Introduction
Recombinant human endostatin (Endostar, Simcere
Pharmaceutical Group, China) is a modified human endostatin with a
nine amino acid sequence at the N-terminus (MGGSHHHHH) that
significantly exhibits increased heat stability and proteolytic
resistance compared with the endogenous protein (1). Endostar, in combination with
vinorelbine plus cisplatin (the NP regimen), significantly
lengthens the progression-free survival of patients with non-small
cell lung cancer (NSCLC) and Endostar plus paclitaxel-carboplatin
improves the overall survival of NSCLC patients (2). It is recommended that Endostar is
administered once per day over four hours. The half-life of
Endostar in vivo is only 10 h, so continuous administration
may augment the anticancer effects. However, it is unclear whether
continuous administration results in additional adverse side
effects. To evaluate the safety of the continuous administration of
Endostar, we conducted a pre-clinical study in nude mice, using a
ubiquitous immunocompromised cancer model.
Materials and methods
Animals
A total of 16 female nude mice (weighing
approximately 20 g) were used in this study. The dosage was
converted from clinical dosage, taking into consideration the body
surface area of the nude mice and the bioavailability of the
intraperitoneal (i.p.) method, and daily dosage was calculated as 2
mg/kg. A total of 12 mice were anesthetized by i.p. injection with
10% chloral hydrate and implanted with an intraperitoneal
mini-osmotic drug pump (ALZET 107D, Durect Corp., Cupertino, CA,
USA) which released drug continuously for seven days. The mice were
randomly divided into three groups of four mice each. The drug
pumps in the continuous administration group were filled with 14
mg/kg Endostar (diluted in 100 μl saline) and the mice were
injected i.p. with 100 μl saline daily. The drug pumps in the
intermittent administration group were filled with 100 μl saline
and the mice were injected i.p. with 2 mg/kg Endostar (diluted in
100 μl saline) daily for seven days. The drug pumps in the saline
group were filled with 100 μl saline and the mice were injected
i.p. with 100 μl saline for seven days. The remaining four mice
constituted the control group. The weight, activity and eating
behaviors of the mice were measured daily. All mice were sacrificed
on the eighth day and whole blood and serum were collected. In
addition, hearts, kidneys and lungs were removed and examined
histochemically for Endostar-induced damage. The experimental
protocol was approved by the Shanghai Medical Experimental Animal
Care Committee.
Determination of Endostar serum
concentrations
The assay was performed as described by Paborsky
et al (3). Endostar was
diluted to 400 ng/ml in serum from the drug-free mice and then
serially diluted to 6.25 ng/ml with a sample dilution buffer to
establish the standard curve. These Endostar and mouse serum
samples were diluted with 100 mM NaAc (pH 5.5), followed by
incubation on Ni-coated plates (Qiagen, Hamburg, Germany) overnight
at 4°C. The serum-Endostar solution was incubated with biotinylated
goat anti-human endostatin antibody (R&D Systems, Minneapolis,
MN, USA) for 2 h at room temperature and then with streptavidin-HRP
(Pierce Biotechnology, Inc., Rockford, IL, USA) for 1 h at room
temperature. Finally, the labeled solution was incubated with the
chromogen 3,3′,5,5′-tetramethylbenzidine (TMB) for 20 min at room
temperature. The Endostar concentration was determined by measuring
the absorbance at 450 nm and calculated by four parameter logistics
according to the standard curve. The assay had been validated by
the Simcere Pharmaceutical Group using standard Endostar with a
detection limit of 12.5–400 ng/ml. The mean recovery was 101.7%,
the mean coefficient of correlation was 1 and the mean RE of every
data point was 0.304% (unpublished data from Simcere Pharmaceutical
Group).
Flow cytometric analysis
Red blood cells from mouse peripheral blood (100 μl)
were lysed and washed. Antibodies against CD11b, CD146 and CD105
were used. The antibodies were conjugated to fluorescein
isothiocyanate (FITC), phycoerythrin (PE) or allophycocyanin (APC).
Appropriate isotype-matched controls were used in all experiments.
All the antibodies and reagents were purchased from Biolegend (San
Diego, CA, USA). The cells were incubated with the antibodies for
25 min at 4°C. Following incubation, the cells were washed twice
and analyzed using a FACScan cytometer with CellQuest software (BD
Biosciences, San Jose, CA, USA).
Histology and immunohistochemical
staining
Formalin-fixed, paraffin-embedded heart, lung and
kidney tissues were routinely stained with hematoxylin and eosin
(H&E). For CD146 staining, the tissue sections were fixed in
10% formalin and treated with anti-CD146 (1:100; Biolegend),
followed by staining of rabbit anti-mouse IgG and development with
3,3-diaminobenzidine (DAB). The sections were counterstained with
hematoxylin and examined under light microscopy (x200).
Microvessel counts
Microvessel counts of the tissues were performed as
previously described (4). Briefly,
any brown stained endothelial cell or cluster was considered to be
a single, countable microvessel. The areas of highest
neovascularization were identified by scanning the tissue sections
at low magnification (×40). After the area of highest
neovascularization was identified, individual microvessel counts
were performed on five fields per specimen (×200). We obtained a
mean value per specimen from these five fields to calculate the
microvessel count for each tissue.
Statistical analysis
Statistical analyses were performed using the SPSS
15.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
All data are reported as the mean ± SD. The differences were
analyzed by one-way analysis of variance (ANOVA) or t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Weight changes in the treatment
groups
The body weight of the mice in the control group
increased after seven days, while the mice in the three injection
groups exhibited no significant changes in body weight over seven
days of Endostar or saline administration. One mouse in each of the
injection groups with i.p. mini-osmotic drug pumps died during the
course of treatment (Table I). No
significant difference in the motor activity or eating behaviors
among the injection groups was observed.
 | Table I.Body weights (g) of the mice in the
different treatment groups. |
Table I.
Body weights (g) of the mice in the
different treatment groups.
| Control group
(n=4) | Saline group
(n=3) | Intermittent
administration group (n=3) | Continuous
administration group (n=3) | P-value |
|---|
| Prior to
Endostar | 19.4±1.2 | 18.6±0.7 | 18.9±2.7 | 20.6±0.4 | 0.445 |
| Following
Endostar | 21.4±1.1 | 18.9±1.3 | 18.4±3.8 | 21.2±0.2 | 0.189 |
| P-value | 0.047 | 0.739 | 0.852 | 0.052 | |
Endostar concentration in serum
The concentration of Endostar in serum samples from
each mouse in the intermittent and continuous administration groups
was measured 24 h after the termination of drug administration. In
both groups, only residual traces of Endostar were detected and the
average serum concentration was not significantly different between
the groups (20.6±13.9 vs. 26.3±5.5 ng/ml, P=0.547)
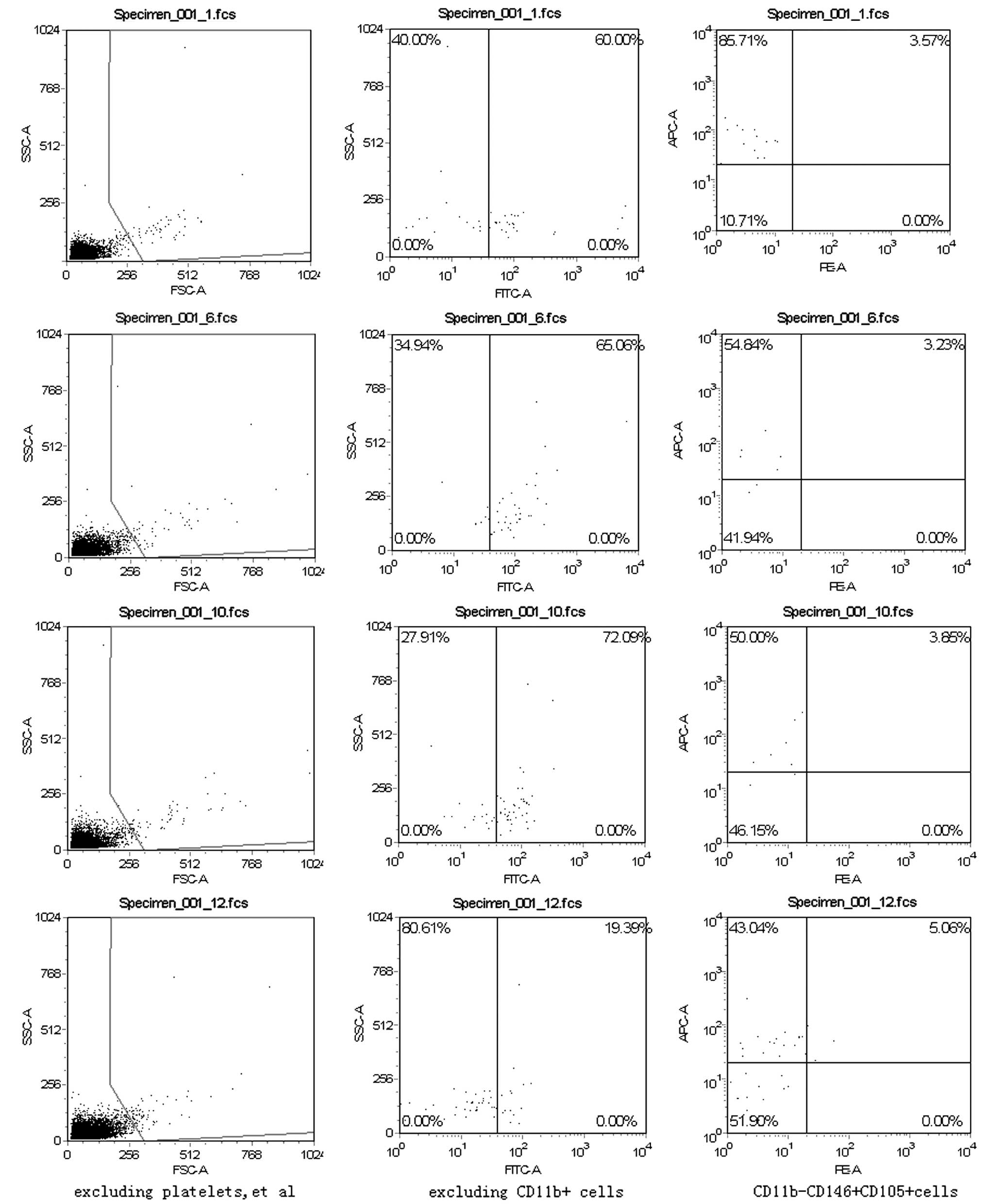
Fraction of
CD11b−CD146+CD105+ cells in the
peripheral blood nucleated cell population
The fraction of
CD11b-CD146+CD105+ cells in the peripheral
blood nucleated cell population in the continuous administration
group (4.41±1.46%) was higher than that in the intermittent
(1.05±0.15%), saline (1.16±0.55%) and control groups (1.49±1.30%;
P=0.011; Fig. 1).
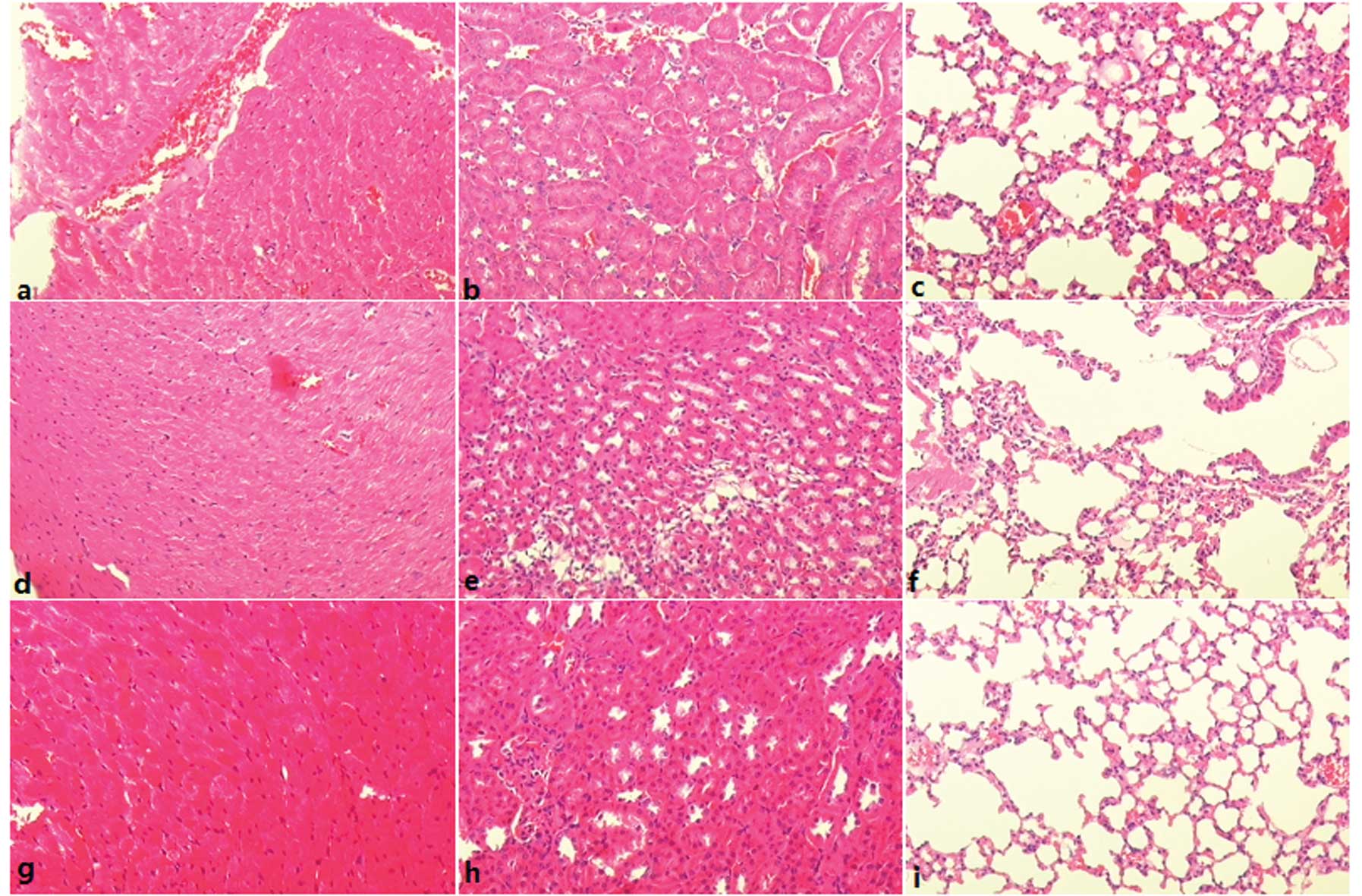
Histology
Tissue histology of the hearts, kidneys and lungs
revealed no clear signs of injury in any of the treatment groups
(Fig. 2). Edema, necrosis and
hemorrhage were not noted in the tissues of heart, kidney and lung
in the intermittent and continuous administration groups, similar
to tissues in the saline group.
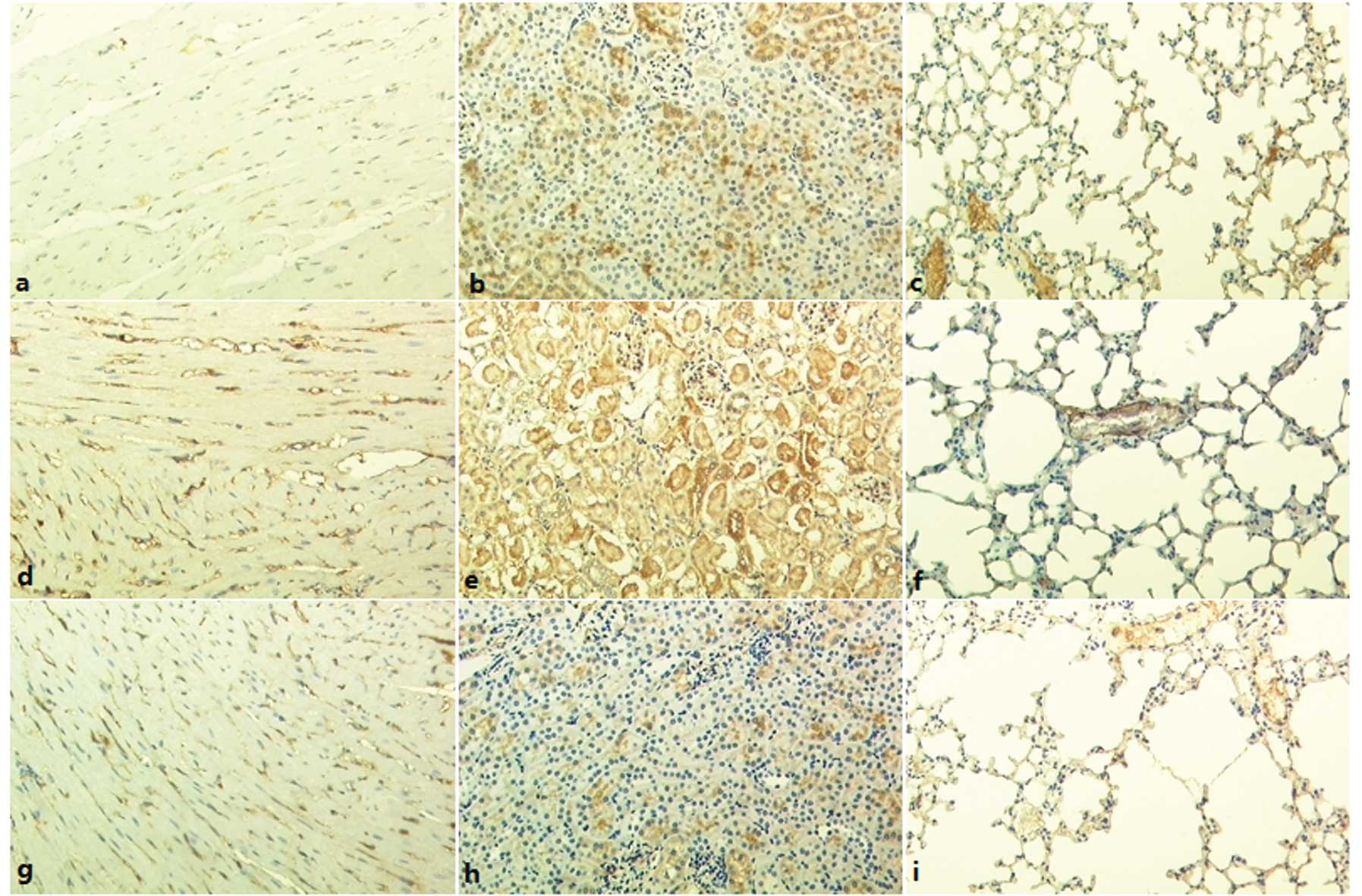
Microvessel counts
The number of CD146-labeled microvessels in heart,
kidney and lung tissues were not found to be significantly
different among the four groups (Table
II, Fig. 3).
 | Table II.CD146-labeled microvessels in heart,
kidney and lung tissue. |
Table II.
CD146-labeled microvessels in heart,
kidney and lung tissue.
| Organ | Control group
(n=4) | Saline group
(n=3) | Intermittent
administration group (n=3) | Continuous
administration group (n=3) | P-value |
|---|
| Myocardium | 132.3±8.1 | 131.3±10.0 | 125.3±13.1 | 127.7±8.7 | 0.820 |
| Kidney | 74.0±6.9 | 72.3±4.0 | 69.3±12.5 | 77.3±4.2 | 0.638 |
| Lung | 6.3±2.1 | 6.0±2.0 | 5.3±1.5 | 6.7±0.6 | 0.806 |
Discussion
Antiangiogenesis therapy has great potential for
cancer treatment, but the clinical efficacy of drugs such as the
humanized vascular endothelial growth factor (VEGF) monoclonal
anti-body bevacizumab and Endostar has not been satisfactory.
Previous efforts have focused on enhancing the treatment efficacy
by modifying the timing, sequence or dose of the anti-angiogenic
drugs in combination with cytotoxic chemotherapy drugs (5,6). In
our previous study concerning the correlation between the dose and
efficacy of Endostar, we found an effective dose range beyond which
the effect on tumor growth decreased (data not shown). In the
present study, we hypothesized that the continuous administration
of Endostar may augment the anti-tumor effects. It is not known,
however, whether the continuous administration of Endostar disrupts
angiogenesis in cancer tissues while sparing normal tissues. To
this end, we observed the impact of the continuous administration
of Endostar on normal heart, kidney and lung tissues in nude
mice.
The mini-osmotic pump was used to control the timing
of i.p. injection. It has been demonstrated that the zinc ion
structure of Endostar allows the molecule to withstand higher
temperatures and proteolysis by trypsin, chymotrypsin and
carboxypeptidase, so prolonged drug administration times should not
impact drug activity (1). In order
to preclude the effects of residual drug released from the osmotic
pump 24 h after termination of pump flow, we compared the
concentration of Endostar in serum in the intermittent
administration group with that in the continuous administration
group at that time point. Only nanogram amounts of drug were
detected in the serum of the two groups and there was no
difference, indicating that the difference between the two groups
was not due to the residual Endostar in the pump.
No significant difference was observed in the motor
activity, weight or eating behaviors between mice in the
intermittent administration, continuous administration and saline
groups, although the weight of the control mice did increase, which
may be the result of implantation surgery. This suggests that
intermittent drug administration, continuous drug administration
and saline administration did not have a significant impact on the
general health or feeding habits of the surviving test mice. The
mortality of the mice was 1/4 in the intermittent administration,
continuous administration and the saline groups and all the mice
were implanted with mini-pumps. Moreover, these deaths all occurred
within 24 h of pump implantation, suggesting that mortality arose
from i.p. implantation surgery rather than from drug injection.
We determined the relative number of vascular
endothelial cells with the expression pattern
CD11b−CD146+CD105+ in the
peripheral blood. The phenotyping of vascular endothelial cells by
surface antigens remains controversial, especially for mice, so we
selected CD146 as one of the antigens as it is a vascular
endothelial cell marker common to humans and mice. The surface
expression of CD146 is found in human T and B lymphocytes, while it
is expressed in the CD11b+ natural killer (NK) cells and
neutrophils of mice (7). Thus, the
CD11b−CD146+ expression pattern should
exclude NK cells and neutrophils in mice. Vascular endothelial
cells which are involved in angiogenesis are CD105+
(8,9). The number of CD105+
vascular endothelial cells in peripheral blood may reflect vascular
injury and renewal. In normal tissues, only 0–1% of vascular
endothelial cells renew daily. Moreover, the number of vascular
endothelial cells in peripheral blood is small in samples from
healthy mice, while this number increases significantly following
vascular injury (10–13). The present study found a
significant increase in CD11b-CD146+CD105+
cells in the continuous administration group, while no significant
difference was found among the control, saline and intermittent
administration groups after seven days. This suggested that
intermittent Endostar delivery did not significantly impact the
vascular endothelium, while continuous Endostar administration may
promote injury of the endothelium.
A randomized, double-blind, placebo-controlled study
found that Endostar plus chemotherapy caused only grade 1 and grade
2 cardiac ischemia in patients, but no significant difference was
noted in overall survival and progressive-free survival between the
treatment and contol groups (2).
Although in the present study the histological structure of
myocardial, kidney and lung tissues was not detectably affected and
immunohistochemical staining analysis did not reveal any
significant differences in microvessel density in these organs
between the groups, we suggest that continuous Endostar
administration injures vascular endothelium, as evidenced by the
increased number of
CD11b−CD146+CD105+ cells in the
peripheral blood compared with the other groups. The safety of
continuous Endostar administration to healthy nude mice is
therefore a valid concern.
In conclusion, the continuous administration of
Endostar increases the number of
CD11b−CD146+CD105+ vascular
endothelial cells in the peripheral blood, suggesting injury to
normal vessels, although no histological injury of myocardium,
kidney or lung was found following seven days of treatment.
Therefore, we suggest that the continuous administration of
Endostar may not be safe for healthy mice.
References
|
1.
|
Jiang LP, Zou C, Yuan X, Luo W, Wen Y and
Chen Y: N-terminal modification increases the stability of the
recombinant human endostatin in vitro. Biotechnol Appl Biochem.
54:113–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Han B, Xiu Q, Wang H, Shen J, Gu A and Luo
Y: A multicenter, randomized, double-blind, placebo-controlled
study to evaluate the efficacy of paclitaxel-carboplatin alone or
with endostar for advanced non-small cell lung cancer. J Thorac
Oncol. 6:1104–1109. 2011. View Article : Google Scholar
|
|
3.
|
Paborsky LR, Dunn KE, Gibbs CS and
Dougherty JP: A nickel chelate microtiter plate assay for six
histidine-containing proteins. Anal Biochem. 234:60–65. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlation with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409. 1993.
|
|
5.
|
Shaked Y, Henke E, Roodhart JM, et al:
Rapid chemotherapy-induced acute endothelial progenitor cell
mobilization: implications for antiangiogenic drugs as
chemosensitizing agents. Cancer Cell. 14:263–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Celik I, Surucu O, Dietz C, et al:
Therapeutic efficacy of endostatin exhibits a biphasic
dose-response curve. Cancer Res. 65:11044–11050. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Despoix N, Walzer T, Jouve N, et al: Mouse
CD146/MCAM is a marker of natural killer cell maturation. Eur J
Immunol. 38:2855–2864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dallas NA, Samuel S, Xia L, et al:
Endoglin (CD105): A marker of tumor vasculature and potential
target for therapy. Clin Cancer Res. 14:19312008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Khan SS, Solomon MA and McCoy JP Jr:
Detection of circulating endothelial cells and endothelial
progenitor cells by flow cytometry. Cytometry B Clin Cytom. 64:1–8.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Abdelmoneim SS, Talwalkar J, Sethi S,
Kamath P, et al: A prospective pilot study of circulating
endothelial cells as a potential new biomarker in portal
hypertension. Liver Int. 30:191–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Goon PK, Boos CJ and Lip GY: Circulating
endothelial cells: markers of vascular dysfunction. Clin Lab.
51:531–538. 2005.PubMed/NCBI
|
|
12.
|
Dome B, Timar J, Ladanyi A, Paku S,
Renyi-Vamos F, Klepetko W, et al: Circulating endothelial cells,
bone marrow-derived endothelial progenitor cells and proangiogenic
hematopoietic cells in cancer: from biology to therapy. Crit Rev
Oncol Hematol. 69:108–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Erdbruegger U, Haubitz M and Woywodt A:
Circulating endothelial cells: A novel marker of endothelial
damage. Clin Chim Acta. 373:17–26. 2006. View Article : Google Scholar : PubMed/NCBI
|