Introduction
Auxiliary heterotopic partial liver transplantation
(AHPLT), which is an important branch of liver transplantation, has
been considered as the best choice for the treatment of acute liver
failure and liver metabolic diseases (1–4).
However, its clinical application is unsatisfactory for the
treatment of chronic end-stage liver failure, for which further
investigation is required to solve issues, including the lack of
ideal operative technique, the liver function competition between
the transplanted and host livers, and other post-operative
complications (5–8). Established AHPLT in the past mainly
used small or normal large animals, and was unable to simulate the
characteristics of auxiliary liver transplantation of end-stage
liver failure (9,10). Thereby motivated to resolve these
issues, our study herein aimed to establish a new operative
technique for AHPLT in minipigs using a model of liver
cirrhosis.
Materials and methods
Experimental animals
Twenty-two Chinese experimental minipigs (either
gender) were purchased from the laboratory animal farm of the Inner
Mongolia Medical College. Fourteen minipigs (weighing 21–25 kg)
were used for the establishment of the cirrhosis model, and 8
minipigs (weighting 19–23 kg) were used as donors for the improved
AHPLT. The experimental protocol was approved by the Laboratory
Animal Ethics Committee of the Inner Mongolia Medical College and
conformed to the NIH Guidelines for the Care and Use of Laboratory
Animals.
Establishment of the cirrhosis model
in minipigs
Our previous study successfully produced liver
cirrhosis in minipigs (11). Liver
cirrhosis was induced by the intraperitoneal injection of carbon
tetrachloride (CCl4) twice a week for 9 weeks. Maize
flour was the only food and a 5% alcohol-water mixture was provided
for the animals. A piece of liver tissue was obtained in the 9th
week and was stained with H&E and Van Gieson’s (VG) stain.
Meanwhile, portal vein pressure (PVP) and hepatic venous pressure
(HVP) were measured by direct puncture with a 27G needle and
pressure tubing attached to the normal central venous pressure
monitoring transducer, and then the portal vein pressure gradient
(PVPG) was directly calculated by PVP and HVP.
Establishment of the donor procedure
model
Under general anaesthesia, the donor abdominal
cavity was exposed via a midline incision. The donor liver was
perfused with lactated Ringer’s solution at 4°C via an abdominal
aorta and portal vein. Once the perfusion was finished, the whole
donor liver was resected. The right lobe was resected along the
line connecting the front side of the median fissure of the liver
with the right edge of the suprahepatic vena cava. The collateral
aorta, porta and cava vessels were ligated. The left lobe was used
for the transplantation. The 2.5-cm abdominal aorta of the donor
was reserved.
Recipient procedure and testing
index
The venous channel was established through the
internal jugular vein, and then general anesthesia and assisted
respiration were carried out. After opening the abdomen, the
condition of the spleen was observed and splenectomy was performed.
The reserved abdominal aorta was anastomosed end-to-side with the
suprahepatic vena cava of the recipient. Latex drainage tube
matching the diameter of the abdominal aorta was adopted. One end
of the common latex drainage tube was inserted into the anastomosed
abdominal aorta and sutured. The donor liver was placed on the
splenic bed, then the portal vein of the transplanted liver was
anastomosed end-to-end with the splenic vein of the recipient,
while the other end of the latex drainage tube was secured with the
suprahepatic vena cava of the transplanted liver. Blood flow to the
portal vein was thus reestablished. The common hepatic artery of
the transplanted liver was end-to-end anastomosed with the splenic
artery of the recipient. The donor’s common bile duct was intubated
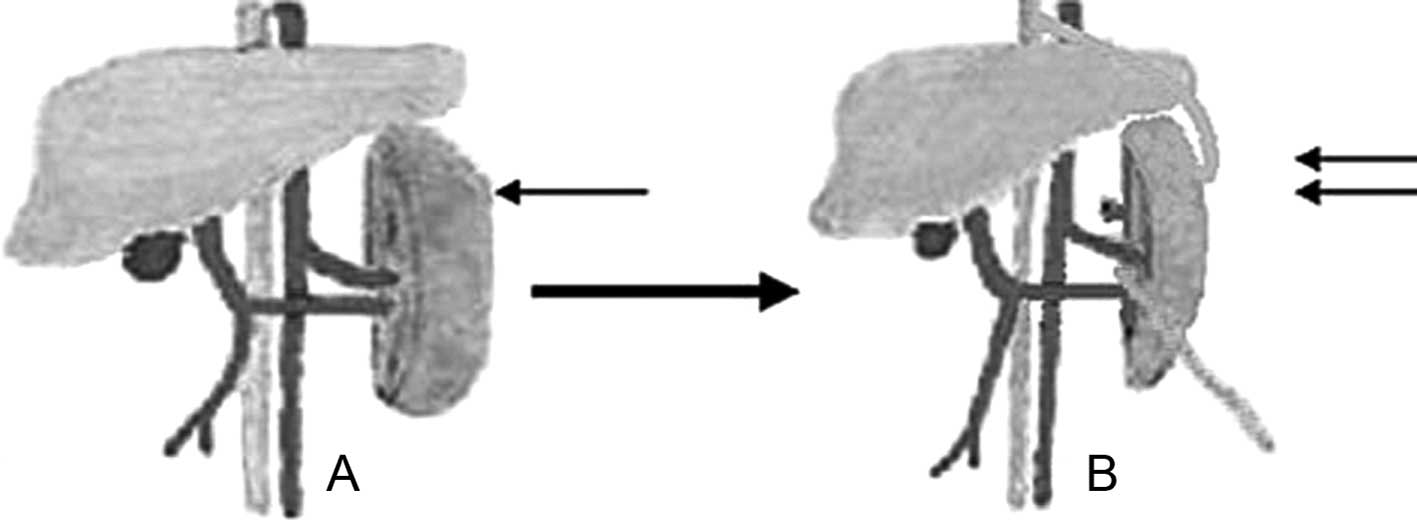
and bile was collected with an extracorporeal bag (Fig. 1). After the operation, fluid
infusion was given for the symptomatic treatment, and
anti-rejection drugs were not administered. Throughout the
transplantation process, vital signs [including the heart rate
(HR), mean arterial pressure (MAP) and central venous pressure
(CVP)] of the recipient pigs were measured by a central venous
catheter in the right internal jugular vein and a catheter in the
right internal carotid artery that was connected to a cardiac
output monitor (NPB4000, USA). The PVP, HVP and PVPG of the
recipients were measured at different time intervals. Additionally,
the PVP, HVP and PVPG of the donors were also measured after the
operation. On the 3rd day after the operation, the blood flow of
every anastomosis was examined with Doppler vascular ultrasound.
After being anesthetized, 2 cases were randomly selected to perform
laparotomy.
Statistical analysis
SPSS13.0 software was used for data analysis.
Results are expressed as the means ± SEM, unless otherwise noted.
All variables were analyzed by two-way ANOVA. P≤0.05 denoted
statistical significance.
Results
Fourteen minipigs were utilized to establish the
cirrhosis model through administration of CCl4 by
intraperitoneal injection. After administration for 9 weeks,
cirrhosis developed in 8 pigs, and 4 cases of ascites were found.
An increasing spleen volume was discerned by visual inspection.
Before the establishment of the cirrhosis model, the PVP, HVP and
PVPG of the experimental animals were 13.66±1.15, 6.73±1.00 and
6.92±1.42 cmH2O, respectively. After 9 weeks, the PVP,
HVP and PVPG of the cirrhotic pigs were 24.52±2.84, 6.81±1.05 and
17.70±2.71 cmH2O, respectively. The normal liver and the
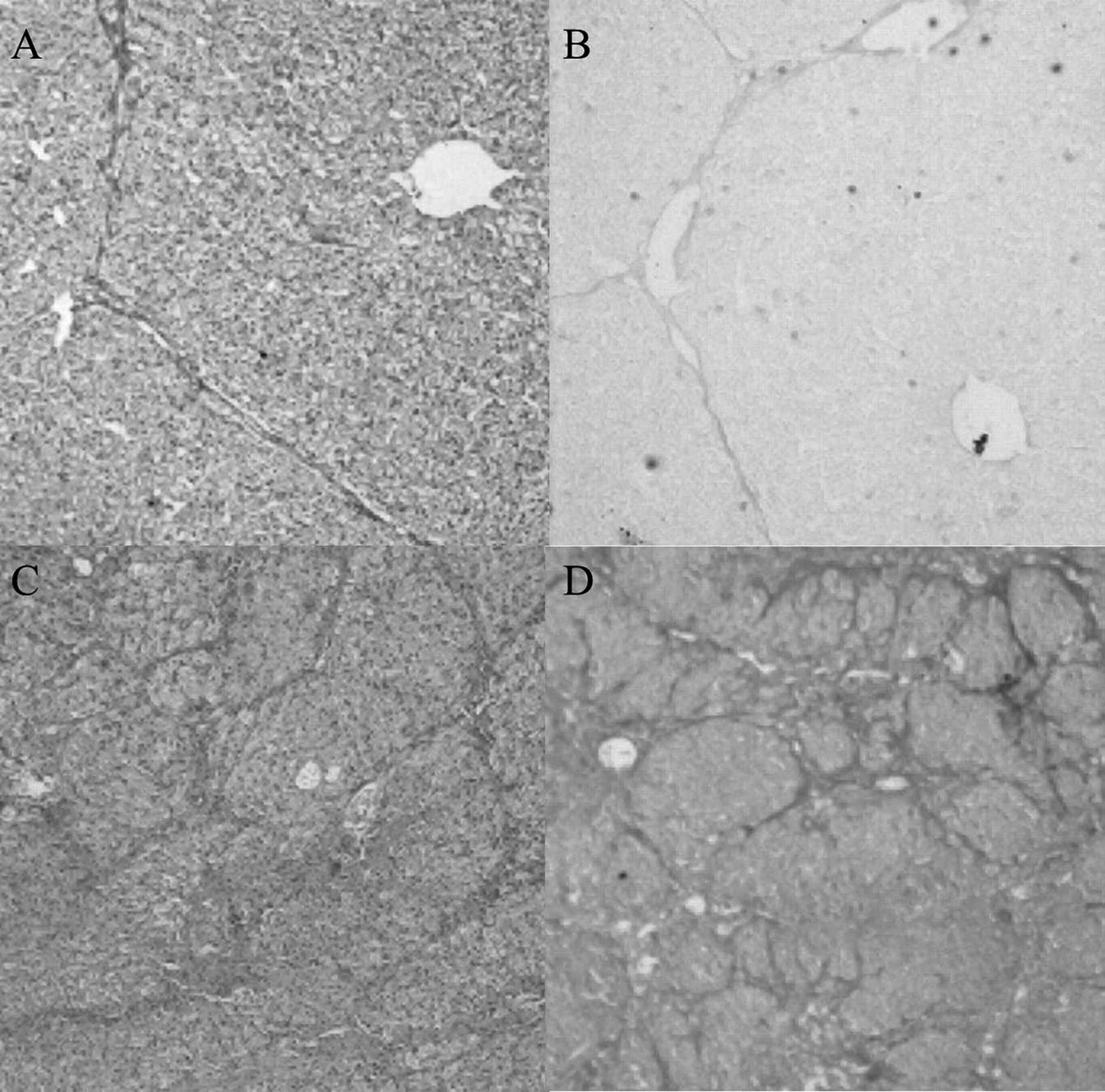
cirrhotic liver stained by H&E and VG are shown in Fig. 2.
Eight cases were used to establish the novel AHPLT
model, 7 of which survived on the 3rd day after the operation,
whereas 1 case died of bleeding of the transplanted liver section
and shock at 5 h after the operation; the surgical success rate was
thus 87.5% (7/8). Changes in the vital signs of the surviving 7
recipients at different time periods during the operation are shown
in Table I, and the PVP, HVP and
PVPG of the recipients are shown in Table II. After the blood flow to the
transplanted liver was regained for 15 min, the PVP, HVP and PVPG
of the donor were 21.59±1.26, 6.08±0.66 and 15.51±1.35
cmH2O, respectively. After the blood flow of the portal
vein was regained for 0.5–3 h, all experimental pigs had bile
excretion. On the 3rd day after the operation, 1 case of abnormal
blood flow between the suprahepatic vena cava of the transplanted
liver and the latex drainage tube was discovered employing Doppler
vascular ultrasound examination. Moreover, the laparotomy showed a
distortion at the anastomosis, but no obvious abnormality in the
blood flow of each anastomosis of the other animals was observed.
Two pigs were vivisected, in which a small amount of thrombosis was
found at the anastomosis between the vena cava of the transplanted
liver and the latex drainage tube.
 | Table I.Changes in the vital signs of the
recipients during liver transplantation (χ̄ ± s, n=8). |
Table I.
Changes in the vital signs of the
recipients during liver transplantation (χ̄ ± s, n=8).
| Time | HR (/min) | MAP (mmHg) | CVP
(cmH2O) |
|---|
| A | 130.75±6.11 | 120.88±4.91 | 5.33±0.37 |
| B | 127.87±4.29 | 119.13±6.10 | 5.31±0.43 |
| C | 136.87±5.89a | 120.50±4.66b | 5.20±0.40c |
 | Table II.Changes in PVP, HVP and PVPG of the
recipients (χ̄ ± s; n=8). |
Table II.
Changes in PVP, HVP and PVPG of the
recipients (χ̄ ± s; n=8).
| Time | PVP
(cmH2O) | HVP
(cmH2O) | PVPG
(cmH2O) |
|---|
| A | 24.01±2.57 | 6.41±0.97 | 17.60±2.52 |
| B | 23.10±1.78 | 6.18±0.93 | 16.93±1.68 |
| C | 21.59±1.26a | 6.08±0.66b | 15.51±1.35c |
Discussion
Since Welch introduced the first case of auxiliary
whole liver transplantation to dogs in 1955 (12), many improvements have been made in
the urgical techniques. However, no satisfactory auxiliary
heterotopic liver transplantation method has been reported
hitherto. In the previous study concerning the auxiliary
heterotopic liver transplantation, donor livers were mostly
transplanted into the abdominal cavity, which led to an increase in
abdominal pressure and circulatory disturbance. Recently, a liver
was transplanted to the splenic bed of a normal animal to solve the
shortage of space in the animal model (13). However, the method suffered from
the limited availability of splenic vessels in the normal
condition. In the present study, the auxiliary heterotopic liver
transplantation model was established using a model of liver
cirrhosis; the spleen of the recipient increased to further expand
the transplantation space. Theoretically, cirrhosis and portal
hypertension leads to hemangiectasis of the spleen, which favors
the anastomosis of the blood vessels of the recipient spleen in the
donor. However, no changes in the blood vessel diameter of the
spleen were observed prior to and after establishment of the liver
cirrhosis model, which is in need of further investigation in the
future.
Moreover, in auxiliary heterotopic liver
transplantation, the function competition between the recipient
liver and the transplanted liver is a long-term complication, which
is the most common problem requiring an urgent solution.
Nevertheless, the underlying mechanism has not been completely
clarified (6,10,14).
Portal vein blood flow of the transplanted liver is inadequate,
which is considered as an important manifestation that results from
the function competition. Once the portal vein blood flowing to the
transplanted liver was restored, the function competition was found
to vanish (15). Portal vein blood
flow is driven by the PVPG, i.e., by the pressure difference
between the portal vein and the hepatic vein. Previous studies were
carried out mostly using rat models of transplantation without
arterial blood supply or the established normal large animal model
of AHPLT, which differs greatly from the pathophysiological
characteristics of clinical auxiliary heterotopic liver
transplantation. The results of the present study (Table II) showed that the established
minipig model of AHPLT on the basis of liver cirrhosis better
simulated the characteristics of liver transplantation for human
end-stage liver failure, which is therefore suitable for further
exploration of the function competition mechanism. Due to the
pressure gradient in the vena system, lower venous pressure occurs
at the position closer to the heart. In our study, the end-to-side
anastomosis of the donor vena cava and the recipient suprahepatic
vena cava replaced the previous method of the donor vena cava and
the recipient renal vein or the infrahepatic inferior vena cava
above its level, which aimed to reduce the outflow tract pressure
of the donor hepatic vein. The interaction of higher PVP and lower
HVP results in a further increase in PVPG of the transplanted
liver. However, in this study, 1 case of suprahepatic vena cava
anastomotic distortion of the transplanted liver and the drainage
tube was noted on the 3rd day after the operation. This may have
resulted from the long outflow tract and the abnormal location of
the liver transplantation, which is a shortcoming of this
operation. Two pigs were vivisected, revealing a small amount of
thrombosis at the anastomosis between the vena cava of the
transplanted liver and the latex drainage tube, which may be
avoided by adopting a man-made blood vessel instead of the common
latex drainage tube in the living donor liver transplantation or
donor conduit (donor aorta, donor vena cava and iliacs) in the
cadaver liver transplantation.
Early studies have proven that AHPLT outweighs other
techniques due to advantages, such as slight injury of the
operation, short time of the operation, no anhepatic phase and
stable hemodynamics (16,17). The results of this study also
supported the above findings; the surgery success rate in the group
was 87.5%, and changes in the vital signs of the recipients at
different time periods were more stable.
In this study, although the observation time was
short (only 3 days), a novel AHPLT technique was successfully
established using a model of liver cirrhosis. In a future study, we
will assess the comparison of the difference between a suprahepatic
and infrahepatic venae cavae drainage model. In addition,
post-transplant aspects will also be observed, including liver
function of the transplanted liver compared to that of the
cirrhotic liver on the long term, evolution of the blood flow and
pressure over time, and the histologic analysis of both the
transplanted and cirrhotic livers over time.
Abbreviations:
|
AHPLT
|
auxiliary heterotopic partial liver
transplantation;
|
|
CVP
|
central venous pressure;
|
|
HR
|
heart rate;
|
|
HVP
|
hepatic venous pressure;
|
|
MAP
|
mean arterial pressure;
|
|
PVP
|
portal vein pressure;
|
|
PVPG
|
portal vein pressure gradient;
|
|
VG
|
Van Gieson’s stain
|
Acknowledgements
The authors are grateful to Jian-liang
Qiao and Rui-Fang Zhang for the technical assistance. This study
was supported by a research fund of the Inner Mongolia Medical
College (no. Y2003ZD001), and the Natural Science Foundation of
Inner Mongolia of China (no. 2010MS1126).
References
|
1.
|
Jaeck D, Pessaux P and Wolf P: Which types
of graft to use in patients with acute liver failure?: (A)
Auxiliary liver transplant (B) Living donor liver transplantation
(C) The whole liver (A) I prefer auxiliary liver transplant. J
Hepatol. 4:570–573. 2007. View Article : Google Scholar
|
|
2.
|
Haberal M, Arda IS, Karakayali H, et al:
Successful heterotopic segmental liver transplantation from a live
donor to a patient with Alagille syndrome. J Pediatr Surg.
4:667–671. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rela M, Battula N, Madanur M, et al:
Auxiliary liver transplantation for propionic acidemia: a 10-year
follow-up. Am J Transplant. 9:2200–2203. 2007.PubMed/NCBI
|
|
4.
|
O’Grady J: Modern management of acute
liver failure. Clin Liver Dis. 2:291–303. 2007.
|
|
5.
|
Fern RI, Palenciano CG, Ri A, et al:
Hemodynamic assessment during auxiliary heterotopic liver
transplantation with portal vein arterialization in a swine model:
preliminary report of 10 transplants. Transplant Proc. 8:2603–2605.
2006.
|
|
6.
|
Schleimer K, Stippel DL, Kasper H, et al:
Competition between native liver and graft in auxiliary liver
transplantation in a rat model. Transplant Proc. 4:967–970. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Willemse PJ, Ausema L, Terpstra OT, et al:
Graft regeneration and host liver atrophy after auxiliary
heterotopic liver transplantation for chronic liver failure.
Hepatology. 1:54–57. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tarhan NC, Firat A, Coskun M, et al:
Diagnosis of complications in auxiliary heterotopic partial-liver
transplant recipients: spiral CT findings. Turk J Gastroenterol.
4:192–197. 2002.PubMed/NCBI
|
|
9.
|
Hong IC, Mullen PM, Precht AF, et al:
Non-viral human IL-10 gene expression reduces acute rejection in
heterotopic auxiliary liver transplantation in rats. Microsurgery.
5:432–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Serrou B, Michel H and Gelis C: Study of
the role of the splanchnic organs in hepatic atrophy for the
purpose of auxiliary transplantation of the liver. Ann Surg.
3:274–278. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang JJ, Meng XK, Dong C, et al:
Development of a new animal model of liver cirrhosis in swine. Eur
Surg Res. 1:35–39. 2009.(In Chinese).
|
|
12.
|
Welch CS: A note on transplantation of the
whole liver in dogs. Transpl Bull. 2:54–55. 1955.
|
|
13.
|
Kesen X, Nanhai S, Yuxin C, et al:
Splenectomy and auxiliary liver transplantation. Transplant Proc.
7:2308–2309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lygidakis NJ: Segmental auxiliary liver
transplantation: a new approach to an old problem. J Invest Surg.
3:246–251. 1985.PubMed/NCBI
|
|
15.
|
Kasahara M, Takada Y, Kozaki K, et al:
Functional portal flow competition after auxiliary partial
orthotopic living donor liver transplantation in noncirrhotic
metabolic liver disease. J Pediatr Surg. 39:1138–1141. 2004.
View Article : Google Scholar
|
|
16.
|
Groenland TH, Visser L, Terpstra OT, et
al: Stable hemodynamics during heterotopic auxiliary partial liver
transplantation for end-stage liver cirrhosis. Transplant Proc.
20:538–540. 1988.
|
|
17.
|
Terpstra OT, Schalm SW, Weimar W, et al:
Auxiliary partial liver transplantation for end-stage chronic liver
disease. N Engl J Med. 23:1507–1511. 1988. View Article : Google Scholar : PubMed/NCBI
|