Introduction
Although the incidence and mortality of gastric
cancer (GC) have been steadily declining over several decades in
most countries, GC remains one of the most common causes of
cancer-related mortality (1). In
China, 300,000 cases of mortality and 400,000 new cases associated
with GC occur every year (2).
Although surgical resection is essential to treat this malignancy,
adjuvant (postoperative) chemotherapy should be considered for all
patients who are at high risk for recurring GC and have undergone
curative resection (3). Moreover,
postoperative adjuvant chemotherapy that is based on fluorouracil
regimens is associated with reduced risk of mortality in GC
compared with surgery alone (4).
Therefore, screening for GC patients who are likely to benefit from
fluorouracil regimens is being investigated.
Maspin is a 42-kDa protein that is a member of the
ovalbumin clade of serine protease inhibitors (serpins). Maspin was
first considered to be a tumor suppressor (5). However, conflicting opinions
concerning its function in cancer progression have been reported
(6–9). Maspin may have a significant role in
the progression and metastasis of gastric adenocarcinoma (10,11).
To date, no data on the predictive value of maspin expression for
fluorouracil-based chemotherapy in cases of advanced GC have been
reported.
The aim of the current study was to assess the
prognostic and predictive value of maspin expression for
5-fluorouracil (5-FU)-based chemotherapy in advanced GC
patients.
Materials and methods
Patients and clinical features
Human GC tissues were obtained with informed consent
from 127 patients with advanced GC who underwent radical resection
of GC in 2000 and 2001 at the Department of Surgery at Ruijin
Hospital (Shanghai, China). All diagnoses were confirmed using
histopathology. The stage and grade were established using the TNM
and World Health Organization classification systems. Patients who
had received previous neoadjuvant chemotherapy were excluded. Among
the 127 patients who underwent a radical resection, 53 (41.73%)
received 5-FU-based adjuvant chemotherapy (adjuvant group) and the
remaining 74 (58.27%) did not receive the treatment (surgery group)
due to postoperative complications or patient preference. The
chemotherapy method was performed as follows: leucovorin (200 mg)
was administered for two days via intravenous (i.v.) infusion prior
to 5-FU (400 mg/m2), which was administered as a 10-min
i.v. bolus, followed by 5-FU (600 mg/m2) as a continuous
22-h i.v. infusion with a light shield. This process was repeated
every 3 weeks for 4 to 6 cycles. All patients were followed up
systematically. The follow-up procedure included a complete history
and physical examination, complete blood count, platelet count,
multichannel serum chemistry analysis and additional assessments,
including endoscopy and other radiological studies, every 4 months
for 3 years and annually thereafter. The 127 patients included 82
(64.57%) males and 45 (35.43%) females, ranging from 27 to 74 years
old (mean, 55.4±12.1 years).
Immunohistochemistry (IHC)
The IHC assay was performed on 4-μm sections that
were cut from paraffin-embedded GC samples on adhesive glass
slides. The slides were deparaffinized in xylene, blocked using
endogenous peroxidase in methanol with 0.3% hydrogen peroxide,
washed in 0.01 mol/l sodium citrate and heated in a cooker for 10
min. Nonspecific binding sites were blocked by incubating with 10%
ovalbumin. The samples were incubated with primary mouse monoclonal
antibody against maspin (dilution 1:50; Novocastra,
Newcastle-upon-Tyne, UK) at 37°C for 2 h. Negative control slides
were treated without the primary antibody under equivalent
conditions. For the secondary developing reagents, polymer-HRP/M/R,
which was labeled using the EnVision™ System
(DakoCytomation, Glostrup, Denmark), and the
UltraSensitive™ S-P (Goat) kit (Maixin Bio, Fuzhou,
China) were used. The slides were developed using diaminobenzidine
(DAB; DakoCytomation) and counterstained with hematoxylin.
Pathologists who were blinded to patient outcomes
independently scored the immunostained slides as previously
described (12). Briefly, the
pathologists assigned a score for the percentage of
positive-staining tumor cells (0, none; 1, <1%; 2, 1–10%; 3,
11–33%; 4, 34%–66%; 5, >66%) and an intensity score (0, none; 1,
weak; 2, intermediate; 3, strong). These two scores were then added
to obtain a IHC score for the slides. The cytoplasmic and nuclear
stainings were evaluated separately. The IHC results were grouped
based on the IHC score (<3, negative; 4–6, weakly positive; 7–8,
positive).
Statistical analysis
A χ2 test or two-sided Fisher’s exact
test was used to evaluate the statistical correlation between the
maspin expression patterns and clinicopathological features of the
patients. We also used univariate analysis to evaluate the
correlation between the prognostic factors and overall survival
(OS). Multivariate analyses were performed using the Cox
regression. The differences in the mean values were evaluated using
the Student’s t-test. The survival curves were computed using the
Kaplan-Meier method and were compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
result. Statistical analyses were performed using software from
SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Maspin expression and clinicopathological
features
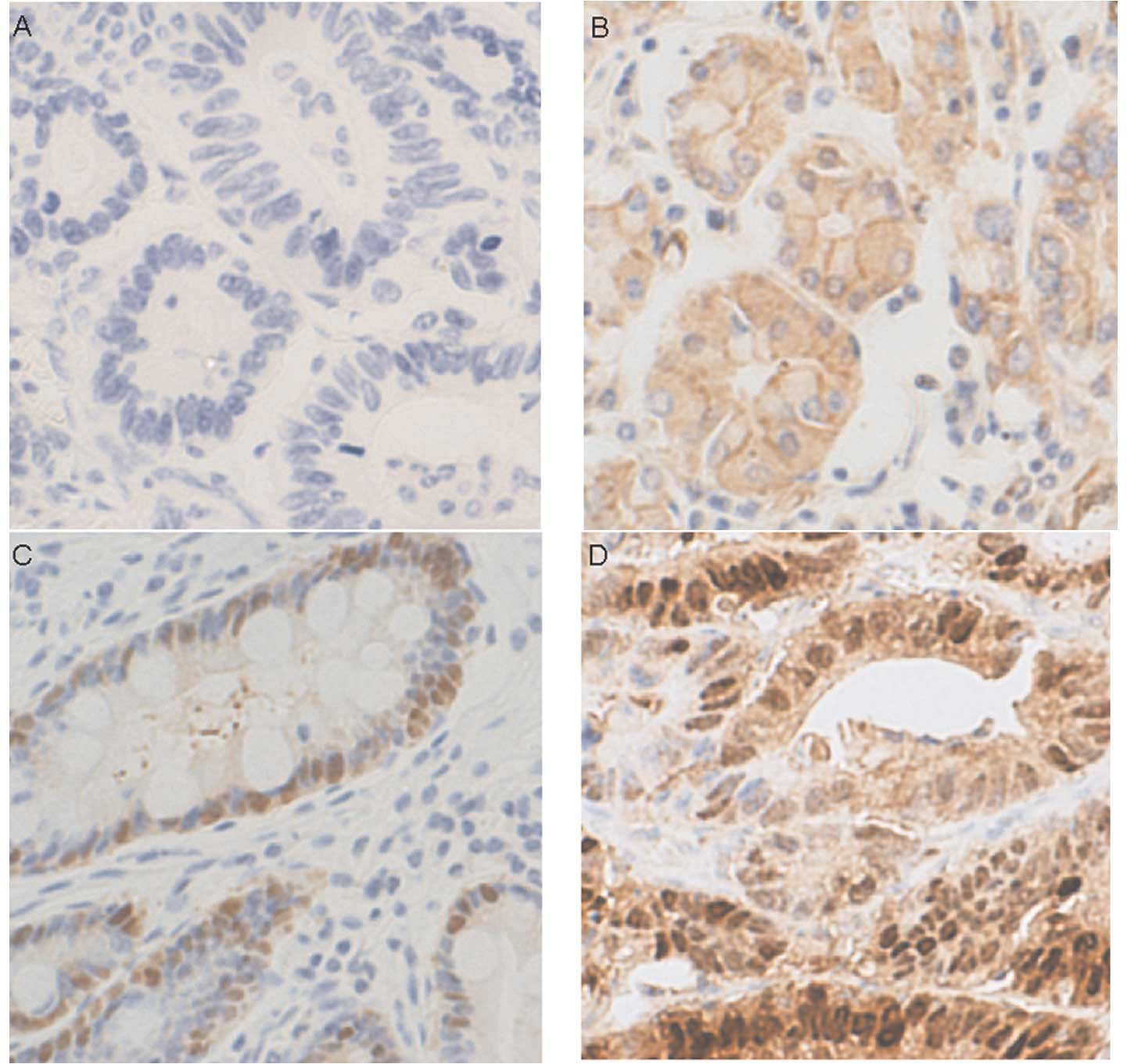
Maspin expression was detected in most patients
(nuclear or cytoplasmic IHC score ≥4). Nuclear and cytoplasmic
maspin expression was detected in 46.5 (59/127) and 68.5% (87/127)
of patients, respectively (Fig.
1). Nuclear maspin immunoreactivity was significantly
associated with larger tumor size (p=0.036), the depth of tumor
invasion (p=0.02) and lymph node metastasis (p=0.002), but not
patient age, gender or tumor cell differentiation (Table I). Cytoplasmic maspin
immunore-activity was associated with tumor cell differentiation
but not with the other clinicopathological variables.
 | Table I.Clinicopathological features with
regard to maspin expression patterns. |
Table I.
Clinicopathological features with
regard to maspin expression patterns.
| Maspin
immunoreactivity
|
|---|
| Nuclear
| Cytoplasmic
|
|---|
| Variable (n=127) | n (127) | Neg (68) | Pos (59) | P-value | n (127) | Neg (40) | Pos (87) | P-value |
|---|
| Age at diagnosis
(years) | | | | | | | | |
| ≤65 | 92 | 48 | 44 | 0.692 | 92 | 30 | 62 | 0.831 |
| >65 | 35 | 20 | 15 | | 35 | 10 | 25 | |
| Gender | | | | | | | | |
| Male | 82 | 44 | 38 | 1 | 82 | 28 | 54 | 0.43 |
| Female | 45 | 24 | 21 | | 45 | 12 | 33 | |
| Tumor size (cm) | | | | | | | | |
| ≤5 | 86 | 52 | 34 | 0.036 | 86 | 26 | 60 | 0.686 |
| >5 | 41 | 16 | 25 | | 41 | 14 | 27 | |
| T (depth of
invasion) | | | | | | | | |
| 2 | 40 | 28 | 12 | 0.02 | 40 | 12 | 28 | 0.96 |
| 3 | 55 | 28 | 27 | | 55 | 18 | 37 | |
| 4 | 32 | 12 | 20 | | 32 | 10 | 22 | |
| N (lymph node
metastasis) | | | | | | | | |
| 0 | 40 | 31 | 9 | 0.002 | 40 | 10 | 30 | 0.317 |
| 1 | 40 | 20 | 20 | | 40 | 11 | 29 | |
| 2 | 28 | 10 | 18 | | 28 | 10 | 18 | |
| 3 | 19 | 7 | 12 | | 19 | 9 | 10 | |
|
Differentiation | | | | | | | | |
|
Differentiated | 14 | 11 | 3 | 0.052 | 14 | 8 | 6 | 0.036 |
|
Undifferentiated | 113 | 57 | 56 | | 113 | 32 | 81 | |
Survival prediction using
clinicopathological factors
To elucidate factors that prolong survival, we
performed an analysis to identify the prognostic factors for OS
using the χ2 test or Fisher’s exact test. Eight factors
were analyzed, including the age and gender of the patients, tumor
size, the depth of tumor invasion, lymph node metastasis, tumor
cell differentiation and nuclear and cytoplasmic maspin
immunoreactivity. Based on the results of these univariate
analyses, five factors were significantly associated with OS
(Table II): tumor size
(p<0.001), the depth of tumor invasion (p=0.024), lymph node
metastasis (p=0.015), tumor cell differentiation (p=0.022) and
nuclear maspin immunoreactivity (p<0.001; Table II). In multivariate analysis,
nuclear maspin immunoreactivity retained an independent prognostic
factor for OS (p=0.002; Table
III).
 | Table II.Univariate analysis of prognostic
factors and overall survival. |
Table II.
Univariate analysis of prognostic
factors and overall survival.
| Overall survival
|
|---|
| Variable | n | Events | P-value |
|---|
| Age at diagnosis
(years) | | | |
| ≤65 | 92 | 35 | 0.122 |
| >65 | 35 | 18 | |
| Gender | | | |
| Male | 82 | 33 | 0.708 |
| Female | 45 | 20 | |
| Tumor size
(cm) | | | |
| ≤5 | 86 | 25 | 0 |
| >5 | 41 | 28 | |
| T (depth of
invasion) | | | |
| 2 | 40 | 11 | 0.024 |
| 3 | 55 | 23 | |
| 4 | 32 | 19 | |
| N (lymph node
metastasis) | | | |
| 0 | 40 | 11 | 0.015 |
| 1 | 40 | 14 | |
| 2 | 28 | 16 | |
| 3 | 19 | 12 | |
|
Differentiation | | | |
|
Differentiated | 14 | 10 | 0.022 |
|
Undifferentiated | 113 | 43 | |
| Nuclear maspin
immunoreactivity | | | |
| Positive | 59 | 37 | 0 |
| Negative | 68 | 16 | |
| Cytoplasmic maspin
immunoreactivity | | | |
| Positive | 87 | 34 | 0.44 |
| Negative | 40 | 19 | |
 | Table III.Multivariate analysis of prognostic
factors and overall survival. |
Table III.
Multivariate analysis of prognostic
factors and overall survival.
| Variable | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age at diagnosis
(years) | 1.096 | 0.604–1.986 | 0.763 |
| Gender | 1.122 | 0.628–2.005 | 0.697 |
| Tumor size | 4.118 | 2.014–8.419 | 0.000 |
| T (depth of
invasion) | 0.652 | 0.356–1.194 | 0.166 |
| N (lymph node
metastasis) | 1.760 | 1.197–2.589 | 0.004 |
|
Differentiation | 0.265 | 0.120–0.586 | 0.001 |
| Nuclear maspin
immunoreactivity | 2.660 | 1.438–4.919 | 0.002 |
| Cytoplasmic maspin
immunoreactivity | 0.967 | 0.527–1.774 | 0.913 |
Predictive impact of nuclear maspin
immunoreactivity
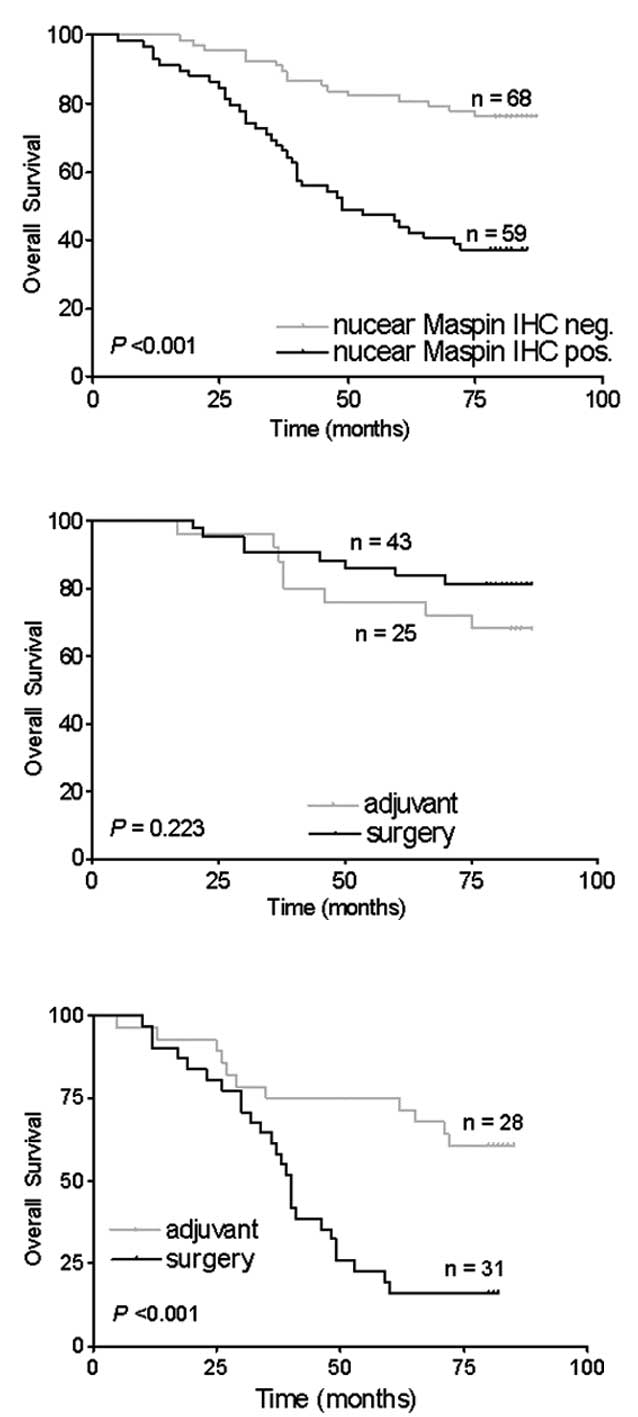
The predictive impact of nuclear maspin
immunoreactivity was evaluated using the Kaplan-Meier survival
analysis. The OS of patients with nuclear maspin expression was
significantly decreased compared with that of patients without
nuclear maspin expression (p<0.001; Fig. 2A). Among patients with or without
nuclear maspin expression, the patient survival was compared
between the surgery and adjuvant groups (Fig. 2B and C). No significant difference
in the OS was detected between the surgery and adjuvant groups in
the 68 patients who lacked nuclear maspin expression (p=0.223;
Fig. 2B). In the 59 patients with
nuclear maspin expression, the patients with 5-FU-based adjuvant
chemotherapy had significantly longer OS than those without
chemotherapy (p<0.001; Fig.
2C).
Discussion
Advanced GC is incurable, but chemotherapy plays an
essential role in the treatment of the disease. In the 1990s,
several chemotherapy regimens were used as active agents, including
5-FU, cisplatin, methotrexate, doxorubicin, etoposide, leucovorin
and mitomycin C. In the 2000s, a few new agents, including oral
fluoropyrimidine (capecitabine, S-1), irinotecan, taxanes
(paclitaxel, docetaxel) and oxaliplatin, were investigated for
treating gastrointestinal cancers (13). Nevertheless, there is no
internationally accepted regimen for the treatment of GC (14). The selection of appropriate
anticancer drugs for individual patients is important. 5-FU has
been widely used as a chemotherapeutic agent in GC and is
considered to be one of the most effective drugs against GC as it
mimics uracil and induces apoptosis in tumor cells (15–17).
However, not all patients with advanced GC respond well to this
anticancer drug. Therefore, the selection of patients with advanced
GC with high chemosensitivity is crucial for the personalized
therapy of GC (18,19).
Nuclear maspin expression has been reported to be
associated with the response to adjuvant 5-FU-based chemotherapy in
patients with stage III colon cancer (20). This finding is consistent with our
results showing that maspin is downregulated in
fluorouracil-resistant colon cancer cells (21). However, no data on the predictive
value of maspin expression in advanced GC have been reported. In
the present study, samples from 127 patients with advanced GC were
assessed to determine the prognostic and predictive value of maspin
expression. Nuclear maspin immunoreactivity was significantly
associated with clinicopathological variables, including tumor
size, the depth of tumor invasion and lymph node metastasis. These
clinicopathological variables and nuclear maspin immunoreactivity
had a significant association with OS. Nuclear maspin expression
was an independent adverse prognostic factor for OS in advanced GC
patients. However, among patients with nuclear maspin expression,
the adjuvant group had significantly longer OS than the surgery
group.
Maspin was first identified by subtractive
hybridization and the differential display method. It was found to
be expressed in normal mammary epithelial cells but not in a number
of mammary carcinoma cell lines and was considered to be a tumor
suppressor (5). As a tumor
suppressor, maspin inhibits the motility, invasive activity and
metastasis of cancer cells (5,22–24)
as well as angiogenesis (25).
However, conflicting opinions concerning its function in cancer
occurrence and progression have been reported. Maspin acts as an
oncogene rather than a tumor suppressor in undifferentiated thyroid
cancer, breast cancer and ductal adenocarcinoma of the pancreas
(6–8). Gastric tumor specimens were found to
have increased maspin expression levels compared with the
corresponding normal tissues and the frequency of maspin induction
was associated with the stage of GC and lymph node metastasis.
Maspin may have a significant role in the progression and
metastasis of gastric adenocarcinoma (10,11).
There are also conflicting opinions concerning the expression
pattern of maspin in different types of human cancer. A previous
study reported that a nuclear signal was present in 96% and a
cytoplasmic signal in 35% of invasive breast cancer cases (26). Invasive ovarian cancers have been
found to be more likely to have predominantly cytoplasmic staining
(9). Cytoplasmic and nuclear
expression of maspin has been identified in 47.6% of squamous cell
cancers of the larynx (27). Two
patterns of immunostaining for maspin have been observed in
maspin-positive GC cases: cytoplasm-only staining (67.0%) and
staining of both cytoplasm and nucleus (33.0%) (28); this is similar to our data, but we
also found nucleus-only staining (12.6%, 16/127 cases) in GC cases.
These different expression patterns of maspin may be due to the use
of different primary antibodies, IHC protocol and tumors. It has
been reported that the nuclear localization of maspin was
associated with increased survival, whereas the cytoplasmic
localization was associated with poor outcome in ovarian carcinoma
(9), although these results were
obtained without excluding the effects of adjuvant therapy. Our
data indicate that nuclear maspin expression is associated with
adverse clinical outcomes, but patients with positive nuclear
maspin expression had a better response to adjuvant 5-FU
chemotherapy. This makes nuclear maspin an attractive therapeutic
target.
In the current study, we revealed that nuclear
maspin expression was an independent adverse prognostic factor for
patients with advanced GC. The significance of nuclear maspin
expression in GC requires further study. In addition, the
significance of the correlation between nuclear maspin expression
and the response to adjuvant 5-FU chemotherapy remains unclear.
Previous studies have revealed that the E2F1-mediated upregulation
of maspin is enhanced by chemotherapeutic drugs and that the
inhibition of maspin expression significantly impairs the ability
of E2F1 to promote chemotherapy-induced apoptosis. Maspin mediates
E2F1-induced sensitivity of cancer cells to chemotherapy (29). Whether maspin mediates E2F1-induced
sensitivity of GC cells to chemotherapy or whether other factors
interact with maspin in 5-FU chemotherapy requires further
investigation.
In conclusion, the present study indicated that
nuclear maspin expression was associated with adverse clinical
outcomes in advanced GC patients. Patients with positive nuclear
maspin expression may exhibit a better response to adjuvant 5-FU
chemotherapy than patients with negative nuclear maspin expression.
Maspin may be a new predictive marker in patients with advanced GC
who are eligible for 5-FU adjuvant chemotherapy.
Acknowledgements
We would like to thank Ms. Yue-Mei Sun
from the Shanghai Institute of Digestive Surgery for assistance
with the patient follow-up, Mr. Jun Ji from the Shanghai Institute
of Digestive Surgery for assistance with the IHC experiment and Mr.
Kang Chen from the Department of Pathology, Ruijin Hospital, for
assistance with the preparation of sections for the IHC experiment.
This study was supported in part by the China National ‘863’
R&D High-Tech Key Project (2006AA02A301 and 2007AA02Z179) and
the National Natural Science Foundation of China (30772107).
References
|
1.
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar
|
|
2.
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
3.
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar
|
|
4.
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research Collaboration) Group; Paoletti X, Oba K, Burzykowski
T, et al: Benefit of adjuvant chemotherapy for resectable gastric
cancer: a meta-analysis. JAMA. 303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zou Z, Anisowicz A, Hendrix MJ, et al:
Maspin, a serpin with tumor-suppressing activity in human mammary
epithelial cells. Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ogasawara S, Maesawa C, Yamamoto M, et al:
Disruption of cell-type-specific methylation at the Maspin gene
promoter is frequently involved in undifferentiated thyroid
cancers. Oncogene. 23:1117–1124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Umekita Y, Ohi Y, Sagara Y, et al:
Expression of maspin predicts poor prognosis in breast-cancer
patients. Int J Cancer. 100:452–455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ohike N, Maass N, Mundhenke C, et al:
Clinicopathological significance and molecular regulation of maspin
expression in ductal adenocarcinoma of the pancreas. Cancer Lett.
199:193–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sood AK, Fletcher MS, Gruman LM, et al:
The paradoxical expression of maspin in ovarian carcinoma. Clin
Cancer Res. 8:2924–2932. 2002.PubMed/NCBI
|
|
10.
|
Kim SM, Cho SJ, Jang WY, et al: Expression
of maspin is associated with the intestinal type of gastric
adenocarcinoma. Cancer Res Treat. 37:228–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Song SY, Son HJ, Kim MH, et al: Prognostic
significance of maspin expression in human gastric adenocarcinoma.
Hepatogastroenterology. 54:973–976. 2007.PubMed/NCBI
|
|
12.
|
Harvey JM, Clark GM, Osborne CK, et al:
Estrogen receptor status by immunohistochemistry is superior to the
ligand-binding assay for predicting response to adjuvant endocrine
therapy in breast cancer. J Clin Oncol. 17:1474–1481. 1999.
|
|
13.
|
Boku N: Perspectives for personalization
in chemotherapy of advanced gastric cancer. Discov Med. 9:84–89.
2010.PubMed/NCBI
|
|
14.
|
Wagner AD, Grothe W, Haerting J, et al:
Chemotherapy in advanced gastric cancer: a systematic review and
meta-analysis based on aggregate data. J Clin Oncol. 24:2903–2909.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Won HJ, Ha TK, Kwon SJ, et al:
Differential effects of 5-fluorouracil on glucose transport and
expressions of glucose transporter proteins in gastric cancer
cells. Anticancer Drugs. 21:270–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jakobsen A, Nielsen JN, Gyldenkerne N, et
al: Thymidylate synthase and methylenetetrahydrofolate reductase
gene polymorphism in normal tissue as predictors of fluorouracil
sensitivity. J Clin Oncol. 23:1365–1369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10(Suppl 3): 49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Park DJ and Lenz HJ: Determinants of
chemosensitivity in gastric cancer. Curr Opin Pharmacol. 6:337–344.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kubota T and Weisenthal L: Chemotherapy
sensitivity and resistance testing: to be ‘standard’ or to be
individualized, that is the question. Gastric Cancer. 9:82–87.
2006.
|
|
20.
|
Dietmaier W, Bettstetter M, Wild PJ, et
al: Nuclear Maspin expression is associated with response to
adjuvant 5-fluorouracil based chemotherapy in patients with stage
III colon cancer. Int J Cancer. 118:2247–2254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zheng Z, Li J, He X, et al: Involvement of
RhoGDI2 in the resistance of colon cancer cells to 5-fluorouracil.
Hepatogastroenterology. 57:1106–1112. 2010.PubMed/NCBI
|
|
22.
|
Sheng S, Truong B, Fredrickson D, et al:
Tissue-type plasminogen activator is a target of the tumor
suppressor gene maspin. Proc Natl Acad Sci USA. 95:499–504. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Dokras A, Gardner LM, Kirschmann DA, et
al: The tumour suppressor gene maspin is differentially regulated
in cytotrophoblasts during human placental development. Placenta.
23:274–280. 2002. View Article : Google Scholar
|
|
24.
|
Shi HY, Zhang W, Liang R, et al: Modeling
human breast cancer metastasis in mice: maspin as a paradigm.
Histol Histopathol. 18:201–206. 2003.PubMed/NCBI
|
|
25.
|
Zhang M, Volpert O, Shi YH, et al: Maspin
is an angiogenesis inhibitor. Nat Med. 6:196–199. 2000. View Article : Google Scholar
|
|
26.
|
Mohsin SK, Zhang M, Clark GM, et al:
Maspin expression in invasive breast cancer: association with other
prognostic factors. J Pathol. 199:432–435. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Marioni G, Blandamura S, Giacomelli L, et
al: Nuclear expression of maspin is associated with a lower
recurrence rate and a longer disease-free interval after surgery
for squamous cell carcinoma of the larynx. Histopathology.
46:576–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Lee DY, Park CS, Kim HS, et al: Maspin and
p53 protein expression in gastric adenocarcinoma and its clinical
applications. Appl Immunohistochem Mol Morphol. 16:13–18.
2008.PubMed/NCBI
|
|
29.
|
Ben Shachar B, Feldstein O, Hacohen D, et
al: The tumor suppressor maspin mediates E2F1-induced sensitivity
of cancer cells to chemotherapy. Mol Cancer Res. 8:363–372.
2010.PubMed/NCBI
|