Introduction
Despite recent advances in diagnostic and
therapeutic techniques, pancreatic carcinoma is one of the most
lethal malignancies among cancers. The 5-year survival rate of
patients having primary pancreatic cancer after complete resection
does not reach 15% (1), while the
overall 5-year survival rates in patients having inoperable
pancreatic cancer are desperately low, ranging from 0.4 to 4%
(2,3).
Currently, gemcitabine is a key drug not only for
treating advanced pancreatic cancer (4) but also as an adjuvant chemotherapy
regimen for resectable pancreatic cancer (5,6).
Furthermore, molecular targeting of epidermal growth factor
receptor (EGFR) or vascular endothelial growth factor (VEGF) has
recently been developed to treat these lesions (7,8).
Moore et al reported in a phase III trial of patients with
advanced pancreatic cancer, that erlotinib, a tyrosine kinase
inhibitor of EGFR, in combination with gemcitabine was superior to
gemcitabine alone when progression-free and overall survival were
compared between the two groups (8).
EGFR, one of the tyrosine kinase receptors of the
ErbB family, is reported to be expressed immunohistochemically in
10–30% of patients with solid tumors including pancreatic carcinoma
(9,10). Tyrosine phosphorylation in EGFR
protein in cancer cells leads to activation of several downstream
intra cellular substrates and plays a pivotal role in tumor
proliferation, invasion and metastasis (11). Recent studies have suggested that
the EGFR gene copy number and expression obtained by fluorescence
in situ hybridization (FISH) and immunohisto chemistry (IHC)
predict the clinical response of a tumor to gefitinib, a tyrosine
kinase inhibitor of EGFR, in patients with non-small cell lung
cancer (12–14). Furthermore, recent studies have
found that mutations of the EGFR gene at the restricted region,
e.g., exon 19 and exon 21, were closely correlated with response to
gefitinib therapy (15–20). However, the relevance of EGFR
expression in pancreatic cancer with therapeutic response has
remained to be verified (8).
Although immunohistochemical expression of EGFR can
also be recognized as positive membranous staining, cytoplasmic
expression of EGFR can frequently be observed in cancer cells of
the pancreas. We previously reported that high cytoplasmic
expression of EGFR in primary pancreatic cancer was significantly
correlated with higher histological grade and poorer survival
(10), suggesting that cytoplasmic
EGFR expression could indicate a potentially aggressive or
metastatic feature of pancreatic cancer. However, it is unclear
whether localization of EGFR expression differs between primary and
metastatic sites of pancreatic cancers at surgically resectable
stages and those at inoperable far advanced stages.
The present study compared immunohistochemically the
levels and localization of EGFR expression between surgically
resected primary pancreatic cancers and far advanced cancers
obtained at autopsy, in order to clarify the clinical impact of
membranous and cytoplasmic EGFR overexpressions in far advanced
pancreatic cancers.
Materials and methods
Patients and tumor specimens
This study was performed with approval by the
Internal Review Board on Ethical Issues of the National Defense
Medical College, Japan. The subjects of this study were 44 patients
who underwent surgery with curative intent for primary pancreatic
cancers between 1987 and 2000 at the National Defense Medical
College Hospital, Tokorozawa, Japan. The clinicopathological
characteristics of these cases are summarized in Table I.
 | Table I.Clinicopathological characteristics of
the patients and tumors. |
Table I.
Clinicopathological characteristics of
the patients and tumors.
| Surgically resected
cancers (n=44)
| Far advanced cancers
(n=20)
|
|---|
| Parameter | n (%) | n (%) |
|---|
| Age (mean ± SD,
years) | 63.3±3.7 | | 57.3±5.7 | |
| <65 | 23 (52) | | 13 (65) | |
| ≥65 | 21 (47) | | 7 (35) | |
| Gender | | | | |
| Male | 34 (77) | | 16 (80) | |
| Female | 10 (23) | | 4 (20) | |
| Tumor site | | | | |
| Head | 36 (82) | | 12 (60) | |
| Body and/or
tail | 8 (18) | | 8 (40) | |
| Stage | | | | |
| I | 1 (2) | | | |
| II | 32 (73) | | | |
| III | 8 (18) | | | |
| IV | 3 (7) | | | |
| Grade | | | | |
| 1 | 12 (27) | 5 (25)a | | 2 (10)b |
| 2 | 28 (64) | 3 (15)a | | 9 (45)b |
| 3 | 4 (9) | 12 (60)a | | 9 (45)b |
| Median survival (mean
± SD, month) | 24.5±10.3 | | | |
The mean patient age was 63.3 years [±3.7 standard
deviation (SD)]. Thirty-four (77.3%) were men and 10 (22.7%) were
women. More than 80% of tumors were located in the head of the
pancreas. As for stage, approximately 90% of the patients were
assigned to stage II or stage III (21). Histologically, all 44 patients had
invasive ductal adenocarcinoma of the pancreas, and the majority of
the patients had moderately differentiated tubular adenocarcinoma.
The median survival time was 24.5 months (±10.3 SD).
In addition, a total of 40 tumor specimens from
primary sites and hepatic metastatic sites were obtained at autopsy
from 20 patients who had died of inoperable far advanced pancreatic
cancer between 1980 and 2001 at the same hospital (Table I).
Using these tumor specimens from a total of 64
patients, formalin-fixed paraffin-embedded tissue blocks were
prepared, and sections were cut and stained with hematoxylin and
eosin (H&E) for routine histopathological examination. Because
surgically resected specimens had been cut routinely for pathology
specimens once a weak periodically, the duration of formalin
fixation of the surgically resected specimens varied from 1 to 6
days. Likewise, the duration of formalin fixation of the autopsied
tissues varied from 1 to 6 days. All specimens were diagnosed as
ductal adenocarcinomas of the pancreas. After a histological review
of the sections by three observers (T.E., H.T. and S.U.), a
representative tissue block was selected from each surgically
resected primary tumor, each primary tumor obtained by autopsy, and
each metastatic tumor obtained by autopsy. These tumor tissue
blocks were subjected to immuno histochemical studies.
Histological classification
The three observers graded the degree of tumor
differentiation. Tumor differentiation was classified into Grade 1
(well-differentiated type), Grade 2 (moderately differentiated
type) and Grade 3 (poorly differentiated type), according to the
degree of tubular formation (21).
The grade of each primary cancer was defined according to the
findings in the widest area of the representative section of the
cancer.
Immunohistochemistry
Immunohistochemical staining for EGFR was performed
using the EGFR pharmDx™ kit (Dako, Carpinteria, CA, USA), a global
standard kit for EGFR assay approved by the US Food and Drug
Administration (US FDA). Sections were deparaffinized in two
sequential xylene baths (5 min), 100% ethanol (3 min) and 95%
ethanol (3 min), followed by a 5-min single wash in wash-buffer
solution (Dako). Subsequently, at room temperature, the section was
rinsed in wash-buffer for 5 min, incubated in proteinase K solution
(Dako) for 5 min, rinsed again in the wash-buffer for 5 min,
incubated in peroxidase blocking agent for 5 min, rinsed, incubated
with the primary EGFR antibody or negative control reagent for 30
min, rinsed, incubated with visualization reagent for 30 min,
rinsed twice with the buffer, incubated with substrate chromogen
solution for 5 min and finally rinsed again with the buffer. Slides
were counterstained with hematoxylin and rinsed gently in reagent
quality water. The positive and negative controls used were
formalin-fixed, paraffin-embedded pellets of HT-29 and CAMA-1 cell
lines, which expressed and did not express EGFR, respectively
(Dako).
Immunohistochemical evaluation
Immunohistochemical evaluation was performed for
both the cell membrane and cytoplasm, separately, for the primary
or metastatic carcinoma samples. The level of membranous EGFR
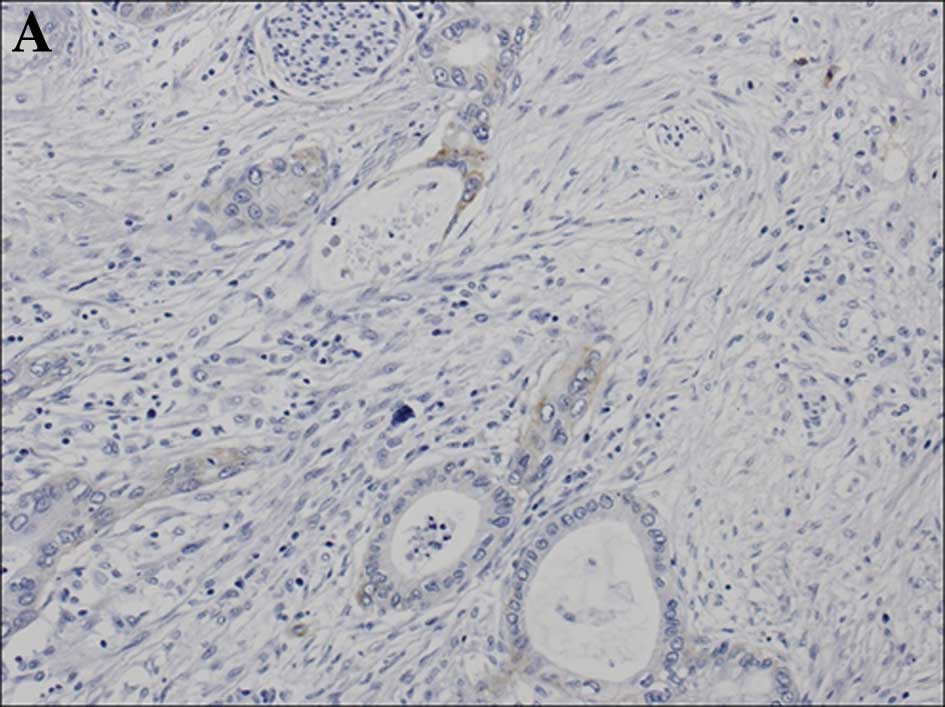
expression was stratified into 4 groups (scores 0, 1+, 2+ and 3+)
according to the criteria for the HER2 test (HercepTest) (22). In detail, when membranous staining
was observed in <10% of the tumor cells, a score of 0 was
assigned, regardless of the intensity of the staining. If faint or
barely perceptible membranous staining was detected in >10% of
the tumor cells, a score of 1+ was assigned. Scores of 2+ and 3+
were assigned when weak to moderate staining and strong staining,
respectively, were observed on the entire membrane in >10% of
the tumor cells (Fig. 1). Cases
showing a score of 2+ or 3+ were defined as showing
overexpression.
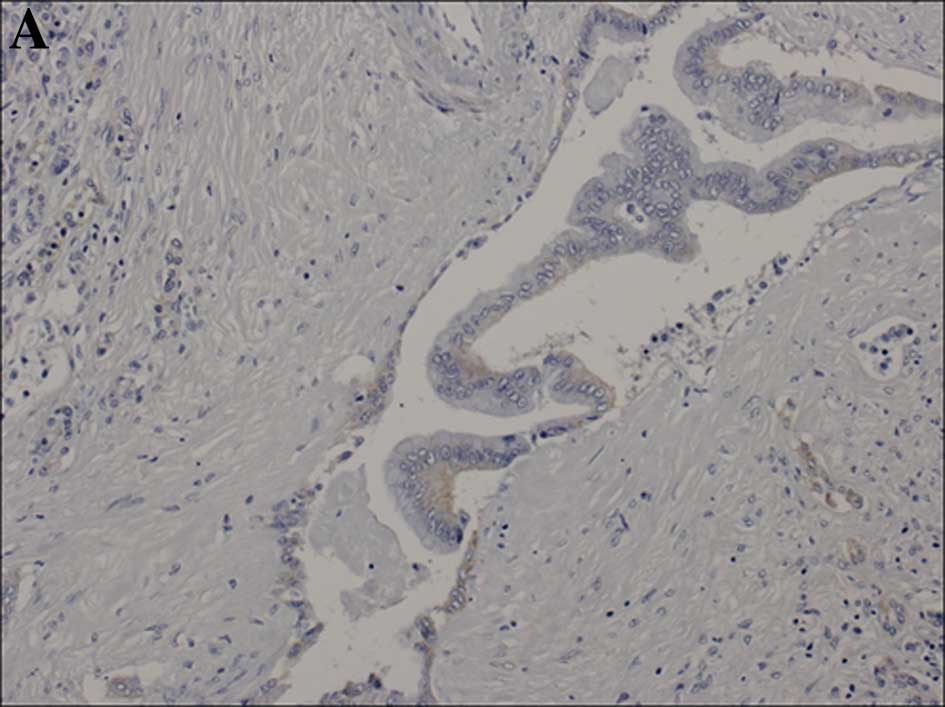
Cytoplasmic staining was divided into 3 grades (0,
1+ and 2+), as grading of the intensity of the immunoreaction was
difficult for the cytoplasm. The level of cytoplasmic staining was
categorized as follows: when cytoplasmic staining was observed in
<10% of the tumor cells, a score of 0 was assigned. If faint or
barely perceptible cytoplasmic staining was detected in >10% of
tumor cells, a score of 1+ was assigned. A score of 2+ was assigned
when moderate or strong staining, respectively, was observed in
>10% of the tumor cells. Cytoplasmic granular staining was also
scored as 2+. Cases showing a score of 2+ were judged as showing
overexpression (Fig. 2).
Statistical analysis
We used the χ2 test or Fisher’s exact
test to determine the correlation between EGFR expression and
histological grade. Differences were considered to indicate
statistical significance at a P-value <0.05. All statistical
analyses were performed using Statview 5.0 software (SAS Institute
Inc., Cary, NC, USA).
Results
The expression profiles of membranous and
cytoplasmic EGFR in both surgically resected cancers and far
advanced cancers obtained at autopsy are shown in Table II. In the 44 surgically resected
cancers, 13 (30%) exhibited membranous overexpression of EGFR,
comprising 1 case (2%) of score 3+ and 12 cases (27%) of score 2+
and 10 (23%) exhibited cytoplasmic overexpression of EGFR.
 | Table II.EGFR immunostaining in the surgically
resected cancers and the far advanced cancers obtained at
autopsy. |
Table II.
EGFR immunostaining in the surgically
resected cancers and the far advanced cancers obtained at
autopsy.
| No. of cases (%)
|
|---|
| Membranous EGFR
reactivity
| Cytoplasmic EGFR
reactivity
|
|---|
| Total | 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ |
|---|
| Surgically resected
cancers | 44 | 24 (55) | 7 (16) | 12 (27) | 1 (2) | 22 (50) | 12 (27) | 10 (23) |
| Far advanced
cancers | 40 | 6 (15) | 15 (38) | 7 (18) | 12 (30) | 4 (10) | 23 (58) | 13 (33) |
| Primary
cancersa | 20 | 3 (15) | 9 (45) | 3 (15) | 5 (25) | 2 (10) | 13 (65) | 5 (25) |
| Hepatic
metastasesa | 20 | 3 (15) | 6 (30) | 4 (20) | 7 (35) | 2 (10) | 10 (50) | 8 (40) |
In the primary tumors in the 20 far advanced
cancers, the percentage of samples with positivity for membranous
EGFR overexpression was 40%, (8 of 20), comprising 3 cases (15%) of
score 2+ and 5 cases (25%) of score 3+, and the percentage of
samples showing positivity for cytoplasmic EGFR overexpresion was
25% (5 of 20). In the hepatic metastases in the 20 far advanced
cancers, the positivity of membrane EGFR overexpression was 55%,
(11 of 20), comprising 4 cases (20%) of score 2+ and 7 cases (35%)
of score 3+, and the positivity of cytoplasmic EGFR overexpresion
was 40% (8 of 20).
In a total of 40 tumors at a far advanced stage, the
percentage of samples showing positivity for membranous EGFR
over-expression was 48% (19 of 40) comprising 7 cases (18%) of
score 2+ and 12 cases (30%) of score 3+, and the percentage of
samples showing positivity for cytoplasmic EGFR overexpresion was
33% (13 of 40). Therefore, the far advanced tumors tended to show
membranous and cytoplasmic EGFR overexpression more frequently than
the surgically resected tumors, although the difference was not
significant.
When these cases were stratified according to
histological grade, higher grade (Grades 2 and 3) cancer tissues
tended to show membranous EGFR overexpression more frequently (12
of 32, 38%) than the lower grade (Grade 1) cancer tissues (1 of 12,
8%) in the surgically resected pancreatic cancers, although the
difference was statistically marginal (P= 0.07). The percentage of
cytoplasmic EGFR overexpression did not differ statistically
between the low grade (Grade 1) tumors (1 of 12, 8%) and higher
grade (Grades 2 and 3) tumors (9 of 32, 28%) in the surgically
resected cases.
The tissues of the far advanced cancers showed
similar rates of membranous and cytoplasmic overexpressions,
regardless of histological grade. In the 40 far advanced tumors,
membranous EGFR overexpression was detected in 3 (43%) of 7 Grade 1
cases and in 16 (48%) of Grade 2 or 3 cases. In these far advanced
tumors, cytoplasmic overexpression of EGFR was detected in 14% (1
of 7) of Grade 1 tumors and 36% (12 of 33) of Grade 2 or 3 tumors
(Table III).
 | Table III.Expression of EGFR stratified
according to histological grading between the surgically resected
cancers and the far advanced cancers obtained at autopsy. |
Table III.
Expression of EGFR stratified
according to histological grading between the surgically resected
cancers and the far advanced cancers obtained at autopsy.
| | No. of tumors (%)
|
|---|
| Histological
grade | Total | Membranous EGFR
overexpression | P-valuea | Cytoplasmic EGFR
overexpression | P-valuea |
|---|
| Surgically resected
cancers | 44 | 13 (30)b | | 10 (23)c | |
| Grade 1 | 12 | 1 (8) | 0.07 | 1 (8) | 0.2 |
| Grade 2/3 | 32 | 12 (38) | | 9 (28) | |
| Far advanced
cancers | 40 | 19 (48)b | | 13 (33)c | |
| Grade 1 | 7 | 3 (43) | 0.8 | 1 (14) | 0.3 |
| Grade 2/3 | 33 | 16 (48) | | 12 (36) | |
Discussion
In the present study, we demonstrated that EGFR
overexpression in the cell membrane and cytoplasm was common in
both surgically resected and far advanced pancreatic carcinomas.
The occurrences of membranous and cytoplasmic EGFR over-expression
tended to be higher in the tumors at far advanced stages than in
the tumors that were at surgically resectable stages as determined
using a global standard kit for EGFR assay.
Cytoplasmic EGFR expression in the far advanced
cancers may be explained by the hypothesis of
epithelial-to-mesenchymal transition which is thought be an
important mechanism for promoting cancer invasion and metastasis
(23). Persistently activated EGFR
can decrease intercellular adhesion between tumor cells and enhance
cancer cell migration. Willmarth et al showed that
EGF-activated EGFR in MCF10A cells enhanced signal transduction
predominantly from the endosomes rather than from the membrane
(24). Barr et al (25) suggested that continuously
EGF-treated EGFR induced endocytosis of E-cadherin, a cell-to-cell
adhesion protein, which enhanced invasiveness in several human
cancer cell lines. Ueda et al previously reported that EGFR
overexpression in the cytoplasm of pancreatic cancers was
associated with poorer clinical outcome of patients (10,26).
The present study corroborated that not only membranous
overexpression but also cytoplasmic overexpression of EGFR is
important for the acquisition of highly aggressive and metastatic
properties of pancreatic carcinomas.
In the present study, the rate of EGFR
overexpression in surgically resected cancers tended to be higher
in higher grade (Grades 2 and 3) tumors than in low grade (Grade 1)
tumors. It is not surprising that poorly differentiated pancreatic
cancers exhibited a higher incidence of EGFR overexpression as the
patients with pancreatic carcinoma with altered EGFR activity tend
to show a more aggressive clinical course and a poorer clinical
outcome (27). Aggressive tumors
appear to require the activation of an EGFR-mediated autocrine
signaling in order to maintain proliferation. Therefore, we suppose
the possibility that cytoplasmic EGFR protein, which is newly
synthesized within the endoplasm reticulum, would be processed at
the cellular surface. Some investigators reported that binding of
EGF to EGFR activates its receptor tyrosine kinases and accelerates
its internalization through clathrin-coated pits followed by the
efficient lysosomal targeting of internalized receptors, which
results in receptor downregulation and degradation. Thus, the
ligand-induced internalization of EGFR, so-called endocytosis
trafficking, is characterized as activated EGFR (28–30).
If the EGFR ligands dissociated EGFR localized in endosomes, EGFR
would be deactivated and recycled to the plasma membrane.
We should consider the possibility that EGFR
localization and its activity in advanced or metastatic pancreatic
cancers may be modulated by chemotherapy or radiation therapy which
those patients had received. It is known that ionizing radiation,
hypoxia and oxidative stress can also phosphorylate EGFR with
ligand independence, which is sequentially internalized and
shuttled into the nucleus (31).
Li et al (32) reported
that the non-small cell lung cancer H226 cells which acquire
resistance to cetuximab, an anti-EGFR antibody, showed decreased
membranous EGFR accompanied by EGFR expressed with nuclear
localization. These findings imply that EGFR localization of cancer
cells may be an important determinant of responsiveness to specific
therapies.
In conclusion, we demonstrated using
immunohistochemistry that membranous and cytoplasmic EGFR
overexpression was frequently noted in surgically resected and far
advanced pancreatic cancers. These findings suggest that membranous
and cytoplasmic overexpression of EGFR may be indicative of the
potential aggressiveness of pancreatic cancers.
Acknowledgements
This study was supported in part by a
grant-in-aid for defense medicine from the Ministry of Defense and
a grant-in-aid from the Foundation for Promotion of Defense
Medicine. The authors thank Dr Tatsuro Takahashi and Mr. Ryuji
Saito, Department of Central Laboratory, Japan Labor Health and
Welfare Organization Kushiro Rosai Hospital, Kushiro, Hokkaido,
Japan, for technical assistance with the immunohistochemistry.
References
|
1.
|
Matsuno S, Egawa S, Fukuyama S, et al:
Pancreatic Cancer Registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004.PubMed/NCBI
|
|
2.
|
Bramhall SR, Allum WH, Jones AG, Allwood
A, Cummins C and Neoptolemos JP: Treatment and survival in 13,560
patients with pancreatic cancer, and incidence of the disease, in
the West Midlands: an epidemiological study. Br J Surg. 82:111–115.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37(Suppl
8): S4–S66. 2001.PubMed/NCBI
|
|
4.
|
Burris HA III, Moore MJ, Andersen J, et
al: Improvements in survival and clinical benefit with gemcitabine
as first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
5.
|
Oettle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar
|
|
6.
|
Ueno H, Kosuge T, Matsuyama Y, et al: A
randomised phase III trial comparing gemcitabine with surgery-only
in patients with resected pancreatic cancer: Japanese Study Group
of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer.
101:908–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kindler HL, Friberg G, Singh DA, et al:
Phase II trial of bevacizumab plus gemcitabine in patients with
advanced pancreatic cancer. J Clin Oncol. 23:8033–8040. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
9.
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ueda S, Ogata S, Tsuda H, et al: The
correlation between cytoplasmic overexpression of epidermal growth
factor receptor and tumor aggressiveness: poor prognosis in
patients with pancreatic ductal adenocarcinoma. Pancreas. 29:e1–e8.
2004. View Article : Google Scholar
|
|
11.
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cappuzzo F, Hirsch FR, Rossi E, et al:
Epidermal growth factor receptor gene and protein and gefitinib
sensitivity in non-small cell lung cancer. J Natl Cancer Inst.
97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hirsch FR, Varella-Garcia M, McCoy J, et
al: Increased epidermal growth factor receptor gene copy number
detected by fluorescence in situ hybridization associates with
increased sensitivity to gefitinib in patients with
bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group
Study. J Clin Oncol. 23:6838–6845. 2005. View Article : Google Scholar
|
|
14.
|
Tsao MS, Sakurada A, Cutz JC, et al:
Erlotinib in lung cancer – molecular and clinical predictors of
outcome. N Engl J Med. 353:133–144. 2005.
|
|
15.
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Han SW, Kim TY, Hwang PG, et al:
Predictive and prognostic impact of epidermal growth factor
receptor mutation in non-small cell lung cancer patients treated
with gefitinib. J Clin Oncol. 23:2493–2501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mitsudomi T, Kosaka T, Endoh H, et al:
Mutations of the epidermal growth factor receptor gene predict
prolonged survival after gefitinib treatment in patients with
non-small cell lung cancer with postoperative recurrence. J Clin
Oncol. 23:2513–2520. 2005. View Article : Google Scholar
|
|
18.
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non-small cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Takano T, Ohe Y, Tsuta K, et al: Epidermal
growth factor receptor mutation detection using high-resolution
melting analysis predicts outcomes in patients with advanced
non-small cell lung cancer treated with gefitinib. Clin Cancer Res.
13:5385–5390. 2007. View Article : Google Scholar
|
|
21.
|
Sobin LH and Wittekind Ch: International
Union Against Cancer (UICC) TNM Classification of Malignant Tumors.
6th edition. Wiley-Liss; New York: 2002
|
|
22.
|
Tsuda H, Akiyama F, Terasaki H, et al:
Detection of HER-2/neu (c-erb B-2) DNA amplification in primary
breast carcinoma. Interobserver reproducibility and correlation
with immunohistochemical HER-2 overexpression. Cancer.
92:2965–2974. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Dembinski JL and Krauss S:
Characterization and functional analysis of a slow cycling stem
cell-like subpopulation in pancreas adenocarcinoma. Clin Exp
Metastasis. 26:611–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Willmarth NE, Baillo A, Dziubinski ML,
Wilson K, Riese DJ II and Ethier SP: Altered EGFR localization and
degradation in human breast cancer cells with an amphiregulin/EGFR
autocrine loop. Cell Signal. 21:212–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Barr S, Thomson S, Buck E, et al:
Bypassing cellular EGF receptor dependence through
epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis.
25:685–693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ueda S, Hatsuse K, Tsuda H, et al:
Potential crosstalk between insulin-like growth factor receptor
type 1 and epidermal growth factor receptor in progression and
metastasis of pancreatic cancer. Mod Pathol. 19:788–796.
2006.PubMed/NCBI
|
|
27.
|
Holbro T, Civenni G and Hynes NE: The ErbB
receptors and their role in cancer progression. Exp Cell Res.
284:99–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Madshus IH and Stang E: Internalization
and intracellular sorting of the EGF receptor: a model for
understanding the mechanisms of receptor trafficking. J Cell Sci.
122:3433–3439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Sorkin A and Goh LK: Endocytosis and
intracellular trafficking of ErbBs. Exp Cell Res. 315:683–696.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sorkin A and von Zastrow M: Endocytosis
and signalling: intertwining molecular networks. Nat Rev Mol Cell
Biol. 10:609–622. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Dittmann K, Mayer C, Kehlbach R and
Rodemann HP: Radiation-induced caveolin-1 associated EGFR
internalization is linked with nuclear EGFR transport and
activation of DNA-PK. Mol Cancer. 7:692008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Li C, Iida M, Dunn EF, et al: Nuclear EGFR
contributes to acquired resistance to cetuximab. Oncogene.
28:3801–3813. 2009. View Article : Google Scholar : PubMed/NCBI
|