma | 21 | 24 | 0.466 | 23 | 22 | 0.945 |
| Squamous cell
carcinoma | 17 | 9 | | 15 | 11 | |
| Large-cell
carcinoma | 2 | 3 | | 3 | 2 | |
| Other | 1 | 1 | | 1 | 1 | |
| Smoking status | | | | | | |
| Smoker | 29 | 27 | 0.826 | 28 | 28 | 0.277 |
| Non-smoker | 12 | 10 | | 14 | 8 | |
| T-factor | | | | | | |
| T1 | 17 | 18 | 0.321 | 19 | 16 | 0.986 |
| T2 | 17 | 12 | | 15 | 14 | |
| T3 | 3 | 6 | | 5 | 4 | |
| T4 | 4 | 1 | | 3 | 2 | |
| N-factor | | | | | | |
| N0 | 14 | 24 | 0.034 | 21 | 17 | 0.788 |
| N1 | 6 | 5 | | 6 | 5 | |
| N2 | 20 | 8 | | 14 | 14 | |
| N3 | 1 | 0 | | 1 | 0 | |
| Recurrence | | | | | | |
| (+) | 25 | 11 | 0.006 | 19 | 17 | 0.861 |
| (−) | 16 | 26 | | 23 | 19 | |
Relation between expression of IGF-1R and
MMP-7 and overall survival
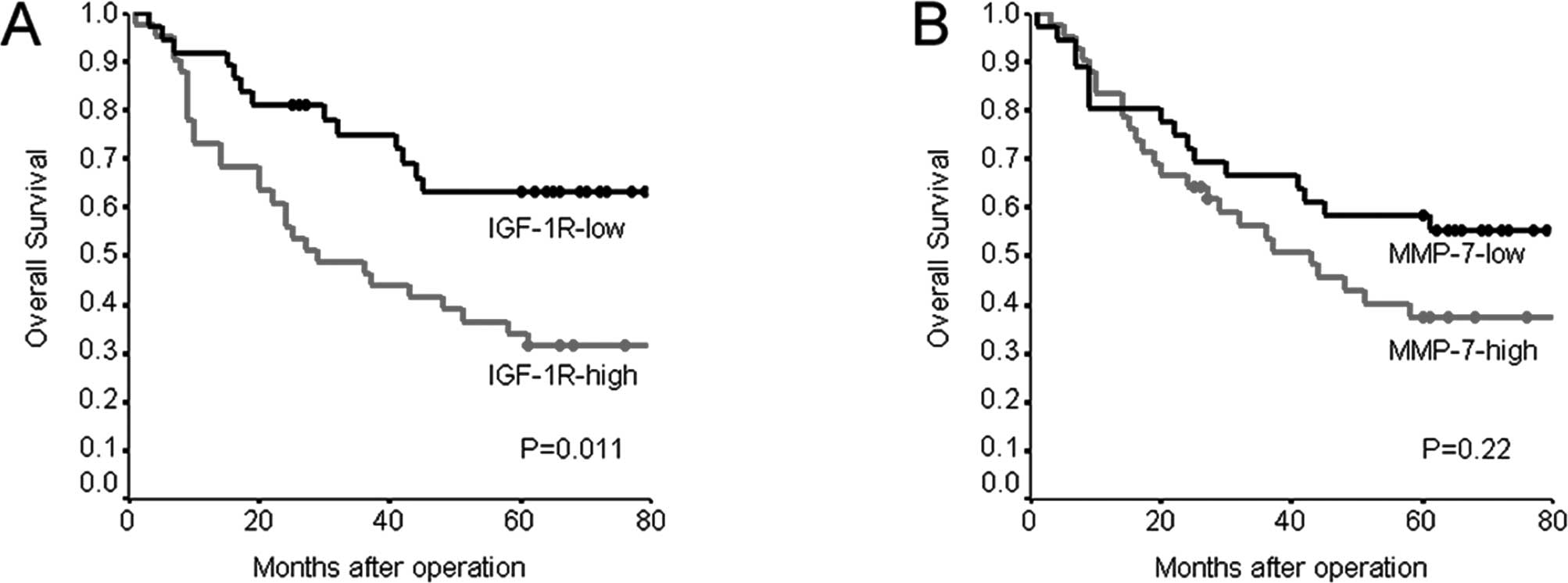
Overall survival was significantly worse in patients
with IGF-1R-high tumors than in those with IGF-1R-low tumors
(P=0.011) (Table III, Fig. 2). The 5-year survival rate was
34.1% in patients with IGF-1R-high tumors, as compared to 63.0% in
those with IGF-1R-low tumors. Overall survival was slightly, but
not significantly, worse in patients with MMP-7-high tumors than in
those with MMP-7-low tumors. The 5-year survival rate was 37.5% in
patients with MMP-7-high tumors, as compared to 58.3% in those with
MMP-7-low tumors (P=0.220) (Fig.
2). Subsequently, we conducted subset analyses to investigate
the prognostic significance of IGF-1R and MMP-7. IGF-1R-high was
associated with worse overall survival than IGF-1R-low in patients
who were male and in those with adenocarcinoma (P=0.022 and 0.016,
respectively) (Table IV).
 | Table III.Univariate analysis of overall
survival in NSCLC. |
Table III.
Univariate analysis of overall
survival in NSCLC.
| Variables | 5-year survival
rate (%) | P-value |
|---|
| Gender | | 0.015 |
| Male | 40.1 | |
| Female | 64.8 | |
| Histological
type | | 0.176 |
|
Adenocarcinoma | 54.2 | |
| Squamous cell
carcinoma | 37.5 | |
| T-factor | | 0.002 |
| T1 | 67.2 | |
| T2,3,4 | 32.5 | |
| N-factor | | 0.280 |
| N0 | 57.2 | |
| N1,2,3 | 38.9 | |
| Smoking status | | 0.109 |
| Smoker | 43.6 | |
| Non-smoker | 57.7 | |
| IGF-1R
expression | | 0.011 |
| High | 34.1 | |
| Low | 63.0 | |
| MMP-7
expression | | 0.220 |
| High | 37.5 | |
| Low | 58.3 | |
 | Table IV.Subset analysis of overall survival
in NSCLC. |
Table IV.
Subset analysis of overall survival
in NSCLC.
| Variables | 5-year survival
rate (%)
|
|---|
| IGF-1R-high | IGF-1R-low | P-value | MMP-7-high | MMP-7-low | P-value |
|---|
| Gender | | | | | | |
| Male | 25.9 | 54.5 | 0.022 | 24.0 | 55.5 | 0.051 |
| Female | 50.0 | 87.5 | 0.064 | 62.2 | 66.6 | 0.955 |
| Histological
type | | | | | | |
|
Adenocarcinoma | 38.1 | 68.4 | 0.016 | 48.9 | 59.0 | 0.608 |
| Squamous cell
carcinoma | 29.4 | 55.5 | 0.586 | 23.3 | 54.5 | 0.478 |
| Pathological
stage | | | | | | |
| I | 53.8 | 72.8 | 0.182 | 61.1 | 71.4 | 0.601 |
| II, III | 25.0 | 53.5 | 0.072 | 22.2 | 50.0 | 0.246 |
| Smoking status | | | | | | |
| Smoker | 31.0 | 57.6 | 0.050 | 28.8 | 57.1 | 0.104 |
| Non-smoker | 41.6 | 77.7 | 0.087 | 54.5 | 62.5 | 0.913 |
Multivariate analysis of overall survival in
patients with NSCLC included the following factors: IGF-1R
expression, T-factor, N-factor, gender and MMP-7 expression. Male
gender (HR=2.598; 95% CI 1.198–5.638, P=0.016), T2–4 disease
(HR=2.540; 95% CI 1.291–4.997, P=0.007) and high expression of
IGF-1R (HR=2.322; 95% CI 1.215–4.436, P=0.011) were significantly
associated with worse overall survival (Table V).
 | Table V.Multivariate analysis of overall
survival in NSCLC. |
Table V.
Multivariate analysis of overall
survival in NSCLC.
| Variables | P-value | Hazard ratio | 95% confidence
interval |
|---|
| Gender | 0.016 | 2.598 | 1.198–5.638 |
| T-factor | 0.007 | 2.540 | 1.291–4.997 |
| IGF-1R | 0.011 | 2.322 | 1.215–4.436 |
Discussion
Overexpression of IGF-1R has been reported to
promote tumor growth, invasion and metastasis in several types of
malignancies (6). Despite previous
studies, however, the clinical significance of IGF-1R expression in
NSCLC remains unclear. MMP-7 has been reported to have multiple
biologic functions related to tumor behavior, such as growth,
invasion, proliferation and apoptosis. In addition, a relation
between MMP-7 expression and postoperative outcomes has been
reported (12–14), but definitive evidence is lacking.
Therefore, we studied immunohistochemically the expression of
IGF-1R and MMP-7 in post-surgical patients with NSCLC.
We assessed the association of IGF-1R and MMP-7
expression with clinicopathological features. Dziadziuszko et
al (17) found that IGF-1R
expression was higher in squamous cell carcinomas than in other
histological types and was associated with disease stage. Cappuzzo
et al (18) reported that a
positive IGF-1R expression was significantly associated with
squamous cell histology and grade III differentiation. Ludovini
et al (19) reported that
IGF-1R protein overexpression was associated with larger tumor
size. Merrick et al (20)
showed that higher IGF-1R scores were associated with
adenocarcinoma and never-smokers. Our results showed that high
IGF-1R expression was significantly related to higher N-factor (P=
0.034) and recurrence (P=0.006). As for histological type, IGF-1R
expression was slightly, but not significantly, higher in squamous
cell carcinomas.
Concerning MMP-7 expression, Liu et al
(12) showed that MMP-7 expression
was significantly higher in squamous cell carcinomas than in
adenocarcinomas. Leinonen et al (13) reported that high MMP-7 expression
was related to lower T-factor and well-differentiated tumors;
moreover, MMP-7 expression was higher in adenocarcinomas than in
other histological subtypes. Sasaki et al (14) demonstrated a trend toward higher
MMP-7 mRNA expression levels in NSCLCs with lymph node metastasis.
By contrast, our results showed no correlation between MMP-7 and
any clinicopathological factor.
We also analyzed the relationship of IGF-1R and
MMP-7 expression to post-surgical outcomes. Previously, Merrick
et al (20) analyzed IGF-1R
expression in 184 surgically treated patients with stage I to IV
NSCLC. In stage I disease, high IGF-1R expression was associated
with significantly shorter survival than low IGF-1R expression.
Dziadziuszko et al (17)
evaluated 189 NSCLCs and showed that the IGF-1R gene copy
number is of prognostic value; nonetheless, IGF-1R protein
expression upon immunohistochemical analysis was not related to
survival. Ludovini et al (19) reported that IGF-1R protein
expression alone was not significantly associated with survival,
although high co-expression of both IGF-1R and epidermal growth
factor receptor was associated with shorter disease-free survival
in resected NSCLC. Cappuzzo et al (18) concluded that IGF-1R expression does
not represent a prognostic factor in resected NSCLC patients.
In the present study, overall survival was
significantly poorer in patients with IGF-1R-high tumors than in
those with IGF-1R-low tumors, and multivariate analysis showed
IGF-1R expression as an independent indicator of poor outcomes. As
for MMP-7, Liu et al (12)
reported that the overall survival rate was significantly lower in
patients with MMP-7-positive NSCLC than in those with
MMP-7-negative NSCLC. On the other hand, Leinonen et al
(13) reported that MMP-7 had no
prognostic value in NSCLC. Our results showed a trend toward poorer
survival in patients with MMP-7-high tumors than in those with
MMP-7-low, but the difference fell short of reaching statistical
significance.
In conclusion, our results suggest that
overexpression of IGF-1R is a useful predictor of lymph node
metastasis and recurrence in patients with NSCLC. Overexpression of
IGF-1R may thus be an important prognostic factor along with gender
and T-factor in patients with NSCLCs.
References
|
1.
|
Dufourny B, Alblas J, van Teeffelen HA, et
al: Mitogenic signaling of insulin-like growth factor I in MCF-7
human breast cancer cells requires phosphatidylinositol 3-kinase
and is independent of mitogen-activated protein kinase. J Biol
Chem. 272:31163–31171. 1997. View Article : Google Scholar
|
|
2.
|
Khandwala HM, McCutcheon IE, Flyvbjerg A
and Friend KE: The effects of insulin-like growth factors on
tumorigenesis and neoplastic growth. Endocr Rev. 21:215–244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Baserga R, Hongo A, Rubini M, Prisco M and
Valentinis B: The IGF-I receptor in cell growth, transformation and
apoptosis. Biochim Biophys Acta. 1332:F105–F126. 1997.PubMed/NCBI
|
|
4.
|
Blakesley VA, Stannard BS, Kalebic T,
Helman LJ and LeRoith D: Role of the IGF-I receptor in mutagenesis
and tumor promotion. J Endocrinol. 152:339–344. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Turner BC, Haffty BG, Narayanan L, et al:
Insulin-like growth factor-I receptor overexpression mediates
cellular radioresistance and local breast cancer recurrence after
lumpectomy and radiation. Cancer Res. 57:3079–3083. 1997.
|
|
7.
|
Long L, Rubin R and Brodt P: Enhanced
invasion and liver colonization by lung carcinoma cells
overexpressing the type 1 insulin-like growth factor receptor. Exp
Cell Res. 238:116–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mott JD and Werb Z: Regulation of matrix
biology by matrix metalloproteinases. Curr Opin Cell Biol.
16:558–564. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Miyata Y, Iwata T, Ohba K, Kanda S,
Nishikido M and Kanetake H: Expression of matrix
metalloproteinase-7 on cancer cells and tissue endothelial cells in
renal cell carcinoma: prognostic implications and clinical
significance for invasion and metastasis. Clin Cancer Res.
12:6998–7003. 2006. View Article : Google Scholar
|
|
11.
|
Kitoh T, Yanai H, Saitoh Y, et al:
Increased expression of matrix metalloproteinase-7 in invasive
early gastric cancer. J Gastroenterol. 39:434–440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liu D, Nakano J, Ishikawa S, et al:
Overexpression of matrix metalloproteinase-7 (MMP-7) correlates
with tumor proliferation, and a poor prognosis in non-small cell
lung cancer. Lung Cancer. 58:384–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Leinonen T, Pirinen R, Bohm J, Johansson
R, Ropponen K and Kosma VM: Expression of matrix metalloproteinases
7 and 9 in non-small cell lung cancer. Relation to
clinicopathological factors, beta-catenin and prognosis. Lung
Cancer. 51:313–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sasaki H, Yukiue H, Moiriyama S, et al:
Clinical significance of matrix metalloproteinase-7 and Ets-1 gene
expression in patients with lung cancer. J Surg Res. 101:242–247.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Miyamoto S, Yano K, Sugimoto S, et al:
Matrix metalloproteinase-7 facilitates insulin-like growth factor
bioavailability through its proteinase activity on insulin-like
growth factor binding protein 3. Cancer Res. 64:665–671. 2004.
View Article : Google Scholar
|
|
16.
|
Adachi Y, Li R, Yamamoto H, et al:
Insulin-like growth factor-I receptor blockade reduces the
invasiveness of gastrointestinal cancers via blocking production of
matrilysin. Carcinogenesis. 30:1305–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dziadziuszko R, Merrick DT, Witta SE, et
al: Insulin-like growth factor receptor 1 (IGF1R) gene copy number
is associated with survival in operable non-small-cell lung cancer:
a comparison between IGF1R fluorescent in situ hybridization,
protein expression, and mRNA expression. J Clin Oncol.
28:2174–2180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cappuzzo F, Tallini G, Finocchiaro G, et
al: Insulin-like growth factor receptor 1 (IGF1R) expression and
survival in surgically resected non-small-cell lung cancer (NSCLC)
patients. Ann Oncol. 21:562–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ludovini V, Bellezza G, Pistola L, et al:
High coexpression of both insulin-like growth factor receptor-1
(IGFR-1) and epidermal growth factor receptor (EGFR) is associated
with shorter disease-free survival in resected non-small-cell lung
cancer patients. Ann Oncol. 20:842–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Merrick DT, Dziadziuszko R and
Szostakiewicz B: High insulin-like growth factor 1 receptor (IGF1R)
expression is associated with poor survival in surgically resected
non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol.
25(Suppl): 75502007.
|